Abstract
The use of microsatellite markers provides a rapid approach for autozygosity mapping in Hermansky-Pudlak syndrome: identification of the second HPS7 mutation in a patient presenting late in life
Hermansky-Pudlak syndrome (HPS) is an autosomal recessive disorder characterised by oculocutaneous hypopigmentation and a bleeding diathesis caused by a lack of dense granules in platelets. HPS is genetically heterogeneous with variable skin, hair, and iris hypopigmentation, and visual impairment. In addition, some forms of HPS give rise to granulomatous colitis and pulmonary fibrosis (1). HPS has been associated with mutations in 9 human genes: HPS1 (mouse model pale ear), AP3B1/HPS2 (pearl), HPS3 (cocoa), HPS4 (light ear), HPS5 (ruby-eye 2), HPS6 (ruby-eye), HPS7/dysbindin (sandy), HPS8/reduced pigmentation and HPS9/pallid (2,3). In addition, genes have been identified for a further 6 mouse models of HPS (mocha, gunmetal, ashen, muted, buff, and subtle gray) (2). In general, HPS is a rare disorder, but HPS1 has a high prevalence in northwest Puerto Rico (1/1800) due to a founder mutation (4).
The large numbers of potential HPS culprit genes (>118 coding exons), together with minimal guidance offered by the genotype-phenotype correlations between HPS genes, provides a significant challenge for the molecular diagnosis of these disorders. In cases with suspected consanguinity, autozygosity mapping can be used as a high speed tool to prioritize mutation screening for a specific HPS gene (5). Further, this can be facilitated using microsatellite markers flanking the HPS genes to exclude genes in the case of heterozygous markers/haplotypes and prioritize direct sequencing of specific genes where autozygosity is noted. We illustrate the proof of principle of this approach in the identification of the second case of a mutation in the HPS7 (encoding Dysbindin) gene in a patient with a bleeding history and hypopigmentation. A 77 year old Caucasian female was referred to the bleeding disorder clinic with a lifelong bleeding tendency (Figure 1A). There was no history of a bleeding disorder in this participant’s half siblings, parents or children. Her parents were first cousins. She had pale skin and hair and had reduced visual acuity and nystagmus throughout life. She also had spontaneous epistaxes as a teenager which lasted for several hours and required medical attention, and several episodes of prolonged bleeding from minor injuries that required surgical haemostasis. She bled for several days after dental extractions in her twenties and thirties and required packing of the tooth sockets to control the bleeding. She had menorrhagia from menarche which eventually necessitated a hysterectomy at the age of 37. She experienced heavy post partum bleeding after all of her three vaginal deliveries. Surgical procedures in this patient, including surgery for an ectopic pregnancy in the 1960s, abdominal hysterectomy and salpingo-oophrectomy in 1971 and excision of a lipoma from her back in 2002, were followed by prolonged bleeding requiring blood product transfusion. She had two episodes of severe per-rectal bleeding. The first one occurred in 1979 and was attributed to a rectal polyp which was surgically removed. She bled significantly after this procedure and required a platelet transfusion. A further episode occurred in 2009 which was attributed to Crohn’s disease. During this episode the patient required red cell and platelet transfusions and tranexamic acid and was being considered for a right hemicolectomy at the time of her haematology referral. Colonic biopsies showed florid granulomatous inflammation with no co-existing infection (Figure 1B). Acid fast bacilli were absent. She was reviewed by a respiratory physician in 2010 and had no evidence of any respiratory disease, with normal lung function tests (FEV1 100% predicted, FVC 95% predicted, TLC 80% predicted and KCO 105% predicted, all within normal ranges). A chest X ray showed no active lung disease, and a CT thorax with contrast showed no convincing features of fibrosis.
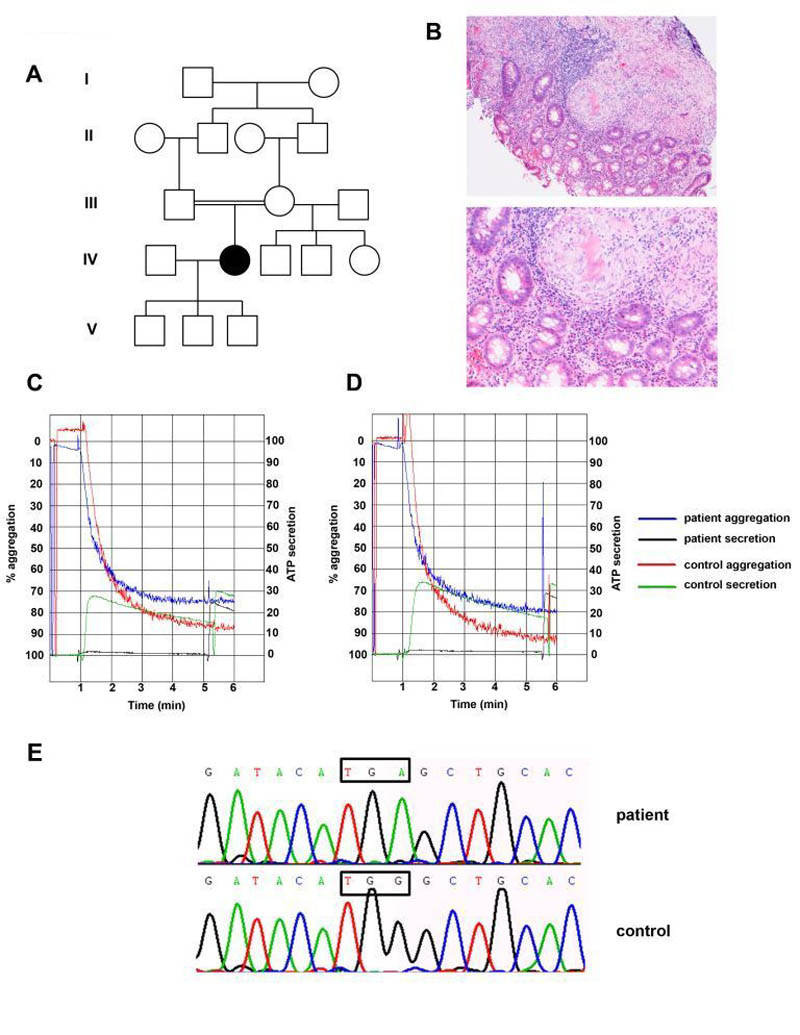
Figure 1. Identification of the second HPS7 mutation in a patient with Hermansky-Pudlak syndrome presenting late in life.
(Panel A) Pedigree of a consanguineous family with Hermansky-Pudlak syndrome. The affected individual is represented by a solid symbol.
(Panel B) Images of a colonic biopsy from the patient in low power (upper panel) and high power (lower panel). Both images show inflammatory infiltrates and granulomata with numerous giant cells, alongside normal bowel mucinous glands. No caseous necrosis is seen, and special stains showed no evidence of micro-organisms (including mycobacteria).
(Panels C and D) Absence of secretion (black trace) to high doses of PAR-1 peptide 100μM (Panel C) and PAR-4 peptide 500μM (Panel D) in this patient. Left sided Y axis depicts percentage aggregation, and right sided Y axis represents platelet ATP secretion assessed using Chronolume®. 1.6nmol of ATP standard were added to each cuvette in order to calculate absolute secretion and secretion normalised to platelet count in PRP for both patient and control.
(Panel E) Identification of a homozygous single base substitution (c.177 G>A) in Dysbindin leading to a premature stop codon (p.Trp59Stop). Sanger sequencing showing wild-type and mutant DTNBP1 sequence traces. The position of the mutation is indicated by the boxed regions.
The patient gave informed consent and was recruited to the GAPP (Genotyping and Phenotyping of Platelets, NIHR ID 9858, Regional Ethics Committee reference 06/MRE07/36) study. Platelet function testing was performed on platelet rich plasma (PRP) by lumiaggregometry using Chronolume® to assess secretion. The response to intermediate concentrations of PAR-1 peptide (30 μM) and collagen (1 μg/ml) was reduced relative to the control on the day and also a panel of over 70 healthy volunteers. Normal aggregation was observed at higher concentrations of these agonists (not shown). A lack of dense granule secretion with the entire panel of agonists tested (6) was noted, as illustrated for PAR-1 and PAR-4 peptides in Figure 1C and D. The lack of platelet ATP secretion was consistent with an absence of platelet dense granules, and in combination with the patient’s clinical features were diagnostic of HPS. Such platelet function testing has previously been shown to be successful in diagnosing other HPS patients with complete lack of secretion from platelet dense granules (5, 6).
DNA was extracted from peripheral blood and genetic studies were undertaken. As the patient’s parents were related by blood, we were able to apply autozygosity linkage mapping by genotyping several microsatellite markers flanking all of the known human HPS genes (Supplementary Table 1). This was carried out to prioritize a limited number of HPS genes for direct sequencing. Strikingly the only HPS locus that displayed autozygosity for both flanking markers and over an extended region of genetic distance was the HPS7 locus. Therefore the most likely candidate HPS gene was HPS7/Dysbindin on chromosome 6p22.3. The 10 coding exons of DTNBP1 (encoding dysbindin) including exon-intron boundaries were PCR-amplified and sequenced. Sequencing of DTNBP1 revealed a homozygous nonsense mutation in exon 4 (c.177 G>A; p.Trp59Stop) confirming a diagnosis of HPS type 7 (Fig 1E).
Dysbindin is important for normal platelet-dense granule and melanosome biogenesis and is mutated in the sandy (sdy) mouse (7). Dysbindin is a component of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) which regulates trafficking to lysosome-related organelles. The BLOC-1 complex is ubiquitously expressed and along with Dysbindin contains seven predicted coiled-coil-forming proteins (pallidin, muted, cappuccino, snapin, BLOC1S1, BLOC1S2 and BLOC1S3) (8), all of which are associated with Hermansky-Pudlak syndrome in mice.
The only previously reported HPS7 mutation in humans was a homozygous nonsense mutation (p.Q103X) found in a 48 year old Portuguese woman with oculocutaneous hypopigmentation, ease of bruising and a bleeding tendency. She had mild shortness of breath on exertion and reduced lung compliance but otherwise normal pulmonary function (8). Although the patient described here has been diagnosed with Crohn’s disease, it is likely that this represents granulomatous colitis given the histological findings reported on colonic biopsy. She has no evidence of pulmonary fibrosis. Colitis has been reported in HPS1, HPS3 and HPS4 and pulmonary fibrosis in HPS1, HPS4 and HPS2 (9, 10, 11).
In conclusion, we report a novel mutation in the HPS7 (Dysbindin) gene causing a premature stop codon which was rapidly identified following autozygosity mapping using microsatellite markers. This high speed technique provides a rapid approach to identify candidates HPS genes for Sanger sequencing in order to identify a disease causing mutation. An alternative method to identify the genetic defect in this patient would have been to utilize second generation sequencing strategies such as whole exome sequencing or custom built arrays, however this would have been far more costly and time consuming given the consanguinity in this family which we exploited to narrow down the culprit gene. Despite having had numerous hospital visits and lifelong excessive bleeding, the cause of this patient’s bleeding was not elucidated until she was in her eighth decade. Mild inherited platelet disorders should therefore be considered in patients presenting with excessive bleeding later in life.
Supplementary Material
Supplementary table 1. Summary of genotypes obtained in the affected patient using microsatellite markers flanking all the 9 known HPS loci reported in humans. The patient only showed extended and flanking autozygosity for the HPS7 locus at chromosome 6p22.3.
Acknowledgements
We would like to thank the patient for her permission to allow us to publish her case history.
This work was funded by the Wellcome Trust (093994), the British Heart Foundation (RG/09/007/27917) and Instituto de Salud Carlos III (ISG, FI10/00535 and JRP, PI10/02594).
Footnotes
Disclosures and competing interests The authors have no relevant conflicts of interest to disclose.
Contributions – G Lowe recruited the patient to the study, performed experimental work, interpreted data and drafted and revised the manuscript. N Morgan performed experimental work, interpreted data and drafted and revised the manuscript. I Sánchez Guiu, M Lordkipanidzé and N Dovlatova performed the experimental work, interpreted data and revised the manuscript. O Chapman and J Wilde referred the patient to the study team and revised the manuscript. J Rivera revised the manuscript. S Watson is chief investigator of the GAPP study, interpreted data and revised the manuscript.
References
- 1.Davies BH, Tuddenham EG. Familial pulmonary fibrosis associated with oculocutaneous albinism and platelet function defect. A new syndrome. Q J Med. 1976;45:219–232. [PubMed] [Google Scholar]
- 2.Dell’Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 4.Witkop CJ, Almadovar C, Pineiro B, et al. Hermansky-Pudlak syndrome (HPS). An epidemiologic study. Ophthalmic Paediatr Genet. 1990;11:245–250. doi: 10.3109/13816819009020986. [DOI] [PubMed] [Google Scholar]
- 5.Morgan NV, Pasha S, Johnson CA, et al. A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8) Am J Hum Genet. 2006;78:160–166. doi: 10.1086/499338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawood BB, Lowe GC, Lordkipanidzé M, et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood. 2012;120:5041–5049. doi: 10.1182/blood-2012-07-444281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swank RT, Sweet HO, Davisson MT, et al. Sandy: a new mouse model for platelet storage pool deficiency. Genet Res. 1991;58:51–62. doi: 10.1017/s0016672300029608. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Zhang Q, Oiso N, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermos CR, Huizing M, Kaiser-Kupfer MI, et al. Hermansky-Pudlak syndrome type 1: gene organization, novel mutations, and clinical-molecular review of non-Puerto Rican cases. Hum Mutat. 2002;20:482. doi: 10.1002/humu.9097. [DOI] [PubMed] [Google Scholar]
- 10.Anderson PD, Huizing M, Claassen DA, et al. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum Genet. 2003;113:10–17. doi: 10.1007/s00439-003-0933-5. [DOI] [PubMed] [Google Scholar]
- 11.Gochuico BR, Huizing M, Golas GA, et al. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Summary of genotypes obtained in the affected patient using microsatellite markers flanking all the 9 known HPS loci reported in humans. The patient only showed extended and flanking autozygosity for the HPS7 locus at chromosome 6p22.3.