Summary
Background and objectives
Recognition of CKD by primary care practitioners is essential in rural communities where nephrology access is limited. This study determined the prevalence of undocumented CKD in patients cared for in rural primary care practices and evaluated characteristics associated with undocumented CKD as well as CKD management.
Design, setting, participants, & measurements
A retrospective cohort study, conducted within the Oregon Rural Practice Based Research Network, consisted of 865 CKD patients with serum creatinine≥1.5 mg/dl in males and ≥1.3 mg/dl in females and an estimated GFR<60 ml/min per 1.73 m2. Documentation of a CKD diagnosis and laboratory values were abstracted by chart review.
Results
Of CKD patients, 51.9% had no documentation of CKD. Undocumented CKD occurred more frequently in female patients (adjusted odds ratio=2.93, 95% confidence interval=2.04, 4.21). The association of serum creatinine reporting versus automating reporting of estimated GFR on CKD documentation was dependent on patient sex, years of practitioner experience, and practitioner clinical training. Hypertensive patients with documented CKD were more likely to have a BP medication change than patients with undocumented CKD (odds ratio=2.07, 95% confidence interval=1.15, 3.73). Only 2 of 449 patients with undocumented CKD were comanaged with a nephrologist compared with 20% of patients with documented CKD (odds ratio=53.20, 95% confidence interval=14.90, 189.90).
Conclusions
Undocumented CKD in a rural primary care setting is frequent, particularly in female patients. Depending on practitioner characteristics, automatic reporting of estimated GFR might improve documentation of CKD in this population.
Introduction
Approximately 26 million people in the United States are estimated to have CKD (1). Often, these patients are managed by primary care practitioners (PCPs) and referred to a nephrologist with advanced CKD (2–4). Early recognition by PCPs is essential to implement treatments to slow CKD progression. Although previous studies have shown poor recognition of CKD, limitations include data collection in single centers and use of International Classification of Disease (ICD) codes to evaluate documentation, which may underestimate practitioner recognition (5–8).
The use of serum creatinine (SCr) as a marker of disease may contribute to poor recognition. The use of automatic reporting of estimated GFR (eGFR) is controversial (9,10), and results evaluating its implementation on improving CKD recognition have been variable (5,7,11).
Compared with their urban counterparts, rural residents have lower incomes and educational attainment, and they travel farther distances to seek medical care (12); these disparities could result in suboptimal CKD care. In 2004, 19% of the US population was estimated to live in a rural area (13). Studies show reduced access to kidney transplantation, home dialysis training, and renal replacement therapy in less-populated areas of the United States (14,15). Although limited nephrology care has been shown in Canadian remote dwellers with CKD (16), little research evaluating predialysis care in US rural communities has been performed.
The objectives of this study were to determine the prevalence of undocumented CKD in rural primary care practices and investigate whether laboratory reporting of eGFR reduces this prevalence. We also investigated the patient and practitioner characteristics that were associated with undocumented CKD and whether undocumented CKD was associated with less adherence to guideline-based care.
Materials and Methods
Study Design and Population
A retrospective cohort study was performed by medical record review within the Oregon Rural Practice Based Research Network; 15 of 46 independent rural practices were chosen based on geographic diversity. Supplemental Tables 1 and 2 summarize additional information about the clinics and practitioners audited.
Interpath generated a list of patients 18 years or older whose PCP was a clinician chosen for the study and who had an SCr measurement between January 1 and December 31, 2006 of ≥1.5 mg/dl (males) or ≥1.3 mg/dl (females). These medical records were audited for possible inclusion. For patients with only one SCr measurement over the 12-month follow-up period, if the SCr value met the above criteria, they were included. In patients with multiple SCr measurements over the follow-up period, as long as two values of SCr met the above criteria by March 1, 2007, the subject was included. Patients with ESRD as defined by receiving dialysis or a history of kidney transplantation were excluded. Medical records of active patients were reviewed until we obtained a maximum of 25 patients per clinician meeting the study inclusion criteria. Although charts were reviewed using these inclusion criteria, to limit the analysis to those patients with more advanced CKD, the final analysis set included only data from patients with an initial eGFR<60 ml/min per 1.73 m2 by the Modification of Diet in Renal Disease (MDRD) Equation (17).
This study was approved by the Oregon Health and Science University Institutional Review Board and performed in adherence to the principles of the Declaration of Helsinki.
Variables
For all subjects included, the primary outcome measured was whether a CKD diagnosis was documented in the medical record including the terms “renal insufficiency,” “renal failure,” or “chronic kidney disease” or an ICD-9 code indicating kidney disease. Study variables included age, sex, race, ethnicity, zip code, comorbidities, antihypertensive medication use, nephrology referral, up to five initial and follow-up BP measurements and SCr values, and CKD laboratory values (urinalysis, proteinuria measurement, calcium, phosphorus, parathyroid hormone, hemoglobin, and iron studies). Zip code was used to categorize a patient’s home address into rural–urban commuting area codes (version 2.0) (18). For all variables, medical records were reviewed for the 12 months after the first SCr measurement that met the inclusion criteria. No data were abstracted before this point.
For each SCr value, if automatic reporting of eGFR was available, the corresponding value was abstracted. For SCr measurements without eGFR reporting, the eGFR was calculated using the four-variable MDRD Equation (17). Patients were categorized by stage of CKD using the initial eGFR. Stage 3 CKD was further categorized into stages 3A and 3B (19). The eight patients with an initial eGFR<15 ml/min per 1.73 m2 were included in stage 4 CKD.
Statistical Analyses
All analyses were performed using SAS 9.3. Associations with CKD documentation were analyzed using logistic regression models. To account for within-clinic correlation, a generalized estimating equation approach was used for parameter estimation (20). Specifically, we assumed a constant correlation among patients within the same clinic. The estimated correlation was 0.14 in the model that did not include practitioner characteristics and 0.11 in the model that included practitioner training and years of experience.
The initial model was built using variables related to patient characteristics and laboratory report format. Because of the strong association of sex with undocumented CKD, variables were tested for inclusion using three different analysis sets: all subjects, male subjects only, and female subjects only (stratified analysis). Variables were added to the initial model if they were significantly associated with undocumented CKD in either of the stratified analyses, and variables with differing strengths of association in the stratified analysis were further tested for an interaction effect in the all-subjects analysis. Because the initial model indicated an association of laboratory report format with undocumented CKD, practitioner characteristics were evaluated with adjustment for the variables identified by the initial model, and laboratory report format was used as a stratification variable in the initial evaluation before modeling interaction terms. Odds ratios and P values for variables involved in interactions were computed using generalized linear model parameterization of categorical variables and estimate statements that included main effects and all interaction terms in which the variable was included.
The analysis of the association of undocumented CKD with compliance to practice guidelines was limited to patients not comanaged with a nephrologist. Logistic regression analyses using generalized estimating equation parameter estimation were performed with the binary outcome of practice guideline compliance. The analyses were adjusted for patient sex, age, minimum recorded eGFR, presence of diabetes or hypertension, practitioner’s years of experience, practitioner degree, and format of eGFR reporting accounting for within-clinician correlation.
Although P values were not adjusted for multiple comparisons, the false discovery rate (FDR) for all comparisons was determined and estimated at 7% (21).
To address the question of whether not limiting the inclusion criteria to two measurements of eGFR<60 ml/min per 1.73 m2 ≥90 days apart affected the results, we performed a sensitivity analysis on the final model using the 594 subjects who met that criterion.
Results
Cohort Characteristics
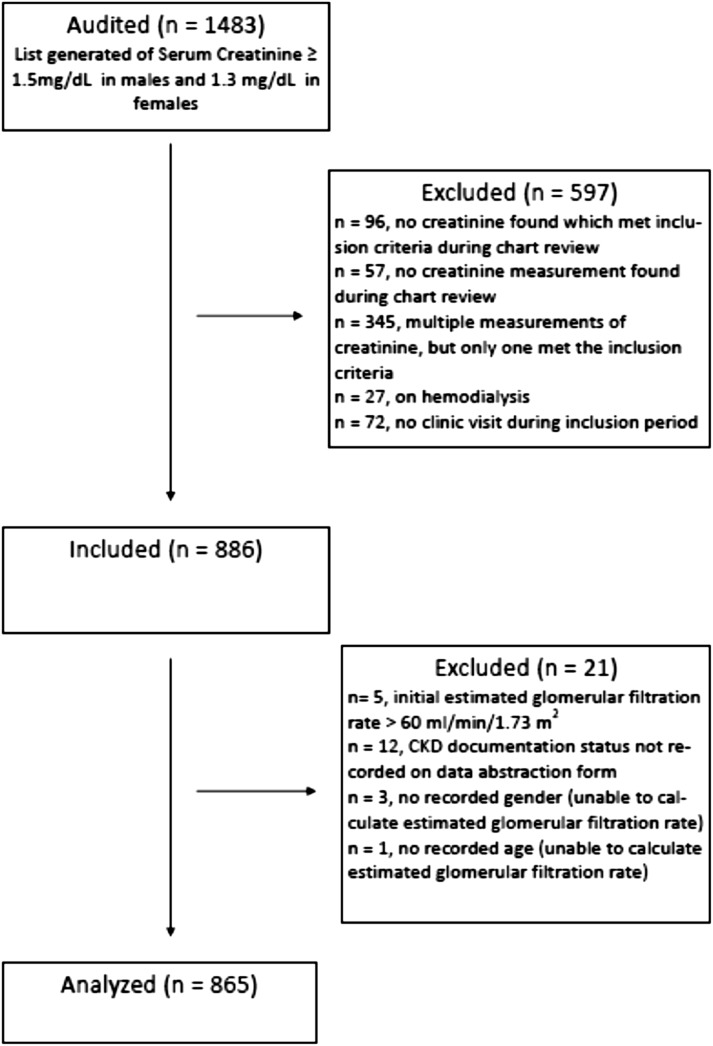
Of 1483 charts audited, data from 865 charts were analyzed (Figure 1).
Figure 1.
Flow diagram of exclusions and inclusions.
Table 1 outlines the demographics of the patient cohort. The average age was 75 years. Less than 1% of individuals were African American, and less than 2% of individuals were Hispanic, although 53.9% of the cohort had no documentation of ethnicity.
Table 1.
Demographics of CKD patient cohort
| Characteristics | Females (n=510; 59%) | Males (n=355; 41%) | Total (n=865) |
|---|---|---|---|
| Mean age (yr ± SD) | 76.0 ± 12.3 | 73.8 ± 12.7 | 75.1 ± 12.5 |
| Age range (yr) | 20–101 | 21–107 | 20–107 |
| Estimated GFR at inclusion (ml/min per 1.73 m2) | |||
| 45–59 | 15 (2.9%) | 126 (35.5%) | 141 (16.3%) |
| 30–44 | 380 (74.5%) | 193 (54.4%) | 573 (66.2%) |
| 15–29 | 107 (21.0%) | 34 (9.6%) | 141 (16.3%) |
| <15 | 8 (1.6%) | 2 (0.6%) | 10 (1.2%) |
| Laboratory report format | |||
| Serum creatinine | 237 (46.5%) | 200 (56.3%) | 437 (50.5%) |
| Automatic estimated GFR | 273 (53.5%) | 155 (43.7%) | 428 (49.5%) |
| Comorbid conditions | |||
| Diabetes mellitus | 195 (38.2%) | 183 (51.6%) | 378 (43.7%) |
| Hypertension | 444 (87.1%) | 283 (79.7%) | 727 (84.0%) |
| Coronary artery disease | 111 (21.8%) | 118 (33.2) | 229 (26.5%) |
| Congestive heart failure | 115 (22.6%) | 95 (26.8%) | 210 (24.3%) |
| Rural–urban commuting area category | |||
| Urban focused (population of 50,000 or more) | 55 (15.5%) | 151 (29.7%) | 206 (23.9%) |
| Large rural town (population of 10,000–49,999) | 104 (29.4%) | 113 (22.2%) | 217 (25.1%) |
| Small rural town (population of 2,500–9,999) | 155 (43.8%) | 178 (35.0%) | 333 (38.6%) |
| Isolated rural town (population of under 2,500) | 40 (11.3%) | 67 (13.2%) | 107 (12.4%) |
| Missing | 1 (0.002%) | 1 (0.003%) | 2 (0.002%) |
The mean eGFR at inclusion was 37.4±8.3 ml/min per 1.73 m2. The mean eGFR in women was 35.1±7.8 ml/min per 1.73 m2, and the mean eGFR in men was 40.7±8.0 ml/min per 1.73 m2.
CKD Documentation and Characteristics Associated with Undocumented CKD
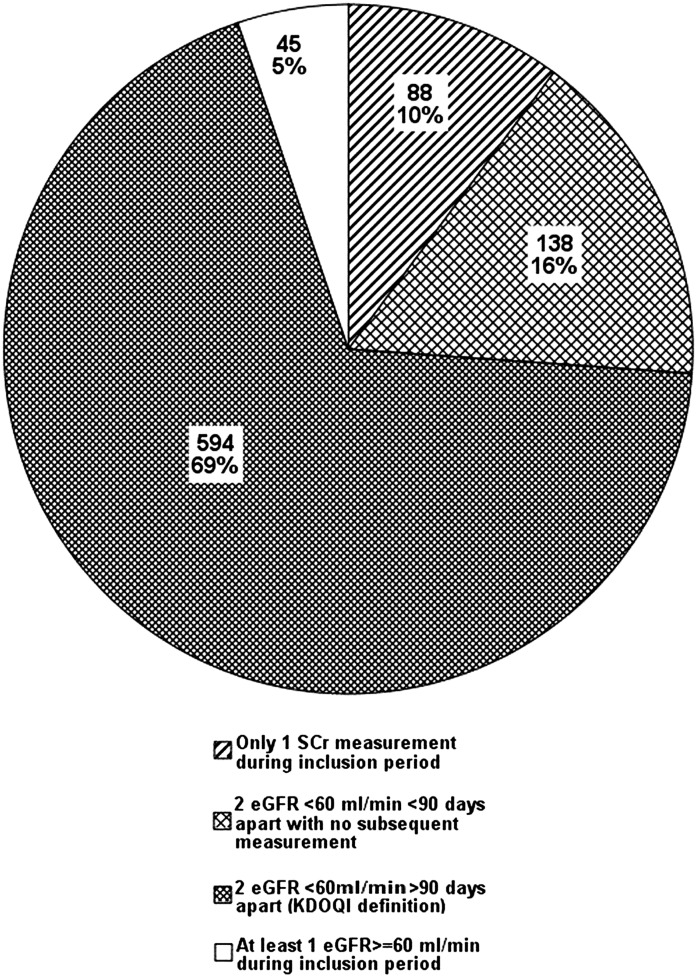
Data from 865 patients with an initial eGFR<60 ml/min per 1.73 m2 were analyzed. Figure 2 shows the proportion of these patients who met the Kidney Disease Outcomes Quality Initiative (KDOQI) definition of CKD (22) and a breakdown by value and number of eGFR measurements during the inclusion period of those patients who did not meet this definition.
Figure 2.
Proportion of included subjects by number and value of estimated GFR measurement during inclusion period.
Overall, 51.9% of all patients analyzed had no CKD diagnosis documented in their charts. Of the patients who strictly met the KDOQI definition of CKD (22), 49.5% had no CKD diagnosis documented; 47.1% of patients with diabetes and 50.1% of patients with hypertension did not have a recorded diagnosis of CKD.
Table 2 outlines the prevalence of undocumented CKD by patient, provider, and laboratory report characteristics. The only patient characteristics associated with undocumented CKD in the unadjusted analysis were sex and eGFR at inclusion. However, age, hypertension, diabetes mellitus, and type of laboratory report were significantly associated with documentation of CKD in the unadjusted analyses stratified by sex. Table 3 outlines a summary of this initial model.
Table 2.
Prevalence of undocumented CKD by patient, practitioner, and laboratory report characteristics
| Predictor | Subjects with Undocumented CKD N (%) | ||||
|---|---|---|---|---|---|
| Overall | By Sex | By Type of Laboratory Report | |||
| Males | Females | SCr-Only Reporting | Automatic Reporting eGFR | ||
| All subjects | 449 (51.9) | 237 (54.2) | 212 (49.5) | ||
| Males | 159 (44.8) | 86 (43.0) | 73 (47.1) | ||
| Females | 290 (56.9) | 151 (65.7) | 139 (50.9) | ||
| eGFR at inclusion (ml/min per 1.73 m2) | |||||
| 40–59 | 88 (62.4) | 74 (58.8) | 14 (93.3) | 43 (60.6) | 45 (64.3) |
| 30–44 | 318 (55.5) | 77 (38.9) | 241 (63.4) | 172 (59.7) | 146 (51.2) |
| <30 | 43 (25.5) | 8 (22.2) | 35 (30.4) | 22 (28.2) | 21 (28.7) |
| Subject age (yr) | |||||
| <60 | 51 (50.5) | 25 (49.0) | 26 (52.0) | 30 (53.6) | 21 (46.7) |
| 60–75 | 108 (44.4) | 38 (37.2) | 70 (49.7) | 53 (46.5) | 55 (42.6) |
| 75–85 | 180 (56.3) | 65 (49.6) | 115 (60.9) | 91 (55.2) | 89 (57.4) |
| >85 | 110 (54.7) | 31 (43.7) | 79 (60.8) | 63 (61.8) | 47 (47.5) |
| Comorbid conditions | |||||
| No diabetes mellitus | 271 (55.7) | 83 (48.3) | 188 (59.7) | 155 (60.3) | 116 (50.4) |
| Diabetes mellitus | 178 (47.1) | 76 (41.5) | 102 (52.3) | 82 (45.6) | 96 (48.5) |
| No hypertension | 85 (61.6) | 36 (50.0) | 49 (74.2) | 45 (72.6) | 40 (52.6) |
| Hypertension | 364 (50.1) | 123 (43.5) | 241 (54.3) | 192 (562) | 172 (48.9) |
| Practitioner training | |||||
| Nurse practitioner or physician assistant | 59 (57.8) | 16 (53.3) | 43 (59.7) | 38 (71.7) | 21 (42.9) |
| Doctor of medicine or osteopathic medicine | 390 (51.1) | 143 (44.0) | 247 (56.4) | 199 (51.8) | 191 (50.4) |
| Practitioner experience (yr) | |||||
| <20 | 212 (47.2) | 68 (37.8) | 144 (53.5) | 161 (53.3) | 51 (34.7) |
| ≥20 | 237 (57.0) | 91 (52.0) | 146 (60.6) | 76 (56.3) | 161 (57.3) |
SCr, serum creatinine; eGFR, estimated GFR.
Table 3.
Association of patient and laboratory report characteristics with undocumented CKD
| Variable | Unadjusted Odds Ratio for Undocumented CKD (95% CI) | Odds Ratio for Undocumented CKD Adjusted for Main Effects (95% CI)a | Adjusted Odds Ratio for Undocumented CKDb from Analysis with Interaction Term | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All Subjects | From Stratified Analysis | All Subjects | From Stratified Analysisc | ||||||
| Males | Females | Males | Females | SCr only | eGFR | Pd | |||
| Sex | Sex × report format 0.04 | ||||||||
| Females (REF: male) | 1.75 (1.35, 2.26) | 2.93 (2.04, 4.21) | 3.81 (2.43, 5.96) | 2.14 (1.37, 3.33) | |||||
| Males | Females | ||||||||
| Laboratory reporting | |||||||||
| SCr only (REF: eGFR) | 0.97 (0.64, 1.49) | 0.69 (0.40, 1.20) | 1.61 (0.99, 2.62) | 0.89 (0.57, 1.40) | 0.66 (0.37, 1.16) | 1.76 (1.02, 3.02) | 0.64 (0.37, 1.12) | 1.14 (0.71, 1.86) | |
| eGFR at inclusion (ml/min per 1.73 m2) | <0.01 | ||||||||
| 40–59 (REF: <30) | 4.17 (2.38, 7.32) | 5.09 (2.14 12.07) | 36.13 (3.27, 398.30) | 9.29 (4.57, 18.87) | 5.63 (2.34, 13.56) | 73.3 (8.72, 616.5) | 9.27 (4.57, 18.78) | ||
| 30–44 (REF: <30) | 3.34 (2.06, 5.39) | 2.50 (1.16, 5.37) | 4.14 (2.35, 7.31) | 4.18 (2.49, 7.03) | 2.83 (1.30, 6.15) | 4.74 (2.65, 8.48) | 4.23 (2.50, 7.18) | ||
| Comorbidity | |||||||||
| No DM (REF: DM) | 1.47 (1.15, 1.90) | 1.29 (0.86, 1.92) | 1.48 (1.04, 2.09) | 1.26 (0.98, 1.62) | 1.20 (0.82, 1.77) | 1.23 (0.81, 1.86) | 1.58 (1.23, 2.03) | 0.08 | |
| No HTN (REF: HTN) | 1.50 (1.01, 2.23) | 1.30 (0.82, 2.08) | 2.19 (1.15, 4.17) | 1.53 (1.03, 2.26) | 1.17 (0.75, 1.83) | 2.19 (1.13, 4.26) | 1.62 (1.10, 2.37) | 0.03 | |
| Age (yr) | 0.03 | ||||||||
| <60 (REF: 85+) | 0.89 (0.50, 1.59) | 1.16 (0.52, 2.60) | 0.69 (0.32, 1.49) | 0.75 (0.41, 1.39) | 0.98 (0.44, 2.21) | 0.33 (0.15, 0.72) | 0.76 (0.41, 1.38) | ||
| 60–74 (REF: 85+) | 0.65 (0.44, 0.94) | 0.64 (0.39, 1.05) | 0.62 (0.36, 1.08) | 0.57 (0.38, 0.86) | 0.50 (0.28, 0.90) | 0.60 (0.32, 1.11) | 0.58 (0.38, 0.88) | ||
| 75–84 (REF: 85+) | 0.97 (0.71, 1.32) | 1.00 (0.59, 1.68) | 0.99 (0.63, 1.55) | 0.97 (0.71, 1.33) | 0.89 (0.53, 1.53) | 1.02 (0.62, 1.68) | 0.99 (0.72, 1.35) | ||
CI, confidence interval; SCr, serum creatinine; eGFR, estimated GFR; REF, reference category for odds ratio; DM, diabetes mellitus; HTN, hypertension.
Adjusted for sex, eGFR at inclusion, age, DM, HTN, and report format.
Adjusted for sex, eGFR at inclusion, age, DM, HTN, report format, and sex × eGFR report. If sex × eGFR at inclusion was added to the model, the significance level for the interaction would be P=0.06.
Separate models stratified by sex adjusted for eGFR at inclusion, age, DM, HTN, and report format.
P values are the significance levels for the factors and interaction terms.
As outlined in Table 3, females were more likely than males to have undocumented CKD, and the sex difference was more pronounced in patients whose laboratory reports reported only SCr values (odds ratio [OR]=3.81, 95% confidence interval [95% CI]=2.43, 5.96 and OR=2.14, 95% CI=1.37, 3.33 for SCr only and eGFR reporting, respectively; P=0.04 for differences in OR). This relation was confirmed in the sensitivity analysis. The probability of undocumented CKD decreased with advancing CKD stage at inclusion. The probability of undocumented CKD was 0.72 in patients with stage 3A (95% CI=0.62, 0.81), 0.55 in patients with stage 3B (95% CI=0.48, 0.61), and 0.22 in patients with stage 4 or higher CKD (95% CI=0.14, 0.33).
As outlined in Table 4, there was a complex interaction between the laboratory format of kidney function reporting and several variables. Specifically, the association of report format on undocumented CKD was dependent on patient sex, years of practitioner experience (<20 versus ≥20 years), and clinical training (nurse practitioner/physician assistant versus doctor of medicine/doctor of osteopathic medicine). In laboratory reports with only SCr reporting, undocumented CKD was significantly associated with clinician training (OR=2.40, 95% CI=1.08, 7.94 for nurse practitioner/physician assistant versus doctor of medicine/doctor of osteopathic medicine) but not years of practitioner experience. With automatic reporting of eGFR, undocumented CKD was significantly associated with years of practice (OR=0.39, 95% CI=0.19, 0.78 for <20 versus ≥20 years) but not the type of degree. Females were more likely than males to have undocumented CKD both with SCr reporting (OR=3.74, 95% CI=2.37, 5.92) and automatic reporting of eGFR (OR=2.27, 95% CI=1.45, 3.56). The complexity of the interactions makes the estimation of the overall association of laboratory report format with undocumented CKD dependent on the distribution of practitioner characteristics in the sample. In this cohort, with 88% of the patients treated by physicians, the association of report format with undocumented CKD is not significant (OR=1.06, 95% CI=0.72, 1.57). In a sample in which equal numbers of patients were treated by physicians and nonphysician practitioners, the average association of report format with undocumented CKD would be significant (predicted OR=1.81, 95% CI=1.16, 2.82). After adjustment for patient and practitioner characteristics, the probability of undiagnosed CKD was 0.73 for stage 3A (95% CI=0.62, 0.81), 0.55 for stage 3B (95% CI=0.48, 0.61), and 0.21 for stage 4 or higher CKD (95% CI=0.14, 0.32).
Table 4.
Interaction of practitioner characteristics and report format with undocumented CKD
| Variable | OR for Undocumented CKD (95% CI): Adjusted OR from Models Stratified by Report Formata | OR for Undocumented CKD (95% CI): Adjusted OR from Model with Interaction Termsb | Pb | |||
|---|---|---|---|---|---|---|
| SCr Only | eGFR | SCr Only | eGFR | |||
| Sex | Sex × format | 0.09 | ||||
| Female (REF: male) | 3.79 (2.28, 6.29) | 2.15 (1.29, 3.56) | Female (REF: male) | 3.74 (2.37, 5.92) | 2.27 (1.45, 3.56) | |
| Experience (yr) | Experience × format | 1.10 (0.60, 2.03) | 0.39 (0.19, 0.78)c | 0.03 | ||
| <20 (REF: 20+) | 0.94 (0.49, 1.80) | 0.34 (0.22, 0.54) | <20 (REF: 20+) | |||
| Clinician training | Training × format | 0.03 | ||||
| NP/PA (REF: MD/DO) | 2.16 (0.96, 4.87) | 0.80 (0.42, 1.54) | NP/PA (REF: MD/DO) | 2.40 (1.08, 7.94) | 0.59 (0.30, 1.20)d | |
| eGFR at inclusion (ml/min per 1.73 m2) | eGFR at inclusion (ml/min per 1.73 m2) | <0.01 | ||||
| 40–59 (REF: <30) | 10.58 (3.85, 29.04) | 9.26 (3.96, 21.64) | 40–59 (REF: <30) | 9.77 (4.77, 20.0) | ||
| 30–44 (REF: <30) | 5.59 (2.63, 11.92) | 3.50 (1.93, 6.36) | 30–44 (REF: <30) | 4.47 (2.61, 7.67) | ||
| Comorbidity | Comorbidity | |||||
| No DM (REF: DM) | 1.38 (0.94, 2.01) | 1.03 (0.68, 1.57) | No DM (REF: DM) | 1.29 (1.01, 1.66) | 0.05 | |
| No HTN (REF: HTN) | 2.45 (1.33, 4.53) | 1.29 (0.75, 2.22) | No HTN (REF: HTN) | 1.52 (1.03, 2.25) | 0.04 | |
| Age (yr) | Age (yr) | 0.02 | ||||
| <60 (REF: 85+) | 0.68 (0.29, 1.59) | 0.80 (0.36, 1.78) | <60 (REF: 85+) | 0.75 (0.40, 1.38) | ||
| 60–74 (REF: 85+) | 0.56 (0.30, 1.02) | 0.53 (0.30, 0.95) | 60–74 (REF: 85+) | 0.55 (0.36, 0.84) | ||
| 75–84 (REF: 85+) | 0.86 (0.56, 1.32) | 1.26 (0.73, 2.17) | 75–84 (REF: 85+) | 0.99 (0.72, 1.36) | ||
OR, odds ratio; CI, confidence interval; SCr, serum creatinine; eGFR, estimated GFR; REF, reference category for OR; NP, nurse practitioner; PA, physician assistant; MD, doctor of medicine; DO, doctor of osteopathic medicine; DM, diabetes mellitus; HTN, hypertension.
Variables included in the model: sex, eGFR at inclusion, age, laboratory report format, presence of DM, presence of HTN, and practitioner degree.
Variables included in the model: sex, eGFR at inclusion, age, laboratory report format, presence of DM, presence of HTN, and practitioner degree. Interactions included in the model: laboratory report format × sex, laboratory report format × practitioner degree, and laboratory report format × clinician experience; P values are the significance levels for the factors and interaction terms.
Differences between OR in SCr only and eGFR (P=0.03).
Differences between OR in SCr only and eGFR (P=0.01).
Relationship between CKD Reporting and Care
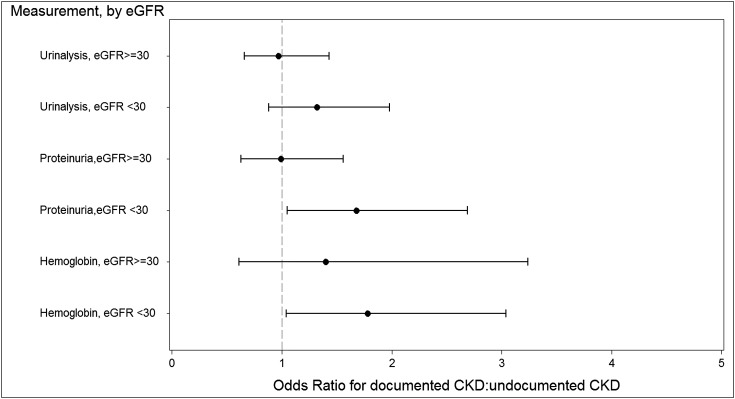
Figure 3 shows the association of a recorded diagnosis of CKD and compliance with KDOQI guideline-recommended care. Having documented CKD was associated with a change in BP medication for a systolic BP>150 mmHg (OR=2.07, 95% CI=1.15, 3.73). Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use in diabetic patients was 74.5% in those patients with documented CKD and 77.0% in those patients with undocumented CKD (OR=0.92, 95% CI=0.57, 1.50). Overall, proteinuria assessment was found in 60.7% of the cohort, with 44% of those patients having a quantitative assessment; 9.8% of patients with stage 3 and 25.2% of patients with stage 4 CKD were referred to a nephrologist, and 33% of patients with proteinuria on a quantitative measurement were referred to a nephrologist. Compared with those patients with undocumented CKD, patients with documented CKD were more likely to be referred in stage 3 (OR=65.70, 95% CI=11.70, 370) and stage 4 CKD (OR=3.76, 95% CI=1.94, 5.59).
Figure 3.
Odds of compliance with treatment guidelines in documented versus undocumented CKD.
Discussion
Of CKD patients, 51.9% had no recorded diagnosis of CKD. Females were more likely than males to have undocumented CKD, regardless of the format of laboratory reporting.
Studies evaluating CKD documentation by PCPs have shown variable results. In an Australian study, PCPs correctly identified 28% of stage 3 and 67% of stage 4 or 5 CKD patients (23). Evaluating documentation using ICD codes, a study conducted in an outpatient Veterans Affairs setting found CKD documentation in 14.6% of subjects (7), and an Italian study found documentation in 15.2% of subjects (6). Our study, which was not limited by poor sensitivity of coding, likely more closely parallels the true lack of recognition by the clinician.
The association of automatic reporting of eGFR on CKD documentation depended on patient sex, practitioner training, and years of practice. In the Veterans Affairs study (7), documentation by ICD code increased from 14.6% to 21.5% after implementation of automatic reporting of eGFR. In another study, implementation of automatic reporting of eGFR with an educational intervention increased CKD documentation from 22.4% to 88.1% (5). A systematic review of the impact of eGFR reporting showed an increase in nephrology referral after implementation in 13 of 16 studies, particularly in populations at risk for under-recognized disease, including women and the elderly (11). However, in our study, 22.2% of males and 30.4% of females with stage 4 CKD or higher (a point at which the SCr would clearly be abnormal) had undocumented CKD, suggesting an issue beyond translation of SCr to eGFR.
Compared with males, females were at particularly high risk for having undocumented CKD, possibly because of a lower SCr level for a given eGFR. In a prior study (5), males were more likely than females to have documented CKD; this difference was eliminated with the implementation of automatic reporting of eGFR and an educational intervention. A cohort study looking at patterns of nephrology referral before and after implementation of automatic eGFR reporting found an increase in the monthly adjusted rate of first nephrology outpatient visits only in females (24). In a systematic review of eGFR reporting, eight of nine studies showed that eGFR reporting led to an increase in the number of female patients referred (11). These studies suggest that automatic reporting of eGFR is important for practitioners recognizing CKD in females. However, in our study, even in subjects with automatic reporting of eGFR, females were more likely than males to have undocumented CKD.
Our data suggest that automatic reporting of eGFR might increase CKD documentation by nonphysician practitioners. A study of US rural community health centers reported 46% of direct clinical care being provided by nonphysician practitioners (25). Automatic reporting of eGFR could have a greater impact in a rural environment, where nonphysician practitioners often function as PCPs.
It is difficult to ascertain whether undocumented CKD is the equivalent of unrecognized CKD and whether this finding translates into suboptimal CKD care. Anemia workup and angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in diabetic subjects were used in a similarly high proportion in both groups. This finding is in contrast to other observations of lesser use of renin-angiotensin system inhibitors and lower rates of urine protein quantification in subjects with undocumented versus documented CKD (8). Proteinuria assessment, an important marker for CKD progression, was low in both groups. This finding suggests that a lack of knowledge of CKD management as opposed to a lack of recognition of CKD alone may be more influential in this underuse. Importantly, there was a difference in rates of BP medication changes for those patients with uncontrolled hypertension in documented versus undocumented CKD patients. Given that BP control is one of the proven modifiable risk factors for progression of CKD (26–28), this difference could translate into worse outcomes in this group.
Nephrology referral rates were low, and those patients with undocumented CKD were less likely to be referred. Although the appropriate timing of nephrology referral is unclear (29), late referral has been associated with a vast array of poor outcomes (3,30–33). Furthermore, early nephrology referral has been shown to slow the progression of CKD (34,35). Our finding that only 25.2% of patients with stage 4 CKD were referred is concerning. The low rate of nephrology referral may reflect inaccessibility to subspecialty care in a rural environment. Poor patient access to nephrology care because of longer distances has been shown to be associated with a lower likelihood of obtaining early nephrology care (36).
The present study has several limitations. Although the majority of the cohort met the KDOQI definition of CKD, some patients with AKI or less advanced CKD may have been included. However, a sensitivity analysis performed on the subjects meeting the KDOQI definition of stage 3 CKD or higher supported the results in the overall cohort. The SCr values were not standardized, and therefore, use of the MDRD formula could have led to spuriously lower eGFR rates, particularly in stage 3A. However, it is unlikely that PCPs are using formulas to standardize SCr, and therefore, although it might have led to misclassification, it is unlikely to explain the lack of CKD documentation that we have reported. Because data collection was limited to the subsequent 12 months from the inclusion SCr value, we missed data that were documented previously in the chart. Furthermore, given our method of medical record review, particularly in practices with paper charts, it is possible that some records were missing. However, by using medical record review, we believe that we increased the likelihood of finding CKD documentation and were able to collect data in a variety of community-based primary care practices, reflecting the setting in which many CKD patients are managed. The 12-month follow-up period was too short to evaluate outcomes such as rate of decline of eGFR, cardiovascular events, or mortality. Finally, multiple comparisons may have resulted in some false positive findings. However, analysis of the probable FDR indicated that the number of comparisons made in the study inflates the expected FDR from the standard 5% to 7%.
In conclusion, this study found that a high proportion of CKD patients, most of whom were being cared for exclusively in primary care, had no documentation of CKD in the medical record. This lack of documentation was particularly prominent in female patients. The effect of automatic reporting of eGFR on CKD documentation is dependent on patient sex, practitioner training, and years of experience. Recognition of disparities, particularly sex, and development of interventions to increase recognition and improve CKD care in primary care present important challenges.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge Diane Devina, Ann Ford, and Monica Goubaud for assistance in gathering data, Nancy Rollins for data management, and LeAnn Michaels for coordinating the project.
This research was supported by a Medical Research Foundation of Oregon Early Clinical Investigator Grant (to M.K.R), a National Kidney Foundation Research Fellowship (to M.K.R), Oregon Clinical and Translational Research Institute Grant 1 UL 1 RRO24140 01 (to C.D.M.), and National Institutes of Health Grant T32 DK 067864 (to S.A.).
Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology October 27–November 1, 2009, in San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02410312/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Patwardhan MB, Samsa GP, Matchar DB, Haley WE: Advanced chronic kidney disease practice patterns among nephrologists and non-nephrologists: A database analysis. Clin J Am Soc Nephrol 2: 277–283, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Avorn J, Bohn RL, Levy E, Levin R, Owen WF, Jr, Winkelmayer WC, Glynn RJ: Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med 162: 2002–2006, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski M, Mann W, Derose S, Selevan D, Pascual N, Diesto J, Crooks P: Implementing KDOQI CKD definition and staging guidelines in Southern California Kaiser Permanente. Am J Kidney Dis 53[Suppl 3]: S86–S99, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Akbari A, Swedko PJ, Clark HD, Hogg W, Lemelin J, Magner P, Moore L, Ooi D: Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med 164: 1788–1792, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Minutolo R, De Nicola L, Mazzaglia G, Postorino M, Cricelli C, Mantovani LG, Conte G, Cianciaruso B: Detection and awareness of moderate to advanced CKD by primary care practitioners: A cross-sectional study from Italy. Am J Kidney Dis 52: 444–453, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wyatt C, Konduri V, Eng J, Rohatgi R: Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis 49: 634–641, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chase HS, Radhakrishnan J, Shirazian S, Rao MK, Vawdrey DK: Under-documentation of chronic kidney disease in the electronic health record in outpatients. J Am Med Inform Assoc 17: 588–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glassock RJ, Winearls CG: Routine reporting of estimated glomerular filtration rate: Not ready for prime time. Nat Clin Pract Nephrol 4: 422–423, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Bauer C, Hostetter TH: eGFR: Is it ready for early identification of CKD? Clin J Am Soc Nephrol 3: 1569–1572, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagoma YK, Weir MA, Iansavichus AV, Hemmelgarn BR, Akbari A, Patel UD, Garg AX, Jain AK: Impact of estimated GFR reporting on patients, clinicians, and health-care systems: A systematic review. Am J Kidney Dis 57: 592–601, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Edelman MA, Menz BL: Selected comparisons and implications of a national rural and urban survey on health care access, demographics, and policy issues. J Rural Health 12: 197–205, 1996 [DOI] [PubMed] [Google Scholar]
- 13.WWAMI Rural Health Research Center. RUCA (Rural-urban commuting area codes) data: Using RUCA data. Available at: http://Depts.washington.edu/uwruca/ruca-uses.php Accessed January 8, 2008
- 14.O’Hare AM, Johansen KL, Rodriguez RA: Dialysis and kidney transplantation among patients living in rural areas of the United States. Kidney Int 69: 343–349, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Powe NR, Tarver-Carr ME, Eberhardt MS, Brancati FL: Receipt of renal replacement therapy in the United States: A population-based study of sociodemographic disparities from the Second National Health and Nutrition Examination Survey (NHANES II). Am J Kidney Dis 42: 249–255, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int 79: 210–217, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.WWAMI Rural Health Research Center. RUCA (Rural-urban commuting area codes) data: Version 2.0. Available at: http://Depts.washington.edu/uwruca/ruca-data.php Accessed January 8, 2008
- 19.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22, 1986 [Google Scholar]
- 21.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995 [Google Scholar]
- 22.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 23.Razavian M, Heeley EL, Perkovic V, Zoungas S, Weekes A, Patel AA, Anderson CS, Chalmers JP, Cass A: Cardiovascular risk management in chronic kidney disease in general practice (the AusHEART study). Nephrol Dial Transplant 27: 1396–1402, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Hemmelgarn BR, Zhang J, Manns BJ, James MT, Quinn RR, Ravani P, Klarenbach SW, Culleton BF, Krause R, Thorlacius L, Jain AK, Tonelli M, Alberta Kidney Disease Network : Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA 303: 1151–1158, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Rosenblatt RA, Andrilla CH, Curtin T, Hart LG: Shortages of medical personnel at community health centers: Implications for planned expansion. JAMA 295: 1042–1049, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group : Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 28.de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J, ADVANCE Collaborative Group : Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20: 883–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Coster C, McLaughlin K, Noseworthy TW: Criteria for referring patients with renal disease for nephrology consultation: A review of the literature. J Nephrol 23: 399–407, 2010 [PubMed] [Google Scholar]
- 30.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W, Jr: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Khan SS, Xue JL, Kazmi WH, Gilbertson DT, Obrador GT, Pereira BJ, Collins AJ: Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int 67: 1038–1046, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Stack AG: Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 41: 310–318, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Jones C, Roderick P, Harris S, Rogerson M: Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant 21: 2133–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Ramírez HR, Jalomo-Martínez B, Cortés-Sanabria L, Rojas-Campos E, Barragán G, Alfaro G, Cueto-Manzano AM: Renal function preservation in type 2 diabetes mellitus patients with early nephropathy: A comparative prospective cohort study between primary health care doctors and a nephrologist. Am J Kidney Dis 47: 78–87, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Brooks JM, Flanigan MJ, Chrischilles EA, Pendergast JF, Hunsicker LG: Physician access and early nephrology care in elderly patients with end-stage renal disease. Kidney Int 74: 1596–1602, 2008 [DOI] [PubMed] [Google Scholar]