Summary
Background and objectives
Early identification of frequently relapsing children with idiopathic nephrotic syndrome is desirable.
Design, setting, participants, & measurements
The relapse status and clinical data of patients previously registered (January of 1993 to December of 2001) in a multicenter prospective study of the International Study of Kidney Disease in Children regimen were analyzed for risk of frequent relapsers over a 2-year follow-up period.
Results
Of 166 children with nephrotic syndrome (113 boys and 53 girls; median age=5.1 years), 145 (87.3%, median age=5.5 years) children were steroid-sensitive, and 21 (12.7%, median age=2.9 years) children were steroid-resistant. Of 145 children with steroid-sensitive nephrotic syndrome, 32 (22.1%, median age=4.2 years) children experienced frequent relapses over 2 years. The time to initial response was significantly longer (10 versus 7 days, P<0.001, log-rank test) in the 32 frequent relapsers than in the 106 nonfrequent relapsers. The time from start of initial treatment to first relapse was significantly shorter (2.6 versus 6.1 months, P<0.001, log-rank test) in the 32 frequent relapsers than in the 57 infrequent relapsers. In a Cox regression model, the time to initial response ≥9 days and the duration from start of initial treatment to first relapse <6 months were significant predictors of frequent relapses (unadjusted and adjusted).
Conclusions
Initial remission time ≥9 days and first relapse within 6 months were associated with frequent relapses. These findings may also be useful also in selecting potential frequent relapsers for clinical trials.
Introduction
The standard initial treatment for children with idiopathic nephrotic syndrome (NS), proposed by the International Study of Kidney Disease in Children (ISKDC), consists of an 8-week regimen of corticosteroids (1,2). Although more than 80% of children with idiopathic NS are steroid-sensitive (SS), about 60% of these patients experience relapses. Moreover, a considerable number have frequent relapses and develop corticosteroid toxicities after repeated treatments (1,2). Although some controlled studies (3–6) and a meta-analysis (7) have shown that long-course corticosteroid regimens result in a longer sustained remission of the disease than the ISKDC regimen, the most appropriate treatment of idiopathic NS has not been determined. There have been no recent large-scale reports of the outcome of the ISKDC regimen in patients with idiopathic NS. Early identification of frequently relapsing (FR) NS is desirable. We, therefore, assessed the 2-year outcomes and risks of FRNS after initial therapy based on the ISKDC regimen in children with idiopathic NS.
Materials and Methods
Patients
The study protocol was based on the Declaration of Helsinki and approved by the regional research ethics vetting boards (Wakayama Medical University #799). We analyzed data from children with idiopathic NS who had been in the control group (prednisolone alone) of a randomized control trial (RCT) of the Japanese Study Group of Renal Disease in Children testing Sairei-to, a Chinese herbal medicine, in patients with idiopathic NS to obtain basic data for new RCTs. Detailed information on these RCTs is available on the website http://www.wan.jp/jsrdc (Japanese version only available) and http://www.umin.ac.jp/ctr (UMIN000000747 and UMIN000005103). Between January of 1993 and December of 2001, children newly diagnosed with idiopathic NS at 46 hospitals in Japan were entered into the study. The criteria for NS were in accordance with ISKDC (8) and included (1) heavy proteinuria, ≥40 mg/h per meter2, (2) hypoalbuminemia, ≤2.5 g/dl, and (3) age at diagnosis, ≥1 and <16 years. In addition, none of these children had hematuria (<20 erythrocytes/high-power field), hypertension, hypocomplementemia, or renal insufficiency, and none had received immunosuppressive therapy. Patients/parents were instructed to dip the urine daily and record results.
Treatment Regimen
Initially, all patients received 2.0 mg/kg per day prednisolone (maximum of 80 mg) in three divided doses for 4 weeks followed by 1.3 mg/kg per 2 days in a single dose for 4 weeks. Treatment for each relapse consisted of 2.0 mg/kg per day prednisolone for 4 weeks followed by tapering to 2.0 mg/kg per 2 days in a single dose for 2 weeks, 1.0 mg/kg per 2 days for 2 weeks, and 0.5 mg/kg/2 days for 2 weeks. Prednisolone doses were calculated from the standard body weight per body length.
Clinical Definitions
Remission and relapse were defined in accordance with the guidelines of the ISKDC (8). Response was defined as a reduction in the rate of urinary protein excretion to <4 mg/h per meter2 (dipstick zero to trace with early-morning urine) for 3 consecutive days. SSNS was defined as a response during the initial 8-week prednisolone regimen, and steroid-resistant NS (SRNS) was defined as a failure to respond during the initial 8-week therapy. Relapse was defined as a reappearance of proteinuria≥40 mg/h per meter2 (dipstick≥2+ with early-morning urine) for 3 consecutive days. FRNS was defined as more than or equal to two relapses of NS within 6 months of the initial episode or more than or equal to four relapses within any 12-month period. Renal insufficiency was defined as an estimated GFR calculated using the Schwartz Equation (9) of <60 ml/min per 1.73 m2.
Statistical Analyses
All statistical analyses were performed using JMP9.0 software (SAS Institute Inc., Cary, NC). The clinical characteristics of patient groups were compared using Fisher’s exact test. Continuous variables were compared using the Wilcoxon test. Extended Fisher’s exact test and the Kruskal–Wallis test with posthoc Steel–Dwass test were used to compare differences among the three subpopulations of patients with SSNS. Time-course events of patients with SSNS were analyzed using the Kaplan–Meier method and the log-rank test. A Cox regression model was used to identify factors associated with the risk of FRNS (10). Factors related to FRNS were selected based on clinical importance. A two-tailed P value<0.05 was regarded as significant.
Results
Clinical Course, Onset Age, and Sex Ratio
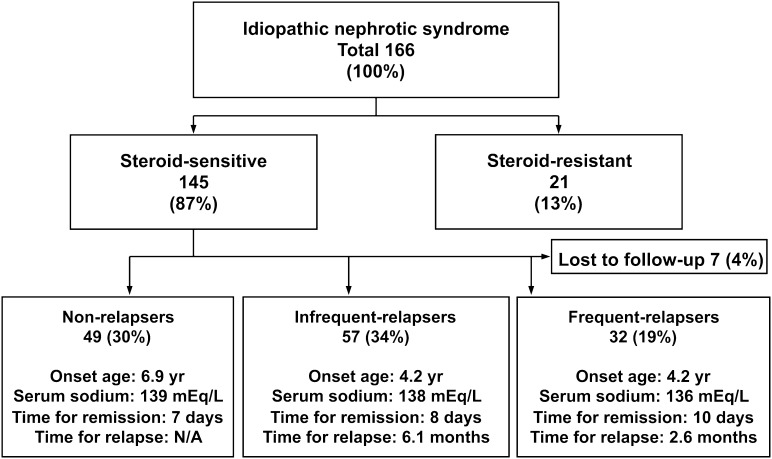
A total of 166 children with idiopathic NS, 113 boys and 53 girls (2.1:1), satisfied the inclusion criteria; their conditions 2 years after the start of initial treatment are shown in Figure 1. Of these 166 children, 145 (87%) children, 98 boys and 47 girls (2.1:1), had SSNS, and 21 (13%) children, 15 boys and 6 girls (2.5:1), had SRNS. There was an overall male preponderance in both groups but no significant difference in sex ratio (P=0.81). Similarly, there were no differences in sex ratio when the SSNS group was subdivided into children with no relapse (31 boys and 18 girls), infrequent relapse (39 boys and 18 girls), and FR (23 boys and 9 girls) (P=0.89). The clinical characteristics of these patients are summarized in Table 1. Median onset age was similar in the SSNS and SRNS groups (P=0.18), but it differed significantly among the three SSNS subgroups (P=0.04), between FRs and nonrelapsers (P=0.03), and between FRs and non- and infrequent relapsers (P=0.05). There was no significant difference in median onset age between males and females in the SSNS and SRNS groups and three SSNS subgroups.
Figure 1.
Status of 166 children with nephrotic syndrome 2 years from the start of initial treatment. Data are shown as median if applicable. N/A, not applicable; time for relapse, time from start of initial treatment to first relapse; time for remission, time from start of initial treatment to disappearance of proteinuria.
Table 1.
Clinical data and status of 166 children with nephrotic syndrome
| Characteristic | Total (n=166) | Steroid-Sensitive (n=145) | Nonfrequent Relapser (n=106) | Nonrelapser (n=49) | Infrequent Relapser (n=57) | Frequent Relapser (n=32) | Steroid-Resistant (n=21) | Pa |
|---|---|---|---|---|---|---|---|---|
| Sex (male/female) | 113/53 | 98/47 | 70/36 | 31/18 | 39/18 | 23/9 | 15/6 | 0.81; 0.67 |
| Onset age (yr) | 5.1 (3.2–9.5) | 5.5 (3.5–9.5) | 6.0 (3.7–9.5) | 6.9 (4.3–9.2) | 4.2 (3.2–9.8) | 4.2 (2.6–7.7) | 2.9 (2.0–10.7) | 0.18; 0.05 |
| Proteinuria (g/d per meter2) | 4.2 (2.7–7.2) | 4.1 (2.7–7.0) | 3.8 (2.5–6.5) | 3.4 (2.7–6.4) | 4.0 (2.5–6.9) | 5.6 (3.0–7.8) | 6.9 (2.8–15.5) | 0.13; 0.12 |
| Estimated GFR (ml/min per 1.73 m2) | 111 (95–124) | 111 (95–124) | 111 (96–125) | 115 (97–128) | 110 (95–119) | 109 (91–124) | 109 (96–132) | 0.93; 0.35 |
| Total protein (g/dl) | 4.1 (3.8–4.4) | 4.1 (3.8–4.4) | 4.1 (3.8–4.5) | 4.1 (3.8–4.6) | 4.1 (3.7–4.5) | 4.0 (3.7–4.3) | 4.1 (3.6–4.6) | 0.91; 0.10 |
| Albumin (g/dl) | 1.6 (1.4–2.0) | 1.6 (1.4–2.0) | 1.6 (1.4–2.0) | 1.7 (1.5–2.1) | 1.6 (1.4–1.9) | 1.6 (1.3–1.9) | 1.7 (1.3–2.3) | 0.63; 0.24 |
| Serum sodium (mEq/L) | 138 (135–140) | 138 (135–140) | 138 (136–140) | 139 (137–140) | 138 (135–140) | 136 (133–139) | 137 (134–140) | 0.36; 0.008 |
| Total cholesterol (mg/dl) | 430 (346–505) | 429 (353–505) | 440 (363–505) | 398 (333–486) | 452 (405–511) | 399 (327–491) | 452 (304–506) | 0.83; 0.25 |
| Time from start of initial treatment to disappearance of proteinuria (d) | N/A | 8 (6–10) | 7 (6–8) | 7 (5–8) | 8 (7–9) | 10 (8–13) | N/A | <0.001b |
| Time from start of initial treatment to first relapse (mo) | N/A | N/A | N/A | N/A | 6.1 (3.2–9.6) | 2.6 (1.5–3.5) | N/A | <0.001c |
Data value median (25%–75%) except sex. N/A, not applicable.
P values are steroid-sensitive versus steroid-resistant in left position and nonfrequent relapser versus frequent relapser in right position.
P values are nonfrequent relapser versus frequent relapser.
P values are infrequent relapser versus frequent relapse.
There was no significant difference in median of proteinuria, estimated GFR, total protein, albumin, and total cholesterol at onset among subgroups. Serum sodium was significant lower in the FR than the nonfrequent relapser group (P=0.008) (Table 1).
Detailed data of onset age are shown in Table 2. Approximately 50% of children with SSNS experienced onset from 2 to less than 6 years of age. The distributions of onset age differed significantly between the SSNS and SRNS groups (P=0.04). Children with SRNS were younger at onset, with 24% presenting with disease before 2 years of age. Two of five SRNS patients with onset age <2 years showed minor glomerular abnormality and FSGS. A renal biopsy was not done in one of five patients. There were no differences in the distributions of onset age between boys and girls in the SSNS (P=0.25) and SRNS (P=0.99) groups and in the entire cohort with NS (P=0.15). Of 145 patients in the SSNS group, 32 (22%) children, 23 boys and 9 girls (2.6:1), developed FRNS over the 2-year follow-up period, with 22 (69%) of these children being ≤5 years at onset.
Table 2.
Subdivision of patients by age at the onset of idiopathic nephrotic syndrome
| Status | Onset Age (yr) | ||||
|---|---|---|---|---|---|
| ≥1 to <2 | ≥2 to <6 | ≥6 to <10 | ≥10 to <14 | ≥14 to <16 | |
| Total | |||||
| Total (n=166) | 13 (8%) | 76 (46%) | 40 (24%) | 28 (17%) | 9 (5%) |
| Male (n=113) | 8 (7%) | 54 (48%) | 24 (21%) | 18 (16%) | 9 (8%) |
| Female (n=53) | 5 (9%) | 22 (42%) | 16 (30%) | 10 (19%) | 0 (0%) |
| Steroid-sensitive | |||||
| Total (n=145) | 8 (6%) | 69 (48%) | 37 (26%) | 24 (17%) | 7 (5%) |
| Male (n=98) | 5 (5%) | 49 (50%) | 22 (22%) | 15 (15%) | 7 (7%) |
| Female (n=47) | 3 (6%) | 20 (43%) | 15 (32%) | 9 (19%) | 0 (0%) |
| Steroid-resistant | |||||
| Total (n=21) | 5 (24%) | 7 (33%) | 3 (14%) | 4 (19%) | 2 (10%) |
| Male (n=15) | 3 (20%) | 5 (33%) | 2 (13%) | 3 (20%) | 2 (13%) |
| Female (n=6) | 2 (33%) | 2 (33%) | 1 (17%) | 1 (17%) | 0 (0%) |
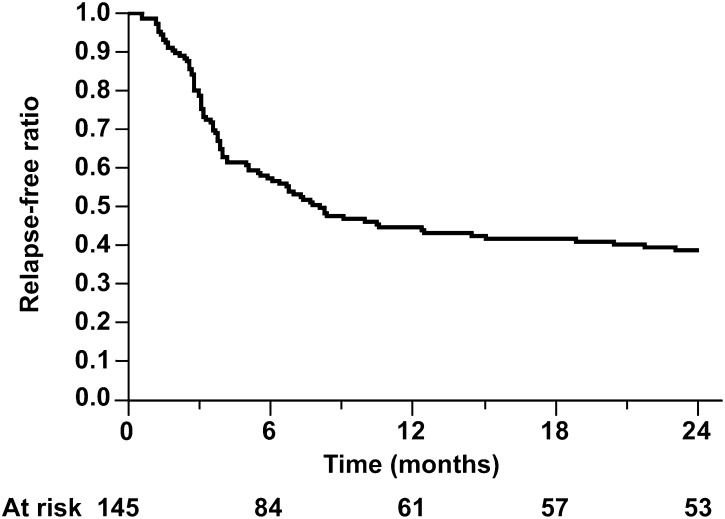
Kaplan–Meier analysis showed that 57%, 44%, 41%, and 39% of the patients in the SSNS group remained relapse-free at 6, 12, 18, and 24 months, respectively, from the start of initial treatment (Figure 2 and Table 3). All patients in the FRNS group had a first relapse within 6 months of the start of initial treatment; 24 (75%) of 32 patients with FRNS showed steroid-dependent NS during the 2-year study period.
Figure 2.
Kaplan–Meier analysis of relapse-free ratio in children with steroid-sensitive nephrotic syndrome.
Table 3.
Relapse-free rates after a standard or long regimen in patients with steroid-sensitive nephrotic syndrome
Time from the Start of Initial Treatment to Disappearance of Proteinuria in the SSNS Group
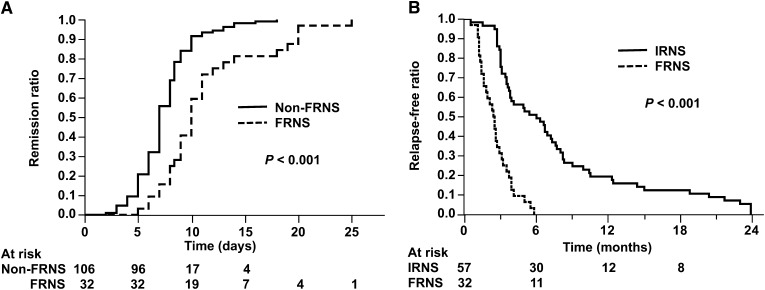
The median (25%–75%) times from the start of initial treatment to the disappearance of proteinuria in the SSNS group and its subgroups are shown in Table 1. This time was significantly longer in the FR (10 [8–13] days) than the nonrelapser (7 [5–8] days) and infrequent relapser (8 [7–9] days) subgroups (P<0.001 each). The time differed significantly between the FR and non-FR (nonrelapser and infrequent relapser) subgroups (10 [8–13] versus 7 [6–8] days, P<0.001). In contrast, the difference between the nonrelapser and infrequent relapser subgroups was not significant (P=0.07). Kaplan–Meier method and log-rank test also showed that the initial response time was significantly longer in the FR than the non-FR (Figure 3A). These findings suggest that the ease of proteinuria disappearance differed in the FRNS and non-FRNS groups. There were no significant differences between males and females in the SSNS group and its subgroups.
Figure 3.
Initial remission ratio and relapse-free ratio in nephrotic syndrome. Kaplan–Meier analysis of time for initial remission (A) and time for first relapse (B). P values are from log-rank test. FRNS, frequent-relapsing nephrotic syndrome; IRNS, infrequent-relapsing nephrotic syndrome.
Time from the Start of Initial Treatment to First Relapse in Patients with SSNS
The median (25%–75%) time from the start of initial treatment to first relapse was significantly shorter in the FR than the infrequent relapser subgroups (2.6 [1.5–3.5] versus 6.1 [3.2–9.6] months, P<0.001) (Table 1). Kaplan–Meier method and log-rank test also showed that the first relapse was significantly earlier in the FR than the infrequent relapser (Figure 3B). There was no significant difference between males and females in either group.
Factors Associated with FRNS
Table 4 presents the results of Cox regression analyses of factors associated with FRNS. A time of initial response ≥9 days and time from start of initial treatment to first relapse <6 months were significant in both unadjusted (P<0.001 in both) and adjusted (P=0.004 and <0.001, respectively) analyses. The adjusted hazard ratio (HR) for an initial response time ≥9 days compared with <9 days was 3.09 (95% confidence interval [CI]=1.42–7.27). The adjusted HR for time from start of initial treatment to first relapse <6 months was 5.09×106 (95% CI=16.56–2.06×10184). Kaplan–Meier method and log-rank test also showed that initial remission time ≥9 days and first relapse within 6 months were associated with frequent relapsing (Figure 4). These findings suggest that an initial response within 9 days and an early first relapse, especially within 6 months, may be significant risk factors for the development of FRNS.
Table 4.
Cox regression model of factors associated with frequently relapsing nephrotic syndrome at 2 years after initial therapy (n=138)
| Factor | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Male (versus female) | 1.32 | 0.63–3.02 | 0.47 | 1.41 | 0.64–3.34 | 0.40 |
| Onset age (<5 to ≥5 yr) | 2.69 | 1.31–5.94 | 0.007 | 1.50 | 0.71–3.39 | 0.29 |
| Serum sodium (<135 to ≥135 mEq/L) | 2.54 | 1.15–5.22 | 0.02 | 1.34 | 0.59–2.83 | 0.47 |
| Time from start of initial treatment to disappearance of proteinuria (≥9 to <9 d) | 6.65 | 3.17–15.19 | <0.001 | 3.09 | 1.42–7.27 | 0.004 |
| Time from start of initial treatment to first relapse (<6 to ≥6 mo) | 8.66×106 | 29.46–N/A | <0.001 | 5.09×106 | 16.56–2.06×10184 | <0.001 |
HR, hazard ratio; CI, confidence interval; N/A, not available.
Figure 4.
Frequent relapse-free ratio stratified by times for initial remission and first relapse. Frequent relapse related with time to initial response (A) and time from start of initial treatment to first relapse (B). P values are from log-rank test.
Discussion
Prolonged initial steroid treatment for more than 3 months has been reported to decrease the risks of relapse in pediatric patients with SSNS (7,11,12). However, even with new corticosteroid regimens, 80%–90% of children with SSNS have relapses, with nearly 50% relapsing frequently (13). Therefore, the initial approach to the treatment of SSNS will likely vary considerably (14).
We have analyzed 2-year outcomes in children with primary NS after initial therapy based on the ISKDC regimen. Large-scale reports describing the outcomes of the ISKDC regimen in children with idiopathic NS have been published, but none have been published recently (2,15). Therefore, our study may provide valuable data on children with idiopathic NS, although they were from over 10 years ago.
Our results suggest that the incidence of FRNS in children with idiopathic NS was not as high as previously reported. We found that the incidence of FRNS was 19% among all children with NS (32 of 166) and 22% among children with SSNS (32 of 145) 2 years after initial treatment. In comparison, a previous study reported that the incidence of FRNS 6 months after initial treatment with prednisone was 28% among children with NS (102 of 363) and 31% among children with SSNS (102 of 334) (2). The Arbeitsgemeinschaft für Pädiatrische Nephrologie study reported that 12 of 37 (32%) patients with SSNS using the ISKDC regimen were FRNS 6 months after initial treatment (4), and a Cochrane meta-analysis found that 110 of 289 (38%) patients using the ISKDC regimen were FRNS (7), a significantly higher percentage than we observed (P<0.001, Fisher's exact test). Relatively small-scale studies from Japan included in the Cochrane analysis showing that the rates of FRNS were high, 43% (13 of 30) in 2000 (16) and 52% (15 of 29) in 1988 (3), suggesting that the differences in relapse rates were not caused by race or study period.
Of our 166 patients with NS, 21 (13%) patients had SRNS. SRNS generally depends on histology. Similarly, the ISKDC reported that 22% (103 of 471) had SRNS, with 7% (25 of 363) having minimal change (17). In addition, the sex ratios and onset age distributions among our patients were similar to those values reported previously (2,15,18).
The relapse-free ratio after initial treatment in our SSNS was intermediate between the standard and long Arbeitsgemeinschaft für Pädiatrische Nephrologie regimens and better than the ratio reported by the ISKDC (Table 3). These findings provide a rationale for reconsidering the ISKDC regimen.
We found that the times from the start of initial treatment to the disappearance of proteinuria differed significantly among our three SSNS subgroups, being significantly longer in FR than non-FR (10 versus 7 days) groups. A time of ≥9 days was significant for FRNS in both unadjusted and adjusted analyses. The adjusted HR for an initial response time ≥9 days compared with <9 days was 3.09 (95% CI=1.42–7.27, P=0.004). These findings suggest that the time from the start of initial treatment to the disappearance of proteinuria may predict whether a patient will develop FRNS. Patients with an initial response time ≥9 days should, therefore, be considered for more intensive treatments, such as a long course of corticosteroids. This time cutoff may also be useful for selecting potential FRs for clinical trials. Interestingly, an initial remission time ≥9 days (odds ratio=3.00, 95% CI=1.20–7.90, P=0.02, n=123) was previously shown to be a significant predictor of steroid dependency in a logistic model (19). Another study, however, reported no correlation between time to initial response and frequency of relapse in 218 SSNS patients who showed minimal change during the 2 years after initial response (8). The reason for these discrepancies is unclear, although they may have been because of differences in the ethnic/racial characteristics of the included patients.
Both unadjusted and adjusted analyses showed that the time from start of initial treatment to first relapse was a significant predictor of FRNS. The adjusted odds ratio for the time from initiation of treatment to first relapse <6 months was 5.09×106 (95% CI=16.56–2.06×10184, P<0.001). These findings suggest that patients who relapse within 6 months after initial remission be considered for more intensive treatment. Based on this finding, an RCT has been designed to examine the efficacy and safety of mizoribine, one of immunosuppressants, to prevent FRNS (UMIN000005103).
A limitation may be the possibility of influence of difference in treatment for each relapse between the original ISKDC regimen and our regimen, although one of the ISKDC RCTs showed that there was no significant difference in the number of relapses during the follow-up period of 6 months between the original ISKDC regimen and the prolonged regimen similar to our regimen (20). Generally, renal biopsies are not indicated at onset when patients fulfill the inclusion criteria of this study. Therefore, renal biopsies were not required for the study. Another limitation is that our study did not include a validation cohort. Therefore, data in the current study should be validated in a separate cohort.
Although we did not examine biochemical parameters such as lipoprotein(a), there is a report suggesting the predictive value of it in nephrotic status (21).
In conclusion, despite this study being prospective and observational in nature rather than a controlled study, our findings suggest the validity of the ISKDC regimen in the treatment of patients with idiopathic NS. Our data also indicated that an initial remission time ≥9 days and first relapse within 6 months were significant risks for the development of FRNS. These findings may also be useful in selecting potential FRs for clinical trials.
Disclosures
None.
Acknowledgments
The authors thank the patients and Drs. Akioka Y (Tokyo), Araki Y (Hokkaido), Awazu M (Tokyo), Baba M (Kanagawa), Furuse A (Kumamoto), Fujinaga S (Saitama), Goto M (Tokyo), Hamada R (Tokyo), Hamahira K (Hyogo), Hamasaki Y (Tokyo), Harada T (Kanagawa), Hatae K (Fukuoka), Hattori M (Tokyo), Hattori S (Kumamoto), Hayashida S (Kumamoto), Higashida K (Yamanashi), Hiramatsu M (Oita), Hiramoto R (Chiba), Igarashi T (Tokyo), Ikeda M (Tokyo), Ikeda T (Kumamoto), Ishihara Y (Kanagawa), Ito S (Kanagawa), Kagami S (Tokushima), Kaku Y (Fukuoka), Kameda A (Hyogo), Kamei K (Tokyo), Kamezaki K (Fukuoka), Kamimaki I (Tochigi), Kamitsuji H (Nara), Kitagawa K (Hyogo), Kobayashi Y (Tochigi), Kodama S (Kagoshima), Konomoto T (Miyazaki), Kosaka T (Tokyo), Maeda E (Gunma), Matsuyama T (Tokyo), Minato T (Hyogo), Mishiku Y (Kanagawa), Miyamoto H (Hyogo), Morita M (Tochigi), Nakanishi N (Osaka), Niimura F (Kanagawa), Nishino M (Osaka), Nozu K (Hyogo), Ochiai R (Tokyo), Ota K (Hyogo), Otsuka Y (Saga), Owada Y (Tochigi), Sako M (Tokyo), Sato T (Saga), So H (Kagoshima), Suehiro F (Hyogo), Suzuki T (Tokyo), Tamura K (Ibaraki), Tanaka R (Hyogo), Tanaka Y (Tokyo), Tsuchida S (Akita), Ushijima T (Kumamoto), Wakaki H (Tokyo), Yamaoka K (Osaka), Yan K (Tokyo), Yoshidome K (Kagoshima), and Yoshiya K (Hyogo) of the Japanese Study Group of Renal Disease in Children for their contributions.
This study was supported in part by the Kidney Foundation, Japan.
Parts of this study were presented at the 37th Annual Meeting of the American Society of Nephrology, October 30, 2004, St. Louis, Missouri, and published in abstract form (Nakanishi et al., J Am Soc Nephrol, 15: 358A, 2004).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.van Husen M, Kemper MJ: New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 26: 881–892, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Ueda N, Chihara M, Kawaguchi S, Niinomi Y, Nonoda T, Matsumoto J, Ohnishi M, Yasaki T: Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr 112: 122–126, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Ehrich JH, Brodehl J: Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 152: 357–361, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Ksiazek J, Wyszyńska T: Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 84: 889–893, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Bagga A, Hari P, Srivastava RN: Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 13: 824–827, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 17: CD001533, 2007 [DOI] [PubMed] [Google Scholar]
- 8.International Study of Kidney Disease in Children : Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101: 514–518, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 10.Cox DR, Oakes D: Analysis of Survival Data, London, Chapman & Hall, 1984 [Google Scholar]
- 11.Hodson EM, Alexander SI: Evaluation and management of steroid-sensitive nephrotic syndrome. Curr Opin Pediatr 20: 145–150, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hodson EM, Craig JC, Willis NS: Evidence-based management of steroid-sensitive nephrotic syndrome. Pediatr Nephrol 20: 1523–1530, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hodson EM, Willis NS, Craig JC: Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev 23: CD002290, 2008 [DOI] [PubMed] [Google Scholar]
- 14.MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, Massie S, Yao L, Nagaraj S, Lin JJ, Wigfall D, Trachtman H, Hu Y, Gipson DS: Management patterns of childhood-onset nephrotic syndrome. Pediatr Nephrol 24: 2193–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 15.White RH, Glasgow EF, Mills RJ: Clinicopathological study of nephrotic syndrome in childhood. Lancet 1: 1353–1359, 1970 [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, Okuhara K, Suehiro F, Ohshima Y, Mayumi M, The West Japan Cooperative Study of Kidney Disease in Children : Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. Kidney Int 58: 1247–1252, 2000 [DOI] [PubMed] [Google Scholar]
- 17.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 [DOI] [PubMed] [Google Scholar]
- 18.International Study of Kidney Disease in Children : Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13: 159–165, 1978 [DOI] [PubMed] [Google Scholar]
- 19.Yap HK, Han EJ, Heng CK, Gong WK: Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 16: 1049–1052, 2001 [DOI] [PubMed] [Google Scholar]
- 20.International Study of Kidney Disease in Children : Nephrotic syndrome in children: A randomized trial comparing two prednisone regimens in steroid-responsive patients who relapse early. Report of the International Study of Kidney Disease in Children. J Pediatr 95: 239–243, 1979 [PubMed] [Google Scholar]
- 21.Kawasaki Y, Suzuki J, Nozawa R, Suzuki S, Suzuki H: Prediction of relapse by plasma lipoprotein(a) concentration in children with steroid-sensitive nephrotic syndrome. Nephron 92: 807–811, 2002 [DOI] [PubMed] [Google Scholar]