Summary
Background and objectives
Kidney transplant recipients are at increased risk for malignancies. One recognized risk for malignancy is ionizing radiation. The purpose of this study was to determine, among kidney transplant recipients, the medical imaging procedures that contribute to radiation exposure and their cumulative radiation exposure, as a result of their pretransplant evaluation.
Design, setting, participants, & measurements
Medical records of patients who received a first, kidney-alone transplant during 2008 at a single transplant center were examined. This study identified medical imaging procedures that were performed as prerequisites for deceased donor wait-listing or receipt of live donor kidney transplants and to maintain active status on the wait list. Frequencies of medical imaging procedures and cumulative effective doses of radiation were calculated.
Results
Among the 172 kidney transplant recipients, 905 procedures were performed. Seventy patients (40.7%) were exposed to low dose (0–20 mSv), 51 (29.7%) were exposed to moderate dose (>20–50 mSv), 28 (16.3%) were exposed to high dose (>50–100 mSv), and 23 (13.4%) were exposed to very high dose (>100 mSv) cumulative effective radiation. Nuclear stress tests accounted for 82.9% of the total radiation exposure. In multivariate analysis, older age, diabetes, and black race were associated with exposure to >20 mSv radiation during the pretransplant evaluation.
Conclusions
Kidney transplant recipients are exposed to large amounts of ionizing radiation from medical imaging during the pretransplant evaluation. The effects of radiation upon malignancy risk and strategies to reduce this radiation exposure warrant further investigation.
Introduction
Among recipients of kidney transplants, cancer is the third leading cause of death with a functioning graft (1). The increased risk of cancer among transplant recipients is multi-factorial in etiology (2). There is a well recognized link to the cumulative dose of immunosuppression and the increased risk for skin cancers, lymphoproliferative disease, and solid organ tumors among transplant recipients.
Ionizing radiation is a well established risk for cancer (3). The United Nations Scientific Committee on the Effects of Atomic Radiation estimated that the individual annual effective radiation dose from natural sources is slightly <3 mSv (4). In the United States, medical radiation exposure is increasingly the largest source of exposure from human-made sources of radiation. Mettler et al. showed essentially equal contributions from medical radiation exposure and natural background radiation exposure in the United States (5). Occupational radiation exposure is monitored and restricted to effective doses of 100 mSv every 5 years (20 mSv/yr), with a maximum of 50 mSv allowed in any given year (6).
Kidney transplant recipients, who already have an increased risk of cancer, may receive high doses of radiation from radiologic procedures. Previous studies have reported increased radiation exposure from medical imaging among the general population (7,8). In more recent studies, increased radiation exposure has been identified in hemodialysis patients and post-transplant recipients (9–11).
Kidney transplant recipients may also receive especially high doses of medical radiation due to their extensive pretransplant testing. In this study, we sought to estimate the cumulative radiation exposure as a result of the pretransplant work-up. In addition, we determined the types and numbers of medical imaging procedures that contribute to radiation exposure, to help identify strategies that may help minimize exposure.
Materials and Methods
Study Design
We conducted a retrospective cohort study of all adults (aged >18 years) who received a kidney-alone transplant from January 1, 2008 through December 31, 2008 at Saint Barnabas Medical Center in Livingston, New Jersey. We excluded recipients of prior kidney transplants, recipients of other solid organ transplants, and recipients of simultaneous pancreas-kidney transplants. The human subjects Institutional Review Board at Saint Barnabas Medical Center approved the study protocol.
Determination of Radiation Exposure
To identify pretransplant exposure to medical radiation, we examined medical records to identify the types and number of medical imaging procedures performed before transplantation. We identified all procedures that expose patients to medical radiation (plain radiography, computed tomography, nuclear medicine imaging, and fluoroscopic procedures) and were performed as prerequisites for placement on our deceased donor waiting list or receipt of a live donor kidney transplant or to maintain active status on the waiting list. We excluded procedures that were not required for placement on our waiting list (e.g., hemodialysis arteriovenous fistulogram) and those specifically delivered for therapeutic purposes (e.g., high dose radiation for breast cancer or prostate cancer).
Certain procedures have a wide range of effective radiation dose dependent upon several factors. Calculation of effective dose of radiation is well described (6,12–16). For nuclear stress tests (NSTs), we calculated the effective dose of radiation using the addenda to International Commission on Radiologic Protection publication 53, to give a more accurate assessment of radiation exposure (17). In cases in which data were missing, we imputed the lowest known amount of radionuclide given to calculate the effective radiation dose. To estimate the radiation exposure for x-rays and noncardiac nuclear medicine scans, we used the lowest estimates of effective dose of radiation measured in millisieverts from published literature. For the computed tomography procedures, the dose length product data were not available, so we used estimates of effective dose of radiation from published literature.
We categorized the cumulative effective radiation doses for the study population into the following groups: low dose (0–20 mSv), moderate dose (>20–50 mSv), high dose (>50–100 mSv), and very high dose (>100 mSv). In univariable logistic regression, our outcome was moderate or higher doses (>20 mSv) of radiation exposure during the pretransplant period.
Analyses were performed using Stata 9.0 statistical software (Stata Corp, College Station, TX). Two-sided P values <0.05 were considered statistically significant. Categorical variables were expressed as proportions, and their values across groups (e.g., total cumulative radiation dose) were compared using chi-squared testing. Continuous variables that were not normally distributed were expressed as medians with 25%–75% interquartile ranges (IQRs) and analyzed as categorical (ordinal) variables. We used logistic regression to model associations between our outcome (exposure to large amounts of cumulative radiation) and possible predictors. Candidate variables with P<0.20 in the unadjusted analyses were eligible for the multivariable model. We generated the multivariable model using a stepwise, backward elimination method. We assessed the significance of each variable in the nested models using the likelihood ratio test and the Wald statistic. Variables with adjusted P<0.10 were retained in the final model.
Results
Study Population
During the study period, 208 kidney transplants were performed at our center, and 172 of the kidney transplant recipients met our inclusion criteria. The demographic and medical characteristics of the 172 patients are shown in Table 1. The median age was 51.2 years (IQR, 43.3–58.0) at evaluation and 54.0 years (IQR, 44.7–60.9) at transplantation. The median number of years of follow-up was 3.7 years (IQR, 2.1–5.2).
Table 1.
Patient demographic and medical characteristics
| Characteristics | Low Dose (0–20 mSv) | Moderate Dose (>20–50 mSv) | High Dose (>50–100 mSv) | Very High Dose (>100 mSv) | P Value | Total |
|---|---|---|---|---|---|---|
| Total participants | 70 (40.7) | 51 (29.7) | 28 (16.3) | 23 (13.4) | 172 | |
| Female | 31 (44.3) | 22 (43.1) | 11 (39.3) | 7 (30.4) | 0.68 | 71 (41.3) |
| Black | 16 (22.9) | 22 (43.1) | 10 (35.7) | 12 (52.7) | 0.07 | 60 (34.9) |
| Age at evaluation (yr) | <0.001 | |||||
| 18–40 | 27 (38.6) | 4 (7.8) | 1 (3.6) | 3 (13.0) | 35 (20.4) | |
| >40–50 | 20 (28.6) | 14 (27.5) | 4 (14.3) | 3 (13.0) | 41 (23.8) | |
| >50–60 | 15 (21.4) | 22 (43.1) | 20 (71.4) | 9 (39.1) | 66 (38.4) | |
| >60 | 8 (11.4) | 11 (21.6) | 3 (10.7) | 8 (34.8) | 30 (17.4) | |
| Etiology of renal disease | 0.002 | |||||
| Diabetes mellitus | 8 (11.4) | 20 (39.2) | 8 (28.6) | 13 (56.5) | 49 (28.5) | |
| Hypertension | 16 (22.9) | 14 (27.5) | 6 (21.4) | 7 (30.4) | 43 (25.0) | |
| GN | 29 (41.4) | 8 (15.7) | 7 (25.0) | 2 (9.7) | 46 (26.7) | |
| Other | 17 (24.3) | 9 (17.7) | 7 (25.0) | 1 (4.4) | 34 (19.8) | |
| Dialysis status at time of evaluation | 0.26 | |||||
| Pre-emptive | 40 (57.1) | 20 (39.2) | 10 (35.7) | 12 (52.2) | 82 (47.7) | |
| On hemodialysis | 26 (37.1) | 29 (56.9) | 16 (57.1) | 11 (47.8) | 82 (47.7) | |
| On peritoneal dialysis | 4 (5.7) | 2 (3.9) | 2 (7.1) | 0 (0) | 8 (4.7) | |
| Transplant type | <0.001 | |||||
| Deceased donor | 27 (34.3) | 30 (58.8) | 18 (64.3) | 18 (78.3) | 90 (52.3) | |
| Living donor | 46 (65.7) | 21 (41.2) | 10 (35.7) | 5 (21.7) | 82 (47.7) | |
| Charlson comorbidity index | 0.07 | |||||
| 2–3 | 49 (70.0) | 23 (45.1) | 16 (57.1) | 6 (26.1) | 94 (54.7) | |
| 4–5 | 17 (24.3) | 17 (33.3) | 6 (21.4) | 11 (47.8) | 51 (29.7) | |
| 6–8 | 4 (5.7) | 11 (21.6) | 6 (21.4) | 6 (26.1) | 27 (15.7) | |
| ABO blood type | 0.08 | |||||
| Group A | 31 (44.3) | 24 (47.1) | 11 (39.3) | 3 (13.0) | 69 (40.1) | |
| Group AB | 5 (7.1) | 0 (0) | 2 (7.1) | 0 (0) | 7 (4.1) | |
| Group B | 8 (11.4) | 7 (13.7) | 4 (14.3) | 7 (30.4) | 26 (15.1) | |
| Group O | 26 (37.1) | 20 (39.2) | 11 (39.3) | 13 (56.5) | 70 (40.7) | |
| Peak panel reactive antibody (%) | 0.11 | |||||
| 0 | 28 (40.0) | 25 (49.0) | 12 (42.9) | 15 (65.2) | 80 (46.5) | |
| 1–19 | 22 (31.4) | 13 (25.5) | 8 (28.6) | 2 (8.7) | 45 (26.2) | |
| 20–79 | 15 (21.4) | 11 (21.6) | 3 (10.7) | 3 (13.0) | 32 (18.6) | |
| 80–100 | 26 (37.1) | 20 (39.2) | 11 (39.3) | 3 (13.0) | 15 (8.7) | |
| Time from initial evaluation to time of transplant (mo) | <0.001 | |||||
| 0–12 | 41 (58.6) | 20 (39.2) | 1 (3.6) | 0 (0) | 62 (36.1) | |
| >12–24 | 11 (15.7) | 9 (17.7) | 7 (25.0) | 0 (0) | 27 (15.7) | |
| >24–48 | 11 (15.7) | 10 (19.6) | 7 (25.0) | 5 (21.7) | 33 (19.2) | |
| >48 | 7 (10.0) | 12 (23.5) | 13 (46.4) | 18 (78.3) | 50 (29.1) |
Data are given as n (%).
Types and Number of Radiologic Tests and Procedures
A total of 905 procedures were performed in 172 patients during the study (Figure 1). Plain radiography (56.8%) and nuclear medicine procedures (28.4%) accounted for the majority of the procedures. The median number of procedures per patient was 5.
Figure 1.
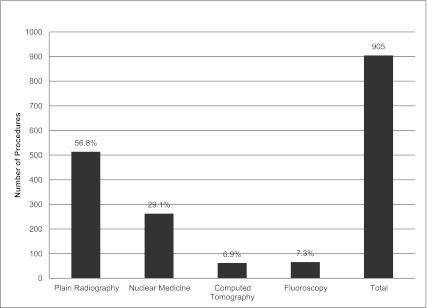
Types and number of radiologic procedures.
Radiation Exposure
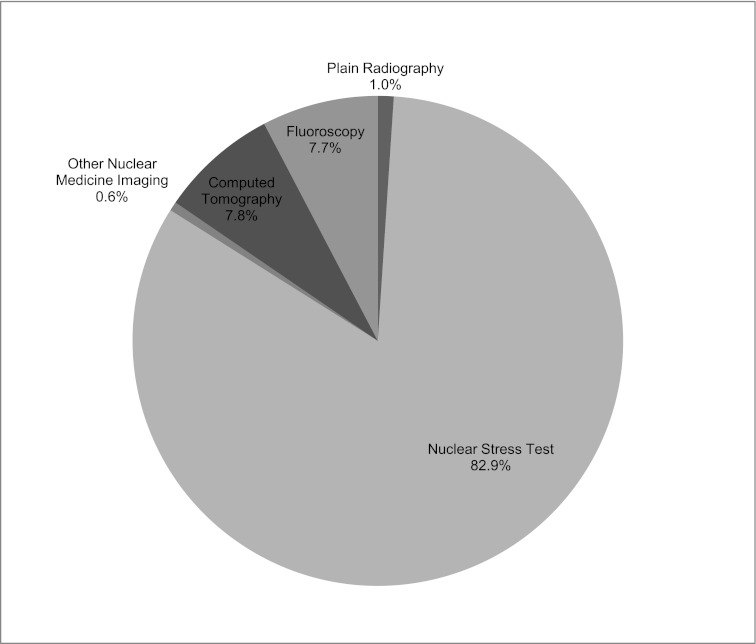
The total radiation exposure before transplant for the study group was approximately 7900.45 mSv. The median cumulative radiation exposure for each patient was 28.8 mSv (IQR, 12.0–60.8) during the 4 years of follow-up. The majority of the total radiation exposure was made up of nuclear medicine testing. The main contributor of radiation exposure from nuclear medicine tests was NST (Figure 2). Although the majority of procedures were from plain radiography, these procedures contributed the least amount of total radiation exposure.
Figure 2.
Percentage of total radiation exposure by type of medical imaging. The key lists the portions of the pie chart in clockwise order, starting with plain radiography (1.0%), nuclear stress test (82.9%), and so forth.
Overall, 70 patients (40.7%) were exposed to low dose (0–20 mSv) cumulative effective radiation, 51 (29.7%) were exposed to moderate dose (>20–50 mSv) radiation, 28 (16.3%) were exposed to high dose (>50–100 mSv) radiation, and 23 (13.4%) were exposed to very high dose radiation (>100 mSv).
We calculated the annual cumulative effective dose of radiation exposure among the 110 transplant recipients with at least 1 year of pretransplant follow-up. We did not calculate annual radiation exposure among the other 62 patients, given that they had <1 year of follow-up between their initial transplant evaluation and receipt of a kidney transplant. Among these 110 transplant recipients, the median annual radiation exposure was 12.6 mSv/yr (IQR, 6.0–27.9 mSv/yr). Of the 110 patients, 15 (13.6%) had 0–3 mSv/yr, 57 (51.8%) had >3–20 mSv/yr, 31 (28.2%) had >20–50 mSv/yr, and 7 (6.4%) had >50–100 mSv/yr of radiation exposure.
Univariable and Multivariable Regression of Baseline Factors Associated with Increased Radiation Exposure
The likelihood of increased dose radiation exposure varied according to demographic and medical factors (Figure 3 and Table 1). We excluded type of transplant and time until transplant from the regression models, however, because these variables are unknown at baseline at the time of the initial transplant evaluation.
Figure 3.
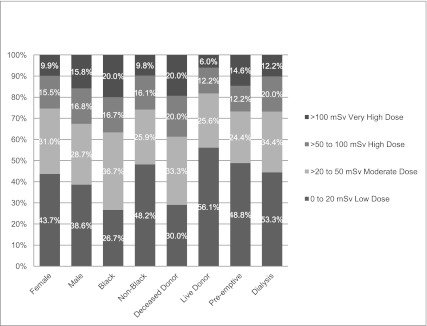
Overall distribution of total effective dose of radiation in study population stratified by sex, race, donor type, and dialysis at time of evaluation.
In univariable logistic regression, moderate or higher doses (>20 mSv) of radiation exposure was more common among patients with the following characteristics: black race, older age, diabetes as the cause of their kidney disease, on maintenance hemodialysis at the time of initial transplant evaluation, and higher Charlson comorbidity index scores. In the multivariable logistic regression model, black race, older age at time of transplant evaluation, and diabetes as the cause of ESRD were independently associated with moderate or higher doses (>20 mSv) of radiation exposure (Table 2).
Table 2.
Multivariable analysis of baseline factors associated with >20 mSv total radiation exposure
| Variable | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Black | 3.39 (1.51–7.59) | 0.003 |
| Age at time of transplant evaluation (vs ≤40) (yr) | ||
| >40 | 6.97 (2.65–18.3) | <0.001 |
| Diabetes as etiology of renal disease (vs cause other than diabetes) | 3.35 (1.39–8.09) | 0.01 |
Discussion
Our study showed that more than half of kidney transplant recipients were exposed to moderate or higher dose (>20 mSv) ionizing radiation during their pretransplant work-up. Compared with the general population, our transplant recipients received 5 times greater cumulative effective radiation exposure per year (7).
The purpose of our study was to assess the radiation exposure before kidney transplant due to transplant evaluation. The study excludes radiation exposure from tests ordered by general nephrologists as part of routine CKD/ESRD care (e.g., fistulogram). Transplant centers have little, if any, control over the procedures ordered as part of routine nephrology care. The radiation exposure of the study patients would potentially be even higher if we included procedures ordered by the general nephrologists (or other health care providers). However, our objective was to determine radiation exposure stemming from tests ordered by the transplant physician, because this is modifiable by an individual transplant center.
NST accounted for the majority of radiation exposure in our study population. The pretransplant protocol at our institution is guided by the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients (18). Our protocol suggests noninvasive cardiac screening annually for diabetic candidates and biennially for candidates aged >50 years with cardiac risk factors. Not surprisingly, ESRD due to diabetes mellitus, older age at time of evaluation, and total years from evaluation to time of transplant resulted in increased risk for exposure to moderate or higher dose (>20 mSv) of ionizing radiation.
Transplant candidates undergo intense cardiovascular evaluation due to the high prevalence of cardiovascular disease (19,20). The optimal frequency of cardiac screening for potential transplant candidates is controversial. Compared with the KDOQI guidelines, the American College of Cardiology/American Heart Association guidelines recommend a less aggressive approach to cardiac screening (21). Friedman et al. demonstrated that this approach can reduce stress testing by 80% but may fail to identify individuals with single vessel coronary artery disease (22). However, the benefit of revascularization for single vessel coronary artery disease is unclear (23). The optimal modality of cardiac screening is also unclear. Indeed, a survey of transplant centers demonstrated a wide variety of preferred cardiac screening, with 7% using exercise stress without imaging, 15% using angiography, and the remainder using stress imaging (24). Stress echocardiography compares favorably to NST as a screening tool for cardiovascular disease and may help reduce radiation exposure in our patient population (25,26). The most favorable cardiac screening test remains controversial, and the preferred test is at the discretion of the individual transplant center (22,24,27).
This study has important limitations. First, this is a single-center retrospective analysis of a small study population. Transplant centers vary in their practices, especially in the use of NST, which was the major contributor to radiation exposure in our study. Our cardiac screening protocol is guided by the KDOQI guidelines and may be similar to other transplant centers’ protocols, but the choice of cardiac screening modality may differ.
Second, this was not a prospective analysis with long-term follow-up; therefore, we cannot determine whether increased cancers resulted from the radiation exposure the study population received. In addition, the majority of the data linking malignancy risk to radiation exposure is a result of a single high dose exposure (28,29). It is unclear if repeated exposure to low dose radiation from medical imaging over many years carries the same risk of malignancy as a single high dose exposure.
Third, we relied on published literature to estimate effective radiation dose for most radiologic procedures. This does not give a precise measurement for the individual or for the specific procedure. However, an individualized dose assessment was calculated for NST, which accounted for the vast majority of radiation exposure in the study. The use of the lowest published estimate of effective radiation dose in our study should minimize the possibility of overestimating the true radiation exposure.
Fourth, the use of mSv imperfectly reflects future malignancy risk due, in part, to the different radiosensitivities of different organs and tissues. However, given the wide variety of radiologic tests and the inability to calculate subject-specific exposure dose for all tests, we believe that effective dose was the best estimate of potential malignancy risk.
In conclusion, renal transplant patients are exposed to a significant amount of ionizing radiation as part of the pretransplant work-up. The cumulative ionizing radiation exposure over time can lead to potential health consequences in the future. The recognition of increased ionizing radiation exposure from medical imaging procedures should prompt clinicians to consider the use of alternative procedures with little or no radiation exposure that provide the same diagnostic capability. These strategies implemented in the pretransplant work-up may reduce the cumulative ionizing radiation exposure and decrease the incidence of potential future cancers among kidney transplant recipients.
Disclosures
None.
Acknowledgments
A portion of this work was presented at the Annual Meeting of the American Society of Nephrology, held November 8–13, 2011, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Ionizing Radiation During Pretransplant Evaluation: Time to Reconsider the Evaluation Process,” on pages 711–713.
References
- 1.United States Renal Data System : US Renal Data System 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Hoover R, Fraumeni JF, Jr: Risk of cancer in renal-transplant recipients. Lancet 2: 55–57, 1973 [DOI] [PubMed] [Google Scholar]
- 3.National Research Council : Health Risks from Exposure to Low Levels of Ionizing Radiation BEIR VII, Phase 2 Board of Radiation Effects Research, Washington, DC, The National Academies Press, 2006 [Google Scholar]
- 4.United Nations Scientific Committee on the Effects of Atomic Radiation: Sources and Effects of Ionizing Radiation: UNSCEAR 2008 Report to the General Assembly with Scientific Annexes, New York, United Nations, 2010 [Google Scholar]
- 5.Mettler FA, Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT: Radiologic and nuclear medicine studies in the United States and worldwide: Frequency, radiation dose, and comparison with other radiation sources—1950-2007. Radiology 253: 520–531, 2009 [DOI] [PubMed] [Google Scholar]
- 6.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37: 1–332, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, Shah ND, Nasir K, Einstein AJ, Nallamothu BK: Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 361: 849–857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinsella SM, Coyle JP, Long EB, McWilliams SR, Maher MM, Clarkson MR, Eustace JA: Maintenance hemodialysis patients have high cumulative radiation exposure. Kidney Int 78: 789–793, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Coyle J, Kinsella S, McCarthy S, MacWilliams S, McLaughlin P, Eustace J, Maher MM: Cumulative ionizing radiation exposure in patients with end stage kidney disease: A 6-year retrospective analysis. Abdom Imaging 37: 632–638, 2012 [DOI] [PubMed] [Google Scholar]
- 10.De Mauri A, Brambilla M, Chiarinotti D, Matheoud R, Carriero A, De Leo M: Estimated radiation exposure from medical imaging in hemodialysis patients. J Am Soc Nephrol 22: 571–578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Mauri A, Brambilla M, Izzo C, Matheoud R, Chiarinotti D, Carriero A, Stratta P, De Leo M: Cumulative radiation dose from medical imaging in kidney transplant patients. Nephrol Dial Transplant 27: 3645–3651, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Einstein AJ: Radiation protection of patients undergoing cardiac computed tomographic angiography. JAMA 301: 545–547, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Martin CJ: The application of effective dose to medical exposures. Radiat Prot Dosimetry 128: 1–4, 2008 [DOI] [PubMed] [Google Scholar]
- 14.ICRP Publication 105. Radiation protection in medicine. Ann ICRP 37: 1–63, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M: Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology 248: 254–263, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ: Radiation dose to patients from cardiac diagnostic imaging. Circulation 116: 1290–1305, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Ann ICRP 28: 1–126, 1998 [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 19.Herzog CA, Ma JZ, Collins AJ: Long-term survival of renal transplant recipients in the United States after acute myocardial infarction. Am J Kidney Dis 36: 145–152, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Collins AJ: Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW: ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol 50: 1707–1732, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Friedman SE, Palac RT, Zlotnick DM, Chobanian MC, Costa SP: A call to action: Variability in guidelines for cardiac evaluation before renal transplantation. Clin J Am Soc Nephrol 6: 1185–1191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group : Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356: 1503–1516, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Danovitch GM, Hariharan S, Pirsch JD, Rush D, Roth D, Ramos E, Starling RC, Cangro C, Weir MR, Clinical Practice Guidelines Committee of the American Society of Transplantation : Management of the waiting list for cadaveric kidney transplants: Report of a survey and recommendations by the Clinical Practice Guidelines Committee of the American Society of Transplantation. J Am Soc Nephrol 13: 528–535, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Parker Ward R, Weiner RB, American College of Cardiology Foundation Appropriate Use Criteria Task Force. American Society of Echocardiography. American Heart Association. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society. Society for Cardiovascular Angiography and Interventions. Society of Critical Care Medicine. Society of Cardiovascular Computed Tomography. Society for Cardiovascular Magnetic Resonance. American College of Chest Physicians : ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 24: 229–267, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, Stainback RF, Blaivas M, Des Prez RD, Gillam LD, Golash T, Hiratzka LF, Kussmaul WG, Labovitz AJ, Lindenfeld J, Masoudi FA, Mayo PH, Porembka D, Spertus JA, Wann LS, Wiegers SE, Brindis RG, Douglas PS, Hendel RC, Patel MR, Peterson ED, Wolk MJ, Allen JM, American College of Cardiology Foundation. American Society of Echocardiography. American College of Emergency Physicians. American Heart Association. American Society of Nuclear Cardiology. Society for Cardiovascular Angiography and Interventions. Society of Cardiovascular Computed Tomography. Society for Cardiovascular Magnetic Resonance : ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: A report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. J Am Coll Cardiol 51: 1127–1147, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Lentine KL, Schnitzler MA, Brennan DC, Snyder JJ, Hauptman PJ, Abbott KC, Axelrod D, Salvalaggio PR, Kasiske B: Cardiac evaluation before kidney transplantation: A practice patterns analysis in Medicare-insured dialysis patients. Clin J Am Soc Nephrol 3: 1115–1124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K: Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res 146: 1–27, 1996 [PubMed] [Google Scholar]
- 29.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K: Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res 160: 381–407, 2003 [DOI] [PubMed] [Google Scholar]