Summary
Background and objectives
The efficacy and safety of immunosuppression for idiopathic membranous nephropathy (IMN) with nephrotic syndrome are still controversial. A systematic review and meta-analysis of randomized controlled trials (RCTs) was performed.
Design, setting, participants, & measurements
The Cochrane Library, PUBMED, EMBASE, Chinese Database, and Clinical Trial Registries (June 2012) were searched to identify RCTs investigating the effect of immunosuppression on adults with IMN and nephrotic syndrome.
Results
This review was an update (36 RCTs, 1762 participants) of the 2004 version (18 RCTs, 1025 participants). Immunosuppression significantly reduced all-cause mortality or ESRD (15 RCTs, 791 participants; risk ratio, 0.58 [95% confidence interval, 0.36–0.95]; P=0.03). However, the result was not consistent when prespecified subgroup analyses were undertaken. Immunosuppression increased complete or partial remission (CR + PR) (16 RCTs, 864 participants; 1.31 [1.01–1.70]; P=0.04) but resulted in more withdrawals or hospitalizations (16 RCTs, 880 participants; 5.35 [2.19–13.02]; P=0.002). Corticosteroids combined with alkylating agents significantly reduced all-cause mortality or ESRD (8 RCTs, 448 participants; 0.44 [0.26–0.75]; P=0.002) and increased CR + PR (7 RCTs, 422 participants; 1.46 [1.13–1.89]; P=0.004) but led to more adverse events (4 RCTs, 303 participants; 4.20 [1.15–15.32]; P=0.03). Cyclophosphamide was safer than chlorambucil (3 RCTs, 147 participants; 0.48 [0.26–0.90]; P=0.02). Cyclosporine and mycophenolate mofetil failed to show superiority over alkylating agents. Tacrolimus and adrenocorticotropic hormone significantly reduced proteinuria.
Conclusions
Alkylating agents plus corticosteroids had long-term and short-term benefits for adult IMN, but resulted in more withdrawals or hospitalizations.
Introduction
Idiopathic membranous nephropathy (IMN) is the most common form of primary nephrotic syndrome in adults (1). Immunosuppression is supposed to induce disease remission and reduce the risk of progression to ESRD or death (1–4). Several meta-analyses were performed >15 years ago (5–7). However, due to the paucity and low quality of evidence, there is still uncertainty regarding the efficacy and safety of immunosuppression. In 2004, we published a meta-analysis of immunosuppression for adults with IMN and nephrotic syndrome based on 18 randomized controlled trials (RCTs) enrolling 1025 participants (8,9). Numerous new RCTs evaluating novel immunosuppressive therapies have subsequently been published. These alternative agents, including tacrolimus (10), mycophenolate mofetil (11), and adrenocorticotropic hormone (12), were expected to be more effective and less toxic. Unfortunately, there has been no evidence related to the favorable effect of adrenocorticotropic hormone on all-cause mortality or risk of ESRD (12,13). Chen et al. failed to show superiority for tacrolimus over cyclophosphamide in complete or partial remission (CR + PR) at the end of follow-up (14). Dussol et al. also failed to demonstrate an effect of mycophenolate mofetil on CR + PR (15). Recent studies evaluating the effects of older immunosuppressive treatments, such as corticosteroids, alkylating agents, cyclosporine, and azathioprine, have also been published (16–18). Tripterygium wilfordii, a traditional Chinese immunosuppressive medicine, has been reported to be effective in Chinese patients (19,20). Thus, substantial new evidence has become available (18 additional RCTs, 737 new participants) to explore whether previous findings for classic immunosuppressive approaches could be modified and/or novel immunosuppressive approaches could be effective.
Materials and Methods
Data Sources and Searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2012 Issue 6), PUBMED (1966 to June 2012), EMBASE (1966 to June 2012), Chinese Database (1978 to June 2012), and Clinical Trial Registries (Clinicaltrials.gov and World Health Organization Clinical Trials Registry) (June 2012) to identify RCTs that evaluated the effects of immunosuppression for adult IMN with nephrotic syndrome. The diagnosis of nephrotic syndrome was defined by the authors in each study. In absence of an explicit definition, a cut-off value of 3.5 g/24 h for proteinuria was used, and the presence of hypoalbuminemia, hyperlipidemia, and edema was not necessary (Supplemental Table 1). A hand-search of references was also performed. The studies were required to have a planned follow-up of at least 6 months. We excluded quasi-RCTs. We included the parallel and first period (at least 6 months) of cross-over randomized studies comparing the following: (1) immunosuppressive treatments with placebo, no treatment, or renin-angiotensin system (RAS) blockers, such as angiotensin converting enzyme inhibitors (ACEIs), and (2) different immunosuppressive treatments with each other. We also included any combination of immunosuppressive drugs. The primary outcomes were composite definite endpoints (all-cause mortality or risk of ESRD), all-cause mortality alone, and risk of ESRD alone (defined as the need for renal replacement therapy). The secondary outcomes were CR + PR, complete remission (CR), partial remission (PR), proteinuria, and adverse events resulting in temporary or permanent withdrawal or hospitalization.
Study Selection, Data Extraction, Quality Assessment, and Data Synthesis
The titles and abstracts, and full texts if necessary, were screened by three authors (Y.C., G.C., and A.P.). Study selection, data extraction, quality assessment, and data synthesis were independently performed by the same authors. Disagreements were resolved by consulting another author (X.C.). Study quality was assessed using the Cochrane-recommended method (21). Dichotomous and continuous outcomes were expressed as the risk ratio (RR) with 95% confidence interval (CI) and mean difference (MD) with 95% CI, respectively. A random-effects model, which is generally considered to be more conservative, was used for data analyses. We used the Cochrane Q test to determine heterogeneity between studies if the threshold P value was <0.10 and the I2 test to further quantify the magnitude of heterogeneity (21). Subgroup analysis was used to explore possible sources of heterogeneity, such as baseline characteristics (impaired baseline renal function and/or resistance to previous corticosteroids ± alkylating agents), industry support, and a priori sample size estimation. Sensitivity analysis, excluding totally/partially unpublished studies and low-quality studies, was also performed. Publication bias was first addressed by using the funnel plots and then further quantified by using the Harbord test if there was an adequate number of identified RCTs (i.e., at least 10 studies). Publication bias was defined as visual asymmetry of the funnel plots or P<0.05 for the Harbord test (21–23). Review Manager (version 5.1), GRADE Profiler (version 3.6) and STATA (version 11.2) software were used.
Results
Literature Search Results and Study Characteristics
Overall, 36 RCTs (n=1762 patients) were included (June 2012) (10–19,24–49) (Figure 1 and Supplemental Table 2). Three trials were included in more than one comparison category (16,17,28) (Supplemental Table 3). The very limited effect of RAS blockers (e.g., ACEI) or corticosteroid monotherapy on the natural history of patients with IMN and nephrotic syndrome, especially for those with heavy proteinuria, has been well recognized (50,51). Thus, we compared corticosteroids plus alkylating agents with no treatment, ACEI, or corticosteroid monotherapy in eight studies (n=448 patients). Furthermore, 18 of 36 trials only used no treatment or ACEI as the control group and therefore were summarized in another new comparison category: immunosuppressive treatments versus no treatment or ACEI. Six studies were only published in conference abstracts (12,28,39,47–49), of which two were added with unpublished data (28,39). All but two studies were published in English. One was published in Chinese (19) and another in Japanese (36). The median sample size was 31 (range, 9–120) and follow-up was 24 months (range, 6–120). Five studies involved patients with impaired renal function (baseline serum creatinine 2.3–2.9 mg/dl or GFR 43–51 ml/min per 1.73 m2) (27,32,34,39,43), and five studies involved patients who were resistant to corticosteroids ± alkylating agents (16,30,36,45,49). Eight studies provided a priori sample size calculation (10,15,30,31,35,40,41,46). Eight studies had industry support (10,11,15,24,30,32,44,49).
Figure 1.
Study selection flow chart. RCT, randomized controlled trial.
The assessment of study quality is presented in Supplemental Figures 1 and 2. Twenty-two studies (61%) specified appropriate methods for random sequence generation. Fifteen studies (42%) reported appropriate allocation concealment methods. Appropriate procedure related to participant blinding was confirmed in six studies (17%), whereas adequate blinding of study personnel and outcome assessors was confirmed in four studies (11%). Thirty-two studies (89%) were considered to have a low risk of bias on the issue of incomplete outcome data. The reporting rates of all-cause mortality or risk of ESRD, CR or PR, proteinuria, and adverse events leading to withdrawal or hospitalization were 83% (30 of 36), 89% (32 of 36), 61% (22 of 36) and 86% (31 of 36), respectively. A total of 17 of 36 studies (47%) were classified as totally or partially unpublished or having a low-quality design (12,16,17,19,26–28,31,36–39,45–49).
Immunosuppressive Treatments versus No Treatment or ACEI
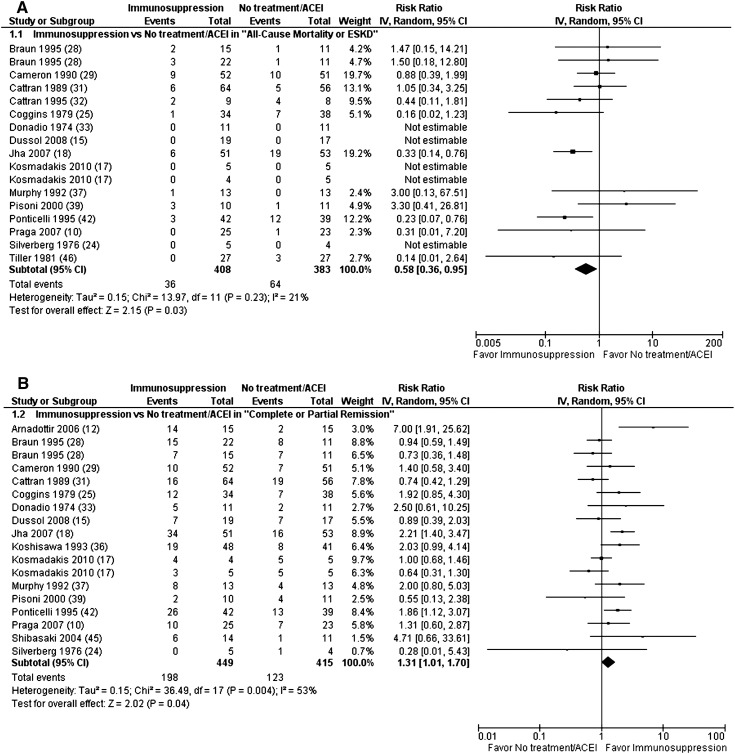
A total of 18 trials (n=935 patients) compared no treatment/ACEI with a variety of immunosuppressive treatments, including corticosteroids, alkylating agents, cyclosporine, tacrolimus, mycophenolate mofetil, adrenocorticotropic hormone, azathioprine, and mizoribine (10,12,15,17,18,24,25,28,29,31–33,36,37,39,42,45,46). Immunosuppression was superior to no treatment or ACEI in the composite definite endpoints (RR, 0.58 [0.36–0.95]; P=0.03) (Figure 2A), risk of ESRD (RR, 0.55 [0.31–0.95]; P=0.03), CR + PR (RR, 1.31 [1.01–1.70]; P=0.04) (Figure 2B), and proteinuria (MD, −0.95 g/24 h [−1.81 to −0.09]; P=0.03) at the end of follow-up (range, 6–120 months) (Supplemental Table 4). Significant heterogeneity was found for CR + PR (I2=53%; heterogeneity P=0.004) and proteinuria (I2=59%; heterogeneity P=0.009). Immunosuppression resulted in a significantly higher risk of adverse events leading to withdrawal or hospitalization (RR, 5.35 [2.19–13.02]; P=0.002).
Figure 2.
Immunosuppression significantly reduced all-cause mortality or risk of ESRD (A) and significantly increased complete or partial remission (B) at the end of follow-up compared with no treatment or ACEI. ACEI, angiotensin converting enzyme inhibitor; CI, confidence interval; IV, inverse variance method.
Sensitivity analysis confirmed that there were no significant changes when nine studies, which provided unpublished data or had a low-quality design, were excluded (12,17,28,31,36,37,39,45,46). The evidence for all-cause mortality or risk of ESRD was not consistent when prespecified subgroup analyses were undertaken (Supplemental Figures 3–5). Subgroup analysis indicated that there was no significant interaction between the effect of immunosuppression and industry support for the study (10,15,24,32). Two trials involved patients with impaired baseline renal function (32,39). There was no significant subgroup difference between the trials with and without participants who had impaired baseline renal function. Four trials provided a priori sample size calculation (10,15,31,46). There was no significant difference in CR in the subgroup with a priori sample size calculation (RR, 0.50 [0.24–1.02]; P=0.06), whereas immunosuppression significantly increased CR in the subgroup without a priori sample size calculation (RR, 2.42 [1.40–4.17]; P=0.002). There was a significant subgroup difference (I2=91.5%; P=0.001), indicating that the presence of a prospectively planned sample size calculation was associated with a more conservative estimate in CR.
Corticosteroid Monotherapy versus No Treatment
There were no significant differences in any of the considered outcomes at the end of follow-up (range, 24–48 months) in three studies (n=295 patients) (25,29,31).
Alkylating Agent Monotherapy versus No Treatment
Among the three studies (n=102 patients), alkylating agent monotherapy resulted in a significantly higher risk of adverse events leading to withdrawal or hospitalization at the end of follow-up (range, 12–36 months) (RR, 7.18 [1.33–38.70]; P=0.02) (33,37,46).
Corticosteroids + Alkylating Agents versus No Treatment, ACEI, or Corticosteroid Monotherapy
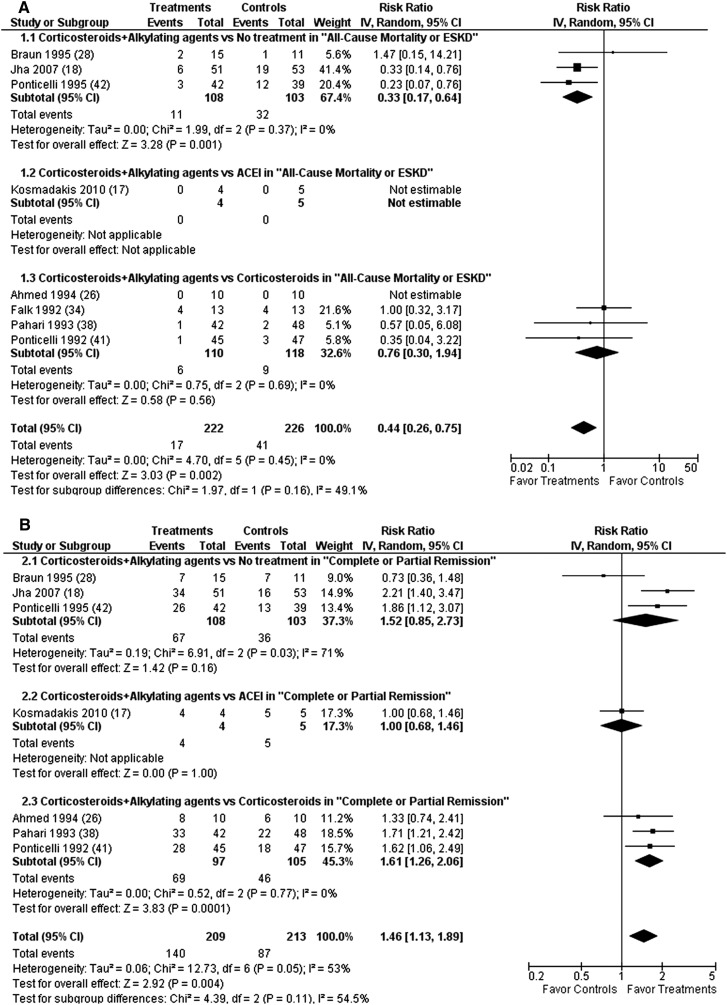
In eight studies (n=448 patients), treatment with corticosteroids plus alkylating agents was superior to no treatment, ACEI, or corticosteroid monotherapy in composite definite endpoints (RR, 0.44 [0.26–0.75]; P=0.002) (Figure 3A), risk of ESRD (RR, 0.45 [0.25–0.81]; P=0.01), CR + PR (RR, 1.46 [1.13–1.89]; P=0.004) (Figure 3B), CR (RR, 2.32 [1.61–3.32]; P<0.001), and proteinuria (MD, −1.25 g/24 h [−1.93 to −0.57]; P=0.001) at the end of follow-up (range, 9–120 months). Significant heterogeneity was found for CR + PR (I2=53%; heterogeneity P=0.05) and proteinuria (I2=50%; heterogeneity P=0.08).
Figure 3.
Alkylating agents plus corticosteroids significantly reduced all-cause mortality or risk of ESRD (A) and significantly increased complete or partial remission (B) at the end of follow-up compared with no treatment or ACEI or corticosteroid monotherapy. ACEI, angiotensin converting enzyme inhibitor; CI, confidence interval; IV, inverse variance method.
Sensitivity analysis, excluding four unpublished or low-quality studies (17,26,28,38), revealed that this regimen led to a significantly higher risk of adverse events leading to withdrawal or hospitalization (RR, 4.20 [1.15–15.32]; P=0.03). No subgroup differences were observed according to a priori sample size estimation, industry support, or impaired baseline renal function. Three studies with 211 patients compared alkylating agents plus corticosteroids with no treatment (18,28,42). This regimen significantly reduced composite definite endpoints (RR, 0.33 [0.17–0.64]; P=0.001), risk of ESRD (RR, 0.31 [0.15–0.65]; P=0.002), increased CR (RR, 3.18 [1.23–8.21]; P=0.02), and decreased proteinuria (MD, -2.06 g/24h [-3.69 to -0.44]; P=0.01) at the end of follow-up (range, 60–120 months). Significant heterogeneity was found for CR + PR (I2=71%; heterogeneity P=0.03) and proteinuria (I2=77%; heterogeneity P=0.04). This regimen resulted in a significantly higher risk of adverse events leading to withdrawal or hospitalization (RR, 9.79 [1.28–75.01]; P=0.03).
Cyclophosphamide + Corticosteroids versus Chlorambucil + Corticosteroids
In three studies (n=147 patients), cyclophosphamide plus corticosteroids led to a significantly lower risk of adverse events leading to withdrawal or hospitalization at the end of follow-up (range, 15–39 months) (RR, 0.48 [0.26–0.90]; P=0.02) (27,40,43).
Cyclosporine ± Corticosteroids versus No Treatment, ACEI, or Corticosteroids ± Alkylating Agents/Azathioprine
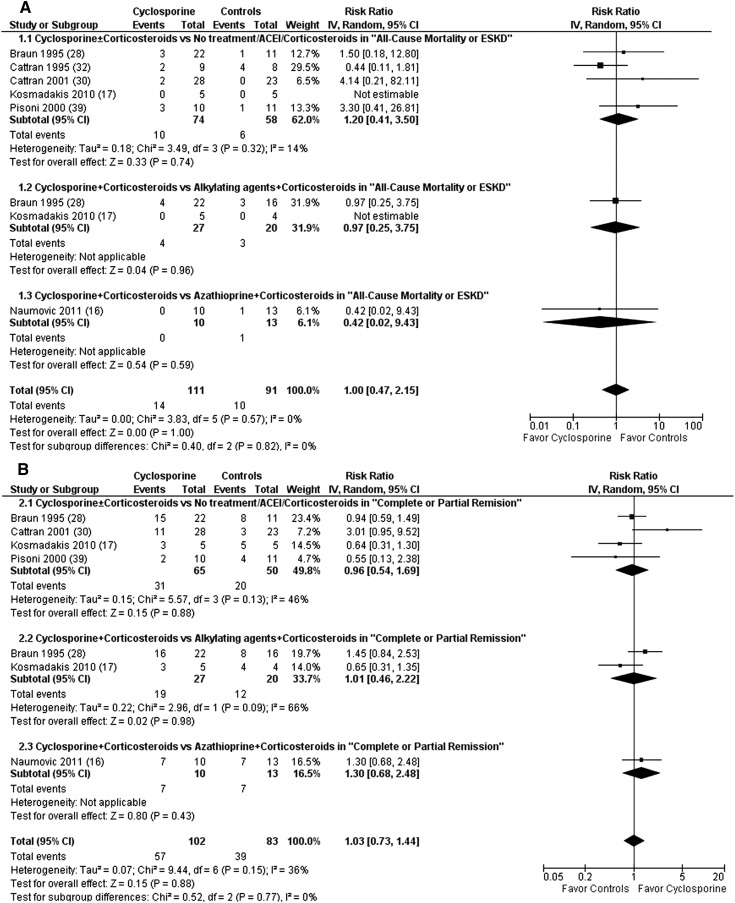
There were no significant differences in any of the considered outcomes at the end of follow-up (range, 9–60 months) in six studies (n=202 patients) (16,17,28,30,32,39) (Figure 4). The subgroup analysis failed to show superiority for cyclosporine ± corticosteroids over no treatment, ACEI, or corticosteroid monotherapy at the end of follow-up (17,28,30,32,39). The subgroup analysis also failed to show superiority for cyclosporine + corticosteroids over alkylating agents + corticosteroids at the end of follow-up (17,28).
Figure 4.
Cyclosporine ± corticosteroids failed to show superiority over no treatment, ACEI, or corticosteroids ± alkylating agents/azathioprine with regard to all-cause mortality or risk of ESRD (A) and complete or partial remission (B) at the end of follow-up. ACEI, angiotensin converting enzyme inhibitor; CI, confidence interval; IV, inverse variance method.
Cyclosporine + Corticosteroids versus Cyclosporine + Corticosteroids
In one study (n=33 patients), cyclosporine (1.5 mg/kg twice a day) significantly reduced proteinuria (MD, −0.70 g/24 h [−0.96 to −0.44]; P<0.001) at the end of follow-up (12 months) compared with cyclosporine (3.0 mg/kg once a day) (49).
Tacrolimus ± Corticosteroids versus No Treatment or Corticosteroids + Alkylating Agents
Tacrolimus significantly reduced proteinuria at the end of follow-up (range, 9–30 months) in three studies (n=145 patients) (MD, -1.06 g/24 h [-1.66 to -0.47]; P<0.001) (10,14,47).
Mycophenolate Mofetil ± Corticosteroids versus No Treatment or Corticosteroids + Alkylating Agents
There were no significant differences in any of the considered outcomes at the end of follow-up (range, 12–24 months) in three studies (n=77 patients) (11,15,44). The subgroup analysis failed to show superiority for mycophenolate mofetil + corticosteroids over alkylating agents + corticosteroids at the end of follow-up (11,44).
Mycophenolate Mofetil + Cyclosporine + Corticosteroids versus Cyclosporine + Corticosteroids
There were no significant differences in any of the considered outcomes at the end of follow-up (12 months) in one study (n=18 patients) of mycophenolate mofetil + cyclosporine (2 mg/kg per day) + corticosteroids versus cyclosporine (5 mg/kg per day) + corticosteroids (48).
Adrenocorticotropic Hormone versus No Treatment or Corticosteroids + Alkylating Agents
In two studies (n=62 patients), adrenocorticotropic hormone significantly reduced proteinuria at the end of follow-up (22 months) (MD, −1.80 g/24 h [−3.19 to −0.41]; P=0.01) (13).
Azathioprine ± Corticosteroids versus Placebo or Cyclosporine + Corticosteroids
There were no significant differences in any of the considered outcomes at the end of follow-up (range, 12–36 months) in two studies (n=32 patients) (16,24).
Mizoribine versus No Treatment
Mizoribine monotherapy significantly increased CR + PR at the end of follow-up (range, 6–24 months) (RR, 2.24 [1.14–4.38]; P=0.02) in two studies (n=114 patients) (36,45).
T. wilfordii
In one study (n=84 patients), T. wilfordii plus corticosteroids significantly increased CR + PR (RR, 2.03 [1.31–3.16]; P=0.002) and CR (RR, 7.63 [1.87–31.13]; P=0.01) at the end of follow-up (12 months) compared with T. wilfordii monotherapy (19).
Early Versus Late Cyclophosphamide + Corticosteroids
There were no significant differences in any of the considered outcomes at the end of follow-up (72 months) in one study (n=26 patients) (35).
Publication Bias
Tests for publication bias have been recommended to be used when at least 10 studies are included in a meta-analysis (22). Thus, the tests were restricted to the comparison of immunosuppression with no treatment or ACEI (n=18 studies). There was no evidence of publication bias for the composite definite endpoints (P=0.38) (Supplemental Figure 6, A and C) and CR + PR (P=0.29) (Supplemental Figure 6, B and D).
Discussion
The original version of our study was published in 2004 (8,9). A total of 18 RCTs, enrolling 1025 patients, were included to evaluate corticosteroids, alkylating agents, cyclosporine, and azathioprine. In this update, we summarized evidence from 36 RCTs with 1762 patients. We identified RCTs investigating immunosuppressive drugs that were not included in the 2004 version, including tacrolimus, mycophenolate mofetil, adrenocorticotropic hormone, mizoribine, and T. wilfordii. More RCTs that evaluated the previous four immunosuppressive drugs were also identified.
In the 2004 version, alkylating agents showed significant beneficial effects only on CR but not on definite endpoints. In this update, a combined alkylating agent and corticosteroid regimen had short- and long-term benefits on adult IMN with nephrotic syndrome. However, this regimen inevitably resulted in more withdrawal or hospitalization. Among alkylating agents, cyclophosphamide was safer than chlorambucil. The superiority of cyclosporine or mycophenolate mofetil plus corticosteroids over alkylating agents plus corticosteroids was not identified, but the above conclusion was based on four small RCTs only, totaling <150 participants. Tacrolimus and adrenocorticotropic hormone significantly reduced proteinuria. The recent identification of M-type phospholipase A2 receptor and the utilization of rituximab represent major milestones in understanding the pathogenesis and searching for new therapeutic strategies for IMN (51–54). Numerous non-RCTs have demonstrated that rituximab is a promising new immunosuppressive drug, but these pioneering RCTs are still ongoing (55,56).
Several factors have decreased the quality of evidence. Methodologic limitations caused by the lack of high-quality trials and imprecise results due to the few events precluded firm conclusions. Prespecified subgroup analyses showed variability in results and failed to demonstrate the consistency of study findings, especially for all-cause mortality or risk of ESRD. Notably, studies with ESRD as the primary outcome may require 7–10 years of follow-up (57). The majority of RCTs (94%) had <7 years of follow-up. Therefore, the follow-up length was inadequate to appropriately assess the definite endpoints. The quality of trial conducting and reporting varied. Forty-seven percent of RCTs were classified as unpublished or having a low-quality design and 78% did not estimate sample size. Approximately 90% of RCTs did not implement double-blind design. Publication bias cannot be excluded for some comparison categories due to the insufficient number of studies. Several baseline characteristics, including race, age, sex, renal pathology, BP, serum creatinine, proteinuria, and responses to previous immunosuppression, have been identified as being associated with the response to the tested immunosuppression and IMN prognosis (1–4). The lack of consistent treatment effects was most likely due to these heterogeneous baseline characteristics. However, the small number of RCTs in each comparison group did not allow us to explore the covariate effects of these baseline characteristics. In addition, the regimens in the treatment and control groups varied among included trials. Furthermore, the included trials were mainly focused on a single ethnicity due to the lack of multinational cooperation, which affected the generalizability of our results.
IMN is a heterogeneous disorder, and no single definitive immunosuppression could be easily identified. The number of included studies doubled in this update; however, the quality of evidence was still suboptimal mainly due to the small sample size and short follow-up in each study, few studies in each comparison category, and the presence of a high risk of bias. More methodologically sound and sufficiently powered studies with adequate follow-up are still urgently needed for clinical decision making, especially for adrenocorticotropic hormone and rituximab. Surrogate outcomes (e.g., remission or proteinuria) should be assessed at least at 1 or 2 years instead of immediately after the cessation of immunosuppression. The priority should be given to definite endpoints (e.g., death or ESRD). The optimal doses, routes, and durations of specific immunosuppression that are the most beneficial and least harmful to patients of different races, ages, and clinical and pathologic severities still remain to be clarified.
Disclosures
None.
Acknowledgments
The authors thank Professor Giuseppe Remuzzi, President Elect of International Society of Nephrology, who had the original idea for this systematic review and revised this manuscript. The authors are also indebted to Dr. Antonietta Chianca, who provided statistical advice, Dr. Lisa A Tjosvold, who helped perform the electronic search, Dr. Luciana Tammuzzo, who hand-searched the Journal of Nephrology, and the principal investigators of the completed and ongoing trials considered in the review, who provided additional information or clarification (Professor Daniel C. Cattran, Professor Peter Mathieson, Dr. Roberto Pisoni, and Professor Teut Risler). The authors also thank Ms. Ruth Mitchell, the Cochrane Trials Search Coordinator, who provided us with the Cochrane Library search strategy and relevant information, and Ms. Narelle Willis, the Cochrane Renal Review Group Coordinator, for her help and support.
This work was partially supported by the Key Science and Technology Planning of Science and Technology Commission Foundation of Beijing (D09050104310000, D09050703560907), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2011BAI10B08), and the National Basic Research Program Project of China (2011CB944004). The sponsors had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
This review is excerpted from a substantive update to a Cochrane Review to be published in The Cochrane Library (www.thecochranelibrary.com). Cochrane Reviews are regularly updated at The Cochrane Library as new evidence emerges in response to comments and criticisms.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07570712/-/DCSupplemental.
References
- 1.Hofstra JM, Wetzels JF: Management of patients with membranous nephropathy. Nephrol Dial Transplant 27: 6–9, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Waldman M, Austin HA, 3rd: Controversies in the treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 5: 469–479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponticelli C, Passerini P: Management of idiopathic membranous nephropathy. Expert Opin Pharmacother 11: 2163–2175, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Hofstra JM, Wetzels JF: Alkylating agents in membranous nephropathy: Efficacy proven beyond doubt. Nephrol Dial Transplant 25: 1760–1766, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Couchoud C, Laville M, Boissel JP: Treatment of membranous nephropathy: A meta-analysis. Nephrol Dial Transplant 9: 469–470, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Hogan SL, Muller KE, Jennette JC, Falk RJ: A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis 25: 862–875, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Imperiale TF, Goldfarb S, Berns JS: Are cytotoxic agents beneficial in idiopathic membranous nephropathy? A meta-analysis of the controlled trials. J Am Soc Nephrol 5: 1553–1558, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Perna A, Schieppati A, Zamora J, Giuliano GA, Braun N, Remuzzi G: Immunosuppressive treatment for idiopathic membranous nephropathy: A systematic review. Am J Kidney Dis 44: 385–401, 2004 [PubMed] [Google Scholar]
- 9.Schieppati A, Perna A, Zamora J, Giuliano GA, Braun N, Remuzzi G: Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev (4): CD004293, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Praga M, Barrio V, Juárez GF, Luño J, Grupo Español de Estudio de la Nefropatía Membranosa : Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chan TM, Lin AW, Tang SC, Qian JQ, Lam MF, Ho YW, Tse KC, Chan KW, Lai KN, Tang CS: Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology (Carlton) 12: 576–581, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Arnadottir M, Stefansson B, Berg L: A randomized study on treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy [Abstract]. J Am Soc Nephrol 17: 571A, 2006 [Google Scholar]
- 13.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Li H, Li XY, Lu FM, Ni ZH, Xu FF, Li XW, Chen JH, Wang HY, Chinese Nephropathy Membranous Study Group : Tacrolimus combined with corticosteroids in treatment of nephrotic idiopathic membranous nephropathy: A multicenter randomized controlled trial. Am J Med Sci 339: 233–238, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Dussol B, Morange S, Burtey S, Indreies M, Cassuto E, Mourad G, Villar E, Pouteil-Noble C, Karaaslan H, Sichez H, Lasseur C, Delmas Y, Nogier MB, Fathallah M, Loundou A, Mayor V, Berland Y: Mycophenolate mofetil monotherapy in membranous nephropathy: A 1-year randomized controlled trial. Am J Kidney Dis 52: 699–705, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Naumovic R, Jovanovic D, Pavlovic S, Stosovic M, Marinkovic J, Basta-Jovanovic G: Cyclosporine versus azathioprine therapy in high-risk idiopathic membranous nephropathy patients: A 3-year prospective study. Biomed Pharmacother 65: 105–110, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kosmadakis G, Filiopoulos V, Smirloglou D, Skarlas P, Georgoulias C, Michail S: Comparison of immunosuppressive therapeutic regimens in patients with nephrotic syndrome due to idiopathic membranous nephropathy. Ren Fail 32: 566–571, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Liu ZH, Li SJ, Wu Y, Zuo K, Wang B, Zeng CH, Li LS: Treatment of membranous nephropathy with tripterygium wilfordii and steroid: A prospective randomized control trial. Chin J Nephrol Dial Transplant 18: 303–309, 2009 [Google Scholar]
- 20.Chen YZ, Gao Q, Zhao XZ, Chen XM, Zhang F, Chen J, Xu CG, Sun LL, Mei CL: Meta-analysis of Tripterygium wilfordii Hook F in the immunosuppressive treatment of IgA nephropathy. Intern Med 49: 2049–2055, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Oxford, UK, The Cochrane Collaboration, 2011 [Google Scholar]
- 22.Harbord RM, Harris RJ, Sterne JAC: Updated tests for small-study effects in meta-analyses. Stata J 9: 197–210, 2009 [Google Scholar]
- 23.Harbord RM, Egger M, Sterne JA: A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Western Canadian Glomerulonephritis Study Group: Controlled trial of azathioprine in the nephrotic syndrome secondary to idiopathic membranous glomerulonephritis. Can Med Assoc J 115: 1209–1210, 1976 [PMC free article] [PubMed] [Google Scholar]
- 25.Coggins CH, Pinn V, Glassock RR, Blades JM, Cotran R, Cohen J, Churg J, Burkholder P, Lemann J, Spargo B: A controlled study of short-term prednisone treatment in adults with membranous nephropathy. Collaborative study of the adult idiopathic nephrotic syndrome. N Engl J Med 301: 1301–1306, 1979 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Rahman M, Alam MR, Islam S, Chowdhury MN, Chowdhury SM, Chowdhury MA: Methyl prednisolone plus chlorambucil as compared with prednisolone alone for the treatment of idiopathic membranous nephropathy - A preliminary study. Bangladesh Ren J 13: 51–54, 1994 [Google Scholar]
- 27.Branten AJ, Reichert LJ, Koene RA, Wetzels JF: Oral cyclophosphamide versus chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency. QJM 91: 359–366, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Braun N, Erley C, Benda N, Zauner I, Kanis R, Grupp C, Ackermann M, Heering P, Hunfstuck R, Risler T: Therapy of membranous glomerulonephritis with nephrotic syndrome. 5 years follow-up of a prospective, randomised multi-centre study. Nephrol Dial Transplant 10: 967, 1995 [Google Scholar]
- 29.Cameron JS, Healy MJ, Adu D, The MRC Glomerulonephritis Working Party : The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. Q J Med 74: 133–156, 1990 [PubMed] [Google Scholar]
- 30.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S: A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med 320: 210–215, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, Morrin PA, Lavoie S, Canadian Glomerulonephritis Study Group : A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int 47: 1130–1135, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Donadio JV, Jr, Holley KE, Anderson CF, Taylor WF: Controlled trial of cyclophosphamide in idiopathic membranous nephropathy. Kidney Int 6: 431–439, 1974 [DOI] [PubMed] [Google Scholar]
- 34.Falk RJ, Hogan SL, Muller KE, Jennette JC, The Glomerular Disease Collaborative Network : Treatment of progressive membranous glomerulopathy. A randomized trial comparing cyclophosphamide and corticosteroids with corticosteroids alone. Ann Intern Med 116: 438–445, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Hofstra JM, Branten AJ, Wirtz JJ, Noordzij TC, du Buf-Vereijken PW, Wetzels JF: Early versus late start of immunosuppressive therapy in idiopathic membranous nephropathy: A randomized controlled trial. Nephrol Dial Transplant 25: 129–136, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Koshisawa S, Sato M, Narita M, Sakai O, Nakajima M: Clinical evaluation of an immunosuppressive drug, mizoribine (HE-69) on steroid-resistant nephrotic syndrome. A multicenter double-blind comparison study with placebo [in Japanese]. Jin To Tohseki (Kidney Dial) 34: 631–650, 1993 [Google Scholar]
- 37.Murphy BF, McDonald I, Fairley KF, Kincaid-Smith PS: Randomized controlled trial of cyclophosphamide, warfarin and dipyridamole in idiopathic membranous glomerulonephritis. Clin Nephrol 37: 229–234, 1992 [PubMed] [Google Scholar]
- 38.Pahari DK, Das S, Dutta BN, Banerjee D: Prognosis and management of membraneous nephropathy. J Assoc Physicians India 41: 350–351, 1993 [PubMed] [Google Scholar]
- 39.Pisoni R, Grinyo JM, Salvadori M, Laurens W, Garini G, Oliver YA, Mezzano S, Ruggenenti P, Remuzzi G: Cyclosporine versus conservative therapy in patients with idiopathic membranous nephropathy (IMN) and deteriorating renal function: Results of the CYCLOMEN trial. J Am Soc Nephrol 11: 95A, 2000 [Google Scholar]
- 40.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Ponticelli C, Zucchelli P, Passerini P, Cesana B, The Italian Idiopathic Membranous Nephropathy Treatment Study Group : Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med 327: 599–603, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C, Bizzarri D, Banfi G: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Reichert LJ, Huysmans FT, Assmann K, Koene RA, Wetzels JF: Preserving renal function in patients with membranous nephropathy: Daily oral chlorambucil compared with intermittent monthly pulses of cyclophosphamide. Ann Intern Med 121: 328–333, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Senthil Nayagam L, Ganguli A, Rathi M, Kohli HS, Gupta KL, Joshi K, Sakhuja V, Jha V: Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: A pilot study. Nephrol Dial Transplant 23: 1926–1930, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Shibasaki T, Koyama A, Hishida A, Muso E, Osawa G, Yamabe H, Shiiki H, Makino H, Sato H, Ishikawa I, Maeda K, Tomita K, Arakawa M, Ishida M, Sato M, Nagase M, Kashihara N, Yorioka N, Koike T, Saito T, Harada T, Mitarai T, Sugisaki T, Nagasawa T, Tomino Y, Nojima Y, Kobayashi Y, Sakai O: A randomized open-label comparative study of conventional therapy versus mizoribine onlay therapy in patients with steroid-resistant nephrotic syndrome (postmarketing survey). Clin Exp Nephrol 8: 117–126, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Tiller DJ, Clarkson AR, Mathew T, Thompson N, Row G, Lauer C, Hobbs J, Seymour A: A prospective randomized trial in the use of cyclophosphamide, dipyridamole and warfarin in membranous and mesangiocapillary glomerulonephritis. Advances in basic and clinical nephrology. In: Proceedings of the 8th International Congress of Nephrology, Athens, Greece, Karger, 1981, pp 345–351 [Google Scholar]
- 47.Xu J, Zhang W, Xu YW, Chen N: A double-blinded prospective randomised study on the efficacy of corticosteroid plus cyclophosphamide or FK506 in idiopathic membranous nephropathy patients with nephrotic syndrome [Abstract]. NDT Plus 3: iii431, 2010
- 48.Jurubita R, Ismail G, Bobeica R, Rusu E, Zilisteanu D, Andronesi A, Motoi O, Ditoiu V, Copaci I, Voiculescu M: Efficacy and safety of triple therapy with MMF, cyclosporine and prednisolone versus cyclosporine and prednisolone in adult patients with idiopathic membranous nephropathy and persistent heavy proteinuria. Presented at the 2012 ERA-EDTA conference, Paris, France, May 24–27, 2012
- 49.Saito T, Watanabe M, Ogahara S, Shuto H, Kataoka Y: Significance of blood concentration of cyclosporine at 2 hours after administration for the treatment of idiopathic membranous nephropathy with steroid resistant nephrotic syndrome. Presented at the World Congress of Nephrology, Milan, Italy, May 23–26, 2009 Milan Italy
- 50.National Kidney Foundation: KDIGO Clinical Practice Guidelines for Glomerulonephritis: Idiopathic membranous nephropathy. Kidney Int Suppl 2[Suppl 2]: 186–197, 2012 [Google Scholar]
- 51.Waldman M, Austin HA, 3rd: Treatment of idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1617–1630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Dahan K: Evaluate Rituximab Treatment for Idiopathic Membranous Nephropathy (GEMRITUX) [ClinicalTrials.gov NCT01508468]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01180036 Accessed January 19, 2013
- 56.Fervenza FC, Jennison SA: Membranous Nephropathy Trial of Rituximab (MENTOR) [ClinicalTrials.gov NCT01180036]. Available at: http://clinicaltrials.gov/ct2/show/NCT01180036 Accessed January 19, 2013
- 57.du Buf-Vereijken PW, Branten AJ, Wetzels JF: Idiopathic membranous nephropathy: Outline and rationale of a treatment strategy. Am J Kidney Dis 46: 1012–1029, 2005 [DOI] [PubMed] [Google Scholar]