Summary
Background and objective
Arteriovenous thigh grafts are a potential vascular access option in hemodialysis patients who have exhausted all upper-limb sites. This study compared the outcomes of thigh grafts with outcomes obtained with dialysis catheters.
Design, setting, participants, & measurements
A prospective vascular access database was queried to identify 209 thigh grafts placed from January 1, 2003, to June 30, 2011. The following were calculated: secondary graft survival (from graft creation to permanent failure), assisted primary graft survival (from graft creation to first thrombosis), and infection-free graft survival (from graft creation to first graft infection). Graft outcomes were compared with those observed with 472 tunneled internal jugular dialysis catheters.
Results
The median duration of patient follow-up was 340 days for grafts and 91 days for catheters. The surgical technical failure rate of thigh grafts was 8.1% and was higher in patients with vascular disease (hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.07–8.04; P=0.03). Secondary and assisted primary graft survival rates at 1, 2, and 5 years were 62%, 54%, and 38% and 38%, 27%, and 17%, respectively. Infection-free graft survival rates at 1, 2, and 5 years were 79%, 73%, and 61%. Secondary survival was much worse for dialysis catheters than thigh grafts (HR, 4.44; 95% CI, 3.65–5.22; P<0.001). Likewise, infection-free survival was far worse for catheters than for thigh grafts (HR, 3.77; 95% CI, 2.80–4.82; P<0.001).
Conclusions
Thigh grafts are a viable vascular option in patients who have exhausted upper-extremity options. Outcomes with thigh grafts are superior to those obtained with dialysis catheters.
Introduction
A proportion of hemodialysis patients exhaust all options for permanent vascular access (fistula or graft) in both upper extremities. Once this occurs, continued hemodialysis requires a choice between placing an arteriovenous graft in the thigh or long-term dependence on tunneled hemodialysis catheters (1). All grafts are prone to recurrent stenosis and thrombosis, which can lead to permanent access failure (2), but there are few publications on the outcomes and complication of thigh grafts. Most published studies reported the outcomes of relatively few thigh grafts, with inconsistent definitions of graft outcomes (3–11). Three larger series (>100 thigh grafts) (12–14) provided information about graft outcomes using standardized definitions (15) but did not comprehensively analyze clinical factors or comorbid conditions that are associated with thigh graft outcomes. Finally, none of the published reports directly compared the outcomes of thigh grafts and tunneled dialysis catheters.
The goals of the present study were to (1) quantify the long-term outcomes of thigh grafts at a large medical center, (2) evaluate the association between graft outcomes and patient characteristics, and (3) compare the outcomes observed with thigh grafts with those obtained with tunneled internal jugular dialysis catheters.
Materials and Methods
Study Population and Vascular Access Management
The University of Alabama at Birmingham supervises in-center hemodialysis provided to approximately 500 patients at five units in metropolitan Birmingham. These patients receive their medical care from clinical nephrologists who are on the University of Alabama at Birmingham nephrology faculty. Six experienced transplant surgeons created all new vascular accesses and revised them as required. Interventional radiologists performed percutaneous access interventions to treat significant stenotic lesions and access thrombosis. Patients were considered for placement of a thigh graft if they had exhausted all options for a fistula or graft in both upper extremities and if they did not have significant peripheral vascular disease. Exclusion criteria for a thigh graft included a history suggestive of peripheral vascular disease (claudication or resting pain; history of lower-extremity angioplasty, peripheral bypass surgery, or amputation; or abnormal findings on physical examination, such as diminished lower-extremity pulses or foot ulcers). All patients underwent preoperative ultrasonographic mapping to assess vein diameter and exclude stenosis or thrombosis. Selected patients underwent noninvasive vascular studies at the surgeon’s discretion. Grafts were typically cannulated 2–3 weeks after their placement. Clotted grafts were treated surgically if the thrombosis occurred within the first month and percutaneously if thrombosis occurred at a later time period (16). Graft infections were treated by systemic antibiotics in conjunction with surgical excision or revision of the infected graft segment (when feasible).
As a comparison group, we identified 472 patients who initiated hemodialysis with a tunneled internal jugular vein catheter and no secondary access (fistula or graft). The rationale for these selection criteria was two-fold. First, by minimizing the likelihood of prior central vein injury, we were able to compare catheters placed in “virgin” central veins with thigh grafts created using “virgin” femoral veins. Second, by excluding patients with a concurrent maturing vascular access, we maximized the duration of catheter follow-up before a censoring event. Tunneled internal jugular venous dialysis catheters were placed by interventional radiology staff under fluoroscopic guidance. Catheters suspected of having dysfunction were treated by instillation of tissue plasminogen activator into both catheter lumens. If this maneuver did not restore patency, the catheter was exchanged over a guidewire. Patients with catheter-related bacteremia (fever or rigors, as well as positive blood cultures) were treated with systemic antibiotics in conjunction with antibiotic lock instillation, in an attempt to salvage the catheter (17). If the patient’s symptoms or bacteremia persisted, the infected catheter was removed. Two full-time vascular access coordinators scheduled all vascular access procedures; tracked access outcomes and complications; and maintained a prospective, computerized access database (18).
Data Analysis
Our local institutional review board approved this retrospective review of existing medical records of patients in our access database. We queried the access database retrospectively to identify all thigh arteriovenous grafts placed between January 1, 2003, and June 30, 2011. There was no overlap between the thigh grafts included in this study and those reported previously from our medical center (5). A total of 255 thigh grafts were placed during the 8.5-year study period, for a mean frequency of approximately 30 grafts annually. If a patient had more than one thigh graft placed, only the first graft was included in our analysis. Thus, the final analysis incorporated 209 patients receiving a first thigh graft. For comparison, we evaluated the outcomes of the first tunneled internal jugular catheter placed in hemodialysis patients during this study period (n=472).
Electronic medical records were reviewed to determine demographic and clinical information, including age, sex, race, diabetes, coronary artery disease, cerebrovascular disease, peripheral vascular disease, and congestive heart failure. The clinical outcome of each thigh graft was followed from the time of surgical placement. Surgical technical failure was defined as inability to create a functional graft in the operating room, which was usually due to severe calcification of the femoral artery. Graft patency was defined according to consensus reporting standards (15). Secondary graft survival was calculated from access creation to permanent failure, regardless of the number of interventions required to maintain long-term patency for dialysis. Assisted primary graft survival was calculated from the time of graft placement until the first episode of graft thrombosis. Finally, infection-free graft survival was calculated from the time of graft placement until the first access infection. Secondary catheter survival was calculated from the initial placement until its removal due to dysfunction or infection but was censored at the time of elective removal due to use of a new fistula or graft. Infection-free catheter survival was calculated from the time of initial placement until the first episode of catheter-related bacteremia.
Statistical Analyses
Secondary graft survival, assisted primary graft survival, and infection-free graft survival were plotted using Kaplan-Meier survival techniques, with patient follow-up censored at the time of death, kidney transplantation, transfer to a nonparticipating dialysis unit, or end of study follow-up (December 31, 2011). Thigh grafts with surgical technical failures were considered to have a survival of 0 days but were included in all the survival analyses. Univariate analysis was used to evaluate the association between graft survival and clinical characteristics. The difference in graft and catheter survival was evaluated by the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Table 1 summarizes the demographic and clinical characteristics of the two patient groups. The median duration of patient follow-up was 340 days for grafts and 91 days for catheters. The two groups were similar in age, sex, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and heart failure. A greater proportion of patients with thigh grafts were black. The median dialysis vintage before receipt of a thigh graft was nearly 3 years. Finally, as expected, most patients receiving a thigh graft had previously undergone vascular access procedures, but 7% had the thigh graft as their initial access because their upper-extremity veins were too small for creation of an access.
Table 1.
Patient characteristics
| Variable | Thigh Grafts | Catheters | P Value |
|---|---|---|---|
| Patients (n) | 209 | 472 | |
| Mean age ± SD (yr) | 52±15 | 54±15 | 0.11 |
| Male sex, n (%) | 90 (43) | 236 (50) | 0.09 |
| Black race, n (%) | 193 (92) | 357 (76) | <0.001 |
| Diabetes, n (%) | 101 (48) | 242 (51) | 0.48 |
| Coronary artery disease, n (%) | 57 (27) | 106 (22) | 0.17 |
| Peripheral vascular disease, n (%) | 29 (14) | 63 (13) | 0.85 |
| Cerebrovascular disease, n (%) | 33 (16) | 66 (14) | 0.54 |
| Any vascular disease, n (%)a | 94 (45) | 173 (37) | 0.04 |
| Congestive heart failure, n (%) | 42 (20) | 94 (20) | 0.96 |
| Median HD vintage (IQR) (yr) | 2.8 (1.1–6.5) | 0 | |
| No. of previous fistulas or grafts, n (%) | NA | ||
| 0 | 14 (7) | ||
| 1 | 47 (22) | ||
| 2 | 66 (31) | ||
| 3 | 47 (22) | ||
| ≥4 | 27 (13) | ||
| Unknown | 8 (4) |
HD, hemodialysis; IQR, interquartile range; NA, not applicable.
Coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
Surgical technical failure (inability to create a functional graft in the operating room) occurred in 17 patients (8.1%). A technical failure was more likely in patients with any vascular disease than in those without vascular disease (12.7% versus 4.3%; hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.07–8.04; P=0.03). Technical failure was not associated with patient age, sex, race, diabetes, or congestive heart failure. Two surgeons placed six or more thigh grafts annually, and four surgeons placed fewer than six thigh grafts annually. The frequency of technical failure was not significantly different between the high- and low-volume surgeons (10.6% versus 5.9%; P=0.31).
None of the patients developed lower-extremity ischemia after graft creation. A graft infection occurred in 45 patients (21%). Most graft infections were early, with 31 (69% of all graft infections) occurring in the first 6 months after their creation. Thus, the cumulative risk of thigh graft infection at 6 months was 15%.
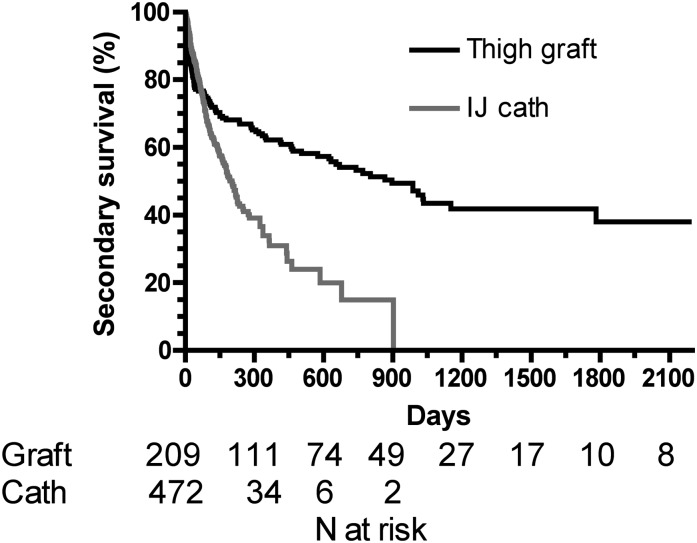
The secondary thigh graft survival rates were 62% at 1 year, 54% at 2 years, and 38% at 5 years (Figure 1). The median secondary graft survival duration was 2.4 years. Secondary survival was much worse for dialysis catheters than thigh grafts (HR, 4.44; 95% CI, 3.65–5.22; P<0.001). One-year secondary survival rate was 31% for catheters versus 62% for thigh grafts. Secondary graft survival was not significantly associated with patient age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, any vascular disease, or congestive heart failure (Table 2).
Figure 1.
Secondary survival of thigh grafts versus tunneled internal jugular (IJ) venous dialysis catheters (calculated from the initial placement to permanent failure). P<0.001.
Table 2.
Association of secondary thigh graft survival and clinical variables
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.00 (0.99–1.02) | 0.63 |
| Sex | 1.50 (0.97–2.25) | 0.07 |
| Race | 0.77 (0.37–1.58) | 0.47 |
| Diabetes | 0.96 (0.64–1.43) | 0.83 |
| Coronary artery disease | 1.36 (0.88–2.08) | 0.16 |
| Peripheral vascular disease | 1.28 (0.75–2.20) | 0.36 |
| Cerebrovascular disease | 0.98 (0.57–1.68) | 0.94 |
| Any vascular diseasea | 1.39 (0.93–2.07) | 0.11 |
| Congestive heart failure | 1.06 (0.65–1.72) | 0.81 |
CI, confidence interval.
Coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
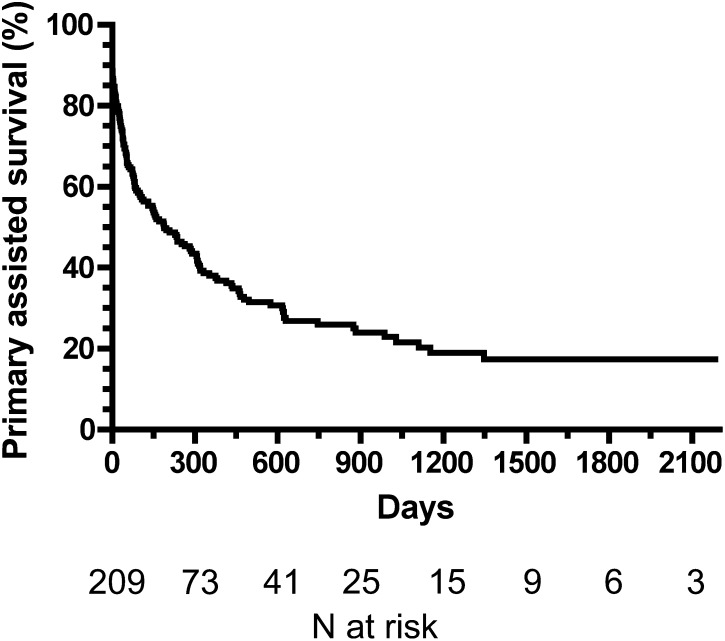
The assisted primary thigh graft survival (time to first thrombosis) rates were 38% at 1 year, 27% at 2 years, and 17% at 5 years (Figure 2). The median secondary graft survival duration was 0.5 year. Assisted primary graft survival was not significantly associated with patient age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, any vascular disease, or congestive heart failure (Table 3).
Figure 2.
Assisted primary survival of thigh grafts (calculated from the initial placement to the first thrombosis or failure due to any cause). The median survival duration was 0.5 year.
Table 3.
Association of assisted primary thigh graft survival and clinical variables
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 0.99 (0.98–1.00) | 0.28 |
| Sex | 1.14 (0.76–1.70) | 0.53 |
| Race | 0.55 (0.28–1.10) | 0.07 |
| Diabetes | 0.90 (0.60–1.34) | 0.60 |
| Coronary artery disease | 0.85 (0.54–1.35) | 0.49 |
| Peripheral vascular disease | 1.00 (0.56–1.80) | 0.99 |
| Cerebrovascular disease | 0.79 (0.46–1.37) | 0.40 |
| Any vascular diseasea | 0.82 (0.55–1.23) | 0.35 |
| Congestive heart failure | 0.77 (0.45–1.31) | 0.33 |
CI, confidence interval.
Coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
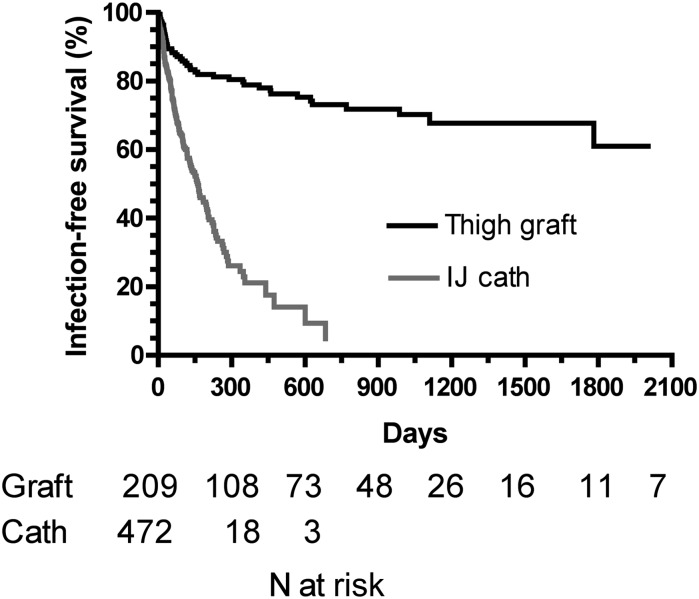
The infection-free thigh graft survival rates were 79% at 1 year, 73% at 2 years, and 61% at 5 years (Figure 3). Infection-free survival was far worse for catheters than thigh grafts (HR, 3.77; 95% CI, 2.80–4.82; P<0.001). The 1-year infection-free survival rate was 21% for catheters versus 79% for thigh grafts. Infection-free graft survival was not significantly associated with patient age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, any vascular disease, or congestive heart failure (Table 4).
Figure 3.
Infection-free survival of thigh grafts versus tunneled internal jugular (IJ) venous dialysis catheters (calculated from the initial placement to the first infection). P<0.001.
Table 4.
Association of infection-free thigh graft survival and clinical variables
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.00 (0.98–1.02) | 0.74 |
| Sex | 1.50 (0.82–2.77) | 0.19 |
| Race | 1.32 (0.32–5.45) | 0.70 |
| Diabetes | 0.67 (0.37–1.23) | 0.19 |
| Coronary artery disease | 1.31 (0.70–2.46) | 0.40 |
| Peripheral vascular disease | 1.20 (0.53–2.68) | 0.66 |
| Cerebrovascular disease | 0.61 (0.24–1.54) | 0.30 |
| Any vascular diseasea | 1.15 (0.64–2.07) | 0.64 |
| Congestive heart failure | 1.45 (0.75–2.81) | 0.27 |
CI, confidence interval.
Coronary artery disease, peripheral vascular disease, or cerebrovascular disease.
Discussion
In a large series of thigh grafts placed at a single large center, we documented acceptable long-term graft patency. The observed secondary graft patency rates of 62%, 54%, and 38% at 1 year, 2 years, and 5 years, respectively, after graft creation is gratifying considering the high comorbidity of the study population. These findings closely agree with those of three large series (>100 thigh grafts) that reported secondary graft survival rates of 68%–78% at 1 year, 54%–64% at 2 years, and 46%–51% at 5 years (Table 5). In fact, two prior observational studies observed secondary survival of thigh grafts to be similar or superior to that obtained with upper-extremity grafts (5,13). Moreover, as our study demonstrated, thigh graft survival is far superior to that obtained with tunneled dialysis catheters.
Table 5.
Comparison of thigh graft outcomes in large series
| Study, Year (Reference) | Grafts (n) | Secondary Graft Survival (%) | Graft Infectiona (%) | ||
|---|---|---|---|---|---|
| 1 yr | 2 yr | 5 yr | |||
| Cull et al, 2004 (12) | 125 | 68 | 54 | NA | 41 |
| Geenen et al., 2010 (14) | 153 | 75 | 64 | 51 | 27 |
| Ram et al., 2010 (13) | 103 | 78 | 63 | 46 | 17 |
| Current study | 209 | 62 | 54 | 38 | 21 |
These series reported outcomes on >100 thigh grafts. NA, not available.
Cumulative infection for life of graft.
Despite the high frequency of vascular disease, including peripheral vascular disease (14%), in our study population, it is notable that none of the patients developed lower-extremity ischemia subsequent to the graft. This gratifying result suggests that the surgical screening process was successful in identifying suitable candidates for placement of a thigh graft. In agreement with our findings, one large series observed distal ischemia after only 1.3% of thigh graft placements (14). In contrast, a second study reported lower-extremity ischemia at 1–65 months after thigh graft placement in 10% of cases, although the long delay after onset of ischemia in some of patients argues against a causal relationship (12).
The technical failure rate was relatively high at 8.1%. This rate is similar to the 3%–8.1% rates reported by two other studies (11,13). We have previously noted a higher technical failure rate in thigh grafts than in upper-extremity grafts (5), whereas another group reported a lower rate in thigh grafts (13). These differences may relate to differences in patient selection. The technical failures occurred in patients with heavy calcification of the femoral artery, which precluded successful anastomosis of the artery to the graft material. We have previously noted a relatively high frequency of technical failure in patients whose pelvic computed tomography revealed severe arterial calcification, and this may be a useful preoperative screening tool to reduce this complication (19).
We observed a relatively high frequency of thigh graft infection, in agreement with other large series (Table 5). Most graft infections occurred within 6 months of the surgery, with a much smaller number occurring in the subsequent years. This timing of graft infections agrees with a previous report from our center (20). Most infected grafts required surgical excision, although a few were salvaged by removal of the infected graft segment and placement of a new jump graft. Some studies have reported a higher likelihood of infection in thigh grafts than upper-extremity grafts (5,20), whereas another showed similar rates (13). However, the cumulative risk of dialysis catheter–related bacteremia at 1 year was 79%, which was considerably higher than the 21% rate of thigh graft infection.
Few publications have examined the association of thigh graft outcomes with clinical patient characteristics. We found no significant association of secondary survival, primary assisted survival, or infection-free graft survival with patient age, sex, race, diabetes, vascular disease, or heart failure. In agreement with our findings, another large series (125 grafts) found no significant association of secondary graft patency with patient age, sex, race, or diabetes, but that study did not look at vascular disease or heart failure (12). Likewise, another large series (153 grafts) observed no significant association between thigh graft infection and patient age, sex, diabetes, or coronary artery disease (14). In contrast, in a smaller series of 37 thigh grafts, the secondary graft survival was significantly shorter in diabetic than nondiabetic patients (median survival, 7 versus 21 months) (11).
The major strengths of this study include the large number of patients studied, the prospective collection of information on graft survival and complications, and the inclusion of all patients in the survival analyses. Our study is limited by being a single-center clinical trial, and the results may not be generalizable to all dialysis centers (although they are similar to those reported in other large series). For example, most of our study patients were black, and the results may not be generalizable to dialysis patients of other races. However, our thigh graft outcomes were similar to those reported from an Australian study, where is it presumed that very few patients were black (14). Finally, interpretation of outcomes in the catheter control group may have been affected by the high censoring event rate (approximately 56% of patients), which was primarily driven by elective catheter removal after successful use of a permanent access. We attempted to minimize early censoring events by excluding patients who initiated dialysis with a catheter and a maturing access. An alternative approach would have been to identify a control group consisting of patients in whom the catheter was a planned “permanent” access (due to lack of options for a fistula or graft). The disadvantages of such an alternative approach include the small number of patients meeting this definition, as well as the likelihood that vascular injury from prior central vein catheters would adversely affect the outcomes of subsequent catheters. If anything, the results obtained with subsequent catheters would probably be inferior to those observed with “first ever” dialysis catheters (which represent the best-case scenario).
In summary, thigh grafts are a viable choice of vascular access in hemodialysis patients who have exhausted all options for a fistula or graft in both upper extremities. With proper patient selection, distal ischemia is rare. Infection remains a relatively common complication of thigh grafts but occurs much less frequently than with the alternative vascular access, a tunneled dialysis catheter.
Disclosures
None.
Acknowledgment
Portions of this manuscript were presented at the American Society of Nephrology meeting in San Diego, California, October 30–November 4, 2012.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Lifting the Veil: Insights into Vascular Access Options,” on pages 708–710.
References
- 1.Vascular Access 2006 Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bhandari S, Wilkinson A, Sellars L: Saphenous vein forearm grafts and gortex thigh grafts as alternative forms of vascular access. Clin Nephrol 44: 325–328, 1995 [PubMed] [Google Scholar]
- 4.Khadra MH, Dwyer AJ, Thompson JF: Advantages of polytetrafluoroethylene arteriovenous loops in the thigh for hemodialysis access. Am J Surg 173: 280–283, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Miller CD, Robbin ML, Barker J, Allon M: Comparison of arteriovenous grafts in the thigh and upper extremities in hemodialysis patients. J Am Soc Nephrol 14: 2942–2947, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Slater ND, Raftery AT: An evaluation of expanded polytetrafluoroethylene (PTFE) loop grafts in the thigh as vascular access for haemodialysis in patients with access problems. Ann R Coll Surg Engl 70: 243–245, 1988 [PMC free article] [PubMed] [Google Scholar]
- 7.Zibari GB, Rohr MS, Landreneau MD, Bridges RM, DeVault GA, Petty FH, Costley KJ, Brown ST, McDonald JC: @HEADA-R:Complications from permanent hemodialysis vascular access. Surgery 104: 681–686, 1988 [PubMed] [Google Scholar]
- 8.Lazarides MK, Iatrou CE, Karanikas ID, Kaperonis NM, Petras DI, Zirogiannis PN, Dayantas JN: Factors affecting the lifespan of autologous and synthetic arteriovenous access routes for haemodialysis. Eur J Surg 162: 297–301, 1996 [PubMed] [Google Scholar]
- 9.Tashjian DB, Lipkowitz GS, Madden RL, Kaufman JL, Rhee SW, Berman J, Norris M, McCall J: Safety and efficacy of femoral-based hemodialysis access grafts. J Vasc Surg 35: 691–693, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Scott JD, Cull DL, Kalbaugh CA, Carsten CG, Blackhurst D, Taylor SM, Snyder BA, York JW, Langan EM: The mid-thigh loop arteriovenous graft: Patient selection, technique, and results. Am Surg 72: 825–828, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Korzets A, Ori Y, Baytner S, Zevin D, Chagnac A, Weinstein T, Herman M, Agmon M, Gafter U: The femoral artery-femoral vein polytetrafluoroethylene graft: A 14-year retrospective study. Nephrol Dial Transplant 13: 1215–1220, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cull JD, Cull DL, Taylor SM, Carsten CG, 3rd, Snyder BA, Youkey JR, Langan EM, 3rd, Blackhurst DW: Prosthetic thigh arteriovenous access: Outcome with SVS/AAVS reporting standards. J Vasc Surg 39: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ram SJ, Sachdeva BA, Caldito GC, Zibari GB, Abreo KD: Thigh grafts contribute significantly to patients’ time on dialysis. Clin J Am Soc Nephrol 5: 1229–1234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geenen IL, Nyilas L, Stephen MS, Makeham V, White GH, Verran DJ: Prosthetic lower extremity hemodialysis access grafts have satisfactory patency despite a high incidence of infection. J Vasc Surg 52: 1546–1550, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Sidawy AN, Gray RJ, Besarab A, Henry M, Ascher E, Silva M, Jr, Miller A, Scher L, Trerotola S, Gregory RT, Rutherford RB, Kent KC: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Mudunuri V, O’Neal JC, Allon M: Thrombectomy of arteriovenous dialysis grafts with early failure: Is it worthwhile? Semin Dial 23: 634–637, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Poole CV, Carlton D, Bimbo L, Allon M: Treatment of catheter-related bacteraemia with an antibiotic lock protocol: Effect of bacterial pathogen. Nephrol Dial Transplant 19: 1237–1244, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Lockhart ME, Robbin ML, McNamara MM, Allon M: Association of pelvic arterial calcification with arteriovenous thigh graft failure in haemodialysis patients. Nephrol Dial Transplant 19: 2564–2569, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Harish A, Allon M: Arteriovenous graft infection: A comparison of thigh and upper extremity grafts. Clin J Am Soc Nephrol 6: 1739–1743, 2011 [DOI] [PubMed] [Google Scholar]