Abstract
Immune cells are important in pathogenesis of acute pancreatitis (AP) and determine disease severity. Results from cytokine-based clinical trials for AP have been disappointing, so strategies that target and alter the behavior of infiltrating immune cells require consideration. Recurrent AP can progress to chronic pancreatitis (CP). CP is a well-described risk factor for pancreatic ductal adenocarcinoma (PDA). However, most patients with CP do not develop PDA, and most patients with PDA do not have history of pancreatitis. Interestingly, CP and PDA tissues have similarities in their desmoplasia and inflammatory infiltrates, indicating overlapping inflammatory responses. Further studies are needed to determine the differences and similarities of these responses, improve our understanding of PDA pathogenesis, and develop specific immune-based therapies. Immune cells in PDA produce immunosuppressive signals that allow tumors to evade the immune response. Unlike single therapeutic agent studies that block immunosuppressive mechanisms, studies of combination therapies that include therapeutic vaccines have provided promising results.
Keywords: acute pancreatitis, chronic pancreatitis, pancreatic ductal adenocarcinoma
Introduction
Premature activation of digestive enzymes in pancreatic acinar cells initiates autodigestion of the pancreas 1. However, acute pancreatitis (AP) is also an inflammatory disorder; a complex cascade of immunologic events affects disease pathogenesis and progression. Although intra-acinar or interstitial activation of trypsinogen is an early event in the development of AP 2, in recent years, many researchers have focused on the roles of leukocytes 3. Macrophages, granulocytes, and T cells have important roles in the pathogenesis of AP. We review the contribution of the different leukocyte populations to pathogenesis and their role in therapy.
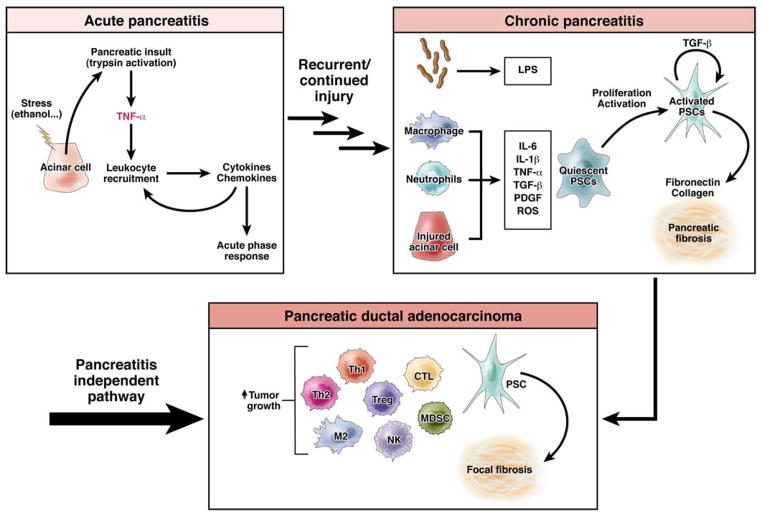
Alcohol and gallstones are the most common etiologic factors in AP. Recurrent AP can lead to chronic pancreatitis (CP), a progressive inflammatory and fibrotic disease (Figure 1). CP eventually leads to irreversible damage and in late stages, pancreatic exocrine and endocrine deficiencies 4. Histologic features of CP include pancreatic fibrosis, acinar cell atrophy, chronic inflammation, and distorted and blocked ducts 5. Population-based studies reported that 20%–45% patients have a recurrence of AP, with the highest rates been among those with alcohol-related AP 6–9. Progression to CP following recurring AP has been reported in 4%–24% of patients—again more commonly among those with alcoholic recurrent AP 7–9. In a recent US population-based study, Yadav et al. found that readmission for a primary diagnosis of AP was 22%, whereas subsequent admission for CP was only 6% 10. Consistent with previous studies, the risk for recurrent AP and progression to CP were greatest among users of alcohol and tobacco. Interestingly, in a long-term prospective study (1976–1992) of patients who had recurring AP and continued to consume alcohol, disease progressed to CP in 78% 11. A 30-year Danish follow-up study found that AP (alcohol-related and idiopathic) progressed to CP with a mean interval of 3.5 years 12.
Figure 1. Immune cell involvement in pancreatic ductal adenocarcinoma, acute and chronic pancreatitis.
Recurrent and continued pancreatic injury can lead to chronic pancreatitis (CP). CP can progress to pancreatic ductal adenocarcinoma (PDA), although most PDA likely arises independent of pancreatitis. Cytotoxic T lymphocyte (CTL), polysaccharide (LPS), myeloid derived suppressor cells (MDSC), natural killer cells (NK), pancreatic stellate cells (PSCs), platelet derived growth factor (PDGF), reactive oxygen species (ROS), regulatory T cells (Tregs), T helper cells (Th).
Chronic inflammatory conditions of the pancreas are risk factors for the development of pancreatic ductal adenocarcinoma (PDA) 13–17. Patients with CP have a higher incidence of PDA18, and individuals with hereditary pancreatitis have a 40% cumulative risk of developing PDA in their lifetime 19. The earliest genetic changes within chronically inflamed pancreatic tissue lead to production of cytokines that initiate a chain of events that results in tumor formation, growth, metastasis, and suppression of anti-tumor immune responses 13–17. Studies have shown that immune responses change as PDAs progress from low-grade pancreatic intraepithelial neoplasias (PanINs) to high-grade PanINs and invasive adenocarcinomas20 (Figure 2). However, most patients with PDA have no history of AP or CP. Since most patients who present with AP have mild and self-limited disease, it is not clear whether patients that develop PDA have experienced bouts of subclinical or asymptomatic pancreatitis. Alternatively, sporadic PanIN lesions could induce a fibro-inflammatory response, similar to that found in patients with CP, that promotes development of PDA. Herein, we review the roles of adaptive and innate immune cells in pancreatitis and pathogenesis of PDA, and their potential as therapeutic targets.
Figure 2. Immune cell infiltrates during pancreatic tumor progression.
Infiltration by immune cells with immunosuppressive activities allows for an environment that fosters tumor growth and progression. Sequential acquisition of mutations in proto-oncogene K-RAS and the tumor suppressors INK4A, TP53, DPC4, BRCA2, and over-expression of cyclin D1 and mesothelin lead to pancreatic tumor progression. Effector T cells (Teff), myeloid derived suppressor cells (MDSC), natural killer cells (NK), pancreatic ductal adenocarcinoma (PDA), pancreatic intraepithelial neoplasia (PanIN), regulatory T cells (Tregs), T helper cells (Th).
Adaptive and Innate Immune Cells and Their Roles in AP
In AP, the initial inflammatory process leads to migration of monocytes and neutrophils into the pancreas, mediated by a multi-step process that involves adhesion molecules 21. Hyperstimulation with caerulein leads to upregulation of intracellular adhesion molecule 1 (ICAM-1) in the pancreas, which mediates adhesion of neutrophils to the site of inflammation 22. Neutrophils have been proposed to have important roles in the early phase of the disease development, contributing to activation of trypsinogen and progression to severe AP 23, 24. In support of this, ICAM-1 deficiency and systemic depletion of neutrophils were each shown to reduce the severity of AP and lung injury 25.
Monocytes and macrophages are key inflammatory cells involved in the pathogenesis of AP; the degree of macrophage activation might be an important determinant of the severity of AP. These infiltrating cells produce cytokines and inflammatory mediators such as tumor necrosis factor (TNF)α, interleukin (IL)1β, IL6, monocyte chemotactic protein (MCP)1, and platelet activating factor (PAF) 26. Some of these mediators are initially released by pancreatic acinar cells, leading to the recruitment of neutrophils and monocytes (Figure 1). These leukocytes, in turn, produce cytokines and chemokines that recruit more leukocytes and increase the inflammatory response in the pancreas, followed by involvement of distant organs such as the lung 27. CC chemokines such as MCP-1, macrophage inflammatory protein (MIP)-1α, and RANTES (CCL5) are believed to activate primarily monocytes. Bhatia et al. showed that administration of bindarit, a blocker of MCP-1 synthesis, significantly reduced the severity of AP, indicating that MCP-1 is an inflammatory mediator during early stages of AP 28. Depletion of macrophages via injection of clodronate liposomes, protected against caerulein mediated pancreatitis in mice 29. Monocyte and macrophage populations, however, are heterogeneous, with great phenotypic and functional plasticity. Recently, CD11bhiGr-1lowCD11c− monocytes (also Ly-6ChiCCR2+), which originate in the bone marrow, were shown to be involved in progression of AP in mice 29.
Although macrophages are a major leukocyte population found in the inflamed pancreas, T cells are also present, in smaller numbers, at sites of inflammation. Based on studies of T-cell depletion and lymphopenic animals, activated T cells appear to be necessary for progression of AP 30. As AP progresses, there are changes in the number and ratio of CD4+ and CD8+ T cells. This could be because CD4+ T cells contribute to activation of macrophage, via antigen presentation and release of inflammatory cytokines 31. In contrast to total depletion of CD4+ T cells, and consistent with functional heterogeneity of CD4+ T cells, recent data indicate that subset of CD4+ IL22+ T cells likely protect against AP in mice 32.
Extra-Pancreatic Immune Responses
Although most episodes of AP are mild, some patients develop severe disease, with local and extra-pancreatic complications33. The incidence of pulmonary complications is high among patients with severe pancreatitis, ranging from 15% to 55% 34. The severity of pulmonary complications ranges from mild hypoxemia, without clinical or radiological abnormalities, to severe acute respiratory distress syndrome. Renal dysfunction is another severe complication; it results from inadequate fluid resuscitation and/or septic complications. In contrast to lung and renal injury, hepatic injury is usually mild, but can still contribute to the systemic inflammatory response 35.
Pancreatic necrosis is the most severe local complication, and is associated with high risk of infectious complications. Infection and bacterial colonization of the necrotic pancreas are the most feared complications, and significantly increase mortality. Bacterial colonization and its associated inflammation likely result from failure of intestinal barrier function and translocation of intestinal microflora or their products into the splanchnic vascular bed 36. Pathogen-associated molecular patterns (PAMPs) derived from the intestinal microflora activate the host innate immune system via pattern recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding domain and leucine-rich repeat containing molecules (NLRs) 37, 38. Activation of TLRs and NLRs likely mediates the mechanism by which bacterial translocation leads to severe AP. Consistent with this, mice that lack TLR4 develop less severe forms of AP 36, and polymorphisms in TLR genes have been associated with susceptibility to AP 39, 40.
Li et al. 41 associated upregulation of TLR4 with increased expression of TNFα in peripheral blood mononuclear cells during early stages of AP, supporting an important role for TLR-4 in the pathogenesis of AP. In mice with caerulein-induced AP, pancreatic inflammation required the presence of intestinal flora, which acted via intracellular PAMP nucleotide-oligomerization domain 1 (NOD1) 42. Furthermore, they found that NOD1 facilitated migration of CCR2+ monocytes to the pancreas in response to MCP-1. Therefore, NOD1 signaling promotes pathogenesis of caerulein-induced AP via its ability to respond to the intestinal commensal bacteria.
Interestingly, although blocking TLR4 through an MyD88-independent pathway protects against AP, MyD88 inhibition exacerbates pancreatic inflammation and neoplastic transformation, by increasing dendritic cell ability to promote development of T-helper (Th)2 cells 43. Dendritic cells could therefore have dual roles, via TLR4 signaling checkpoints, that protect against inflammation but also produce inflammatory responses that lead to neoplastic progression. Thus, dendritic cells might be important therapeutic targets.
Immune Cell-Based Therapy for AP
Despite the important roles of cytokines in development of AP, and the abilities of IL-10, PAF antagonists, and anti-TNFα agents to reduce pancreatic inflammation in experimental models 44, 45, there have been discouraging and/or few results on the effects of cytokine manipulation from clinical studies. There have been conflicting results from randomized double-blind studies of the ability of IL10 to prevent endoscopic retrograde cholangiopancreatography-induced AP. One study found no difference between IL10 and control, and the study population of another, which reported effects of IL10, had an unusually high incidence of AP 46, 47. Similarly, the PAF receptor antagonist (lexipafant) did not reduce local complications, multi-organ failure, or mortality in a trial of patients with severe AP 48.
The high risk for infectious complications has precluded clinical studies of anti-TNF agents in patients with severe AP, although in a case report, infliximab reduced symptoms of AP in a patient with inflammatory bowel disease 49. Cytokine-based therapies might be difficult to develop for patients with AP, because cytokine levels vary among patients and the disease has a wide spectrum of presentation. However, strategies to target and alter the activities of immune cells that contribute to AP could have long-term therapeutic effects for a disease that has a broad range of presentation.
The roles of macrophages in the pathogenesis and progression of experimental AP make these cells interesting therapeutic targets. The heterogeneity and plasticity of macrophages, which sequentially exhibit pro- and anti- inflammatory properties, indicate that they not only mediate inflammatory injury but might also be induced to modify the sequence of events that occur during development of AP. Initial experimental strategies focused on inhibiting or depleting macrophages. Macrophages inhibitors (compounds such as gadolinium chloride, liposome-encapsulated dichloromethylene diphosphonate, and PAF antagonists) were shown to modulate the systemic inflammatory response 50. However, most studies administered the inhibitors before AP was induced, which is clinically less relevant, because most patients present following pancreatic injury.
Other approaches to modify the activities of macrophages have been tested. IL4 and IL13 have been tested for their abilities to convert pancreatitis-activated M1 peritoneal macrophages into reparative M2 macrophages. However, despite their ability to change M1 pro-inflammatory macrophages into M2 reparative cells in vitro, these cytokines were degraded and their activities were lost in vivo, before effects on the macrophages could be observed on experimental AP 51.
Transfer of hemin-activated macrophages protects mice from AP injury, 52 and administration of hemin protects against early and late stages of experimental AP 53. Heme-activated macrophages express high level of hemeoxygenase-1, which promotes production of anti-inflammatory agents such as biliverdin and carbon monxide, which induce IL10, IL22, and p38 MAPK 32, 54. Strategies to change macrophages, either in vivo or ex vivo, into cells with anti-inflammatory properties are therefore viable approaches to therapy. In a study involving a xenogenic system, human bone marrow-derived clonal mesenchymal stem cells (hcMSCs) were administered to rats with mild or severe AP 55. The hcMSCs induced Foxp3+ T regulatory (Treg) cells and suppressed pancreatic infiltration by T cells. Although more studies are needed in this area of research, stem cell-based immunosuppressive strategies could be developed as allogenic therapies for AP.
Roles for Adaptive and Innate Immune Cells in CP
Our understanding of fibrogenesis in the pancreas of patients with CP improved with the finding that pancreatic stellate cells (PSCs) regulate synthesis and degradation of the extracellular matrix proteins that comprise fibrous tissue 56. Under homeostatic conditions, PSCs are quiescent. However, PSCs are activated by toxic factors such as ethanol and its metabolites or by inflammatory cytokines and chemokines (Figure 1), which are up-regulated in pancreatic tissues of patients with CP. Such factors induce PSCs to proliferate and transform into myofibroblast-like cells 57. Moreover, activated macrophages were shown to stimulate collagen and fibronectin synthesis by cultured PSCs 58. The significance of infiltrating myeloid cells, and particularly macrophages, was further demonstrated by the requirement of myeloid (rather than acinar cell) nuclear factor (NF)-κ B p65 subunit to promote fibrosis in experimental CP 59.
Human pancreas samples were found to have significant increases in CD4+ and CD8+ T-cell infiltrates and perforin mRNA-expressing cells in CP lesions, compared with healthy pancreatic tissue, indicating the involvement of cell-mediated cytotoxicity60. Although there were no differences in total leukocyte or T-cell populations, samples from patients with CP had increased numbers of CD4+ and CD8+ central memory T-cell subsets (CCR7+), compared to controls 61. This increase in circulating memory T cells was apparent even in samples from patients with CP who had undergone pancreatic resection 0.5 to 3 years earlier, indicating ongoing, abnormal T-cell responses in patients with CP.
A more recent study investigated pancreas-specific T cell responses to antigens from lysates of human CP lesions, obtained during surgical resection 62. Although T cells from healthy individuals, patients with pancreatic cancer, and patients with CP responded similarly to tetanus toxoid, T cells from CP patients had higher levels of IL10-based (mediated via CD4+CD25highFoxP3+CD127−) responses to pancreatitis-associated antigens. In contrast, T cells from patients with pancreatic cancer responded to pancreatic carcinoma-associated, but not pancreatitis-associated, antigens and produced high levels of interferon-γ. Unlike patients with CP or pancreatic cancer, T cells from healthy individuals did not respond to either antigen, supporting the association between CP and changes in tissue- and disease-specific memory and regulatory T-cell responses. However, there are still no immune-based therapies for CP, probably because the disease is generally considered to be fibrotic and irreversible. This idea is likely to change based on above findings and the roles of PSCs and their interactions with immune and other pancreatic cells.
Acute and Chronic Pancreatitis Animal Models
Several animal models of AP have been created, using methods that range from simple and noninvasive to relatively complex and more invasive63. Although these do not necessarily recapitulate all aspects of human AP, they have increased our understanding of the pathophysiologic mechanisms that contribute to inflammation, leukocyte recruitment, pancreatic acinar cell injury, and necrosis. The relative difficulty of accessing and analyzing human pancreatic tissue makes animal models necessary. Due to variations in responses among the different species and models, use of more than one model should be considered, especially for testing potential therapeutic agents. Experimental approaches that reduce established disease or prevent progression of disease as compared to those that test disease prevention are more likely to lead to effective therapies.
Similar to AP, several animal models of CP have been described and are based on repeat or prolonged pancreatic injury (eg, caerulein injections and duct ligation) 64. Genetic models have also been generated to allow for studies of specific gene products in pathogenesis, such as PRSS1, PRSS2, SPINK1, and CFTR. Transgenic mice that express Elastase-IL-1β at an early age develop CP that resembles the human disease 65. Interestingly, as these mice age, they develop acinar–ductal metaplasia, supporting the role of immune signaling in the development of CP and progression to metaplasia.
Innate and Adaptive Immune Cells in PDA Development
Genetically engineered mouse models of pancreatic cancer have facilitated evaluation of inflammatory factors within the developing tumor microenvironment 66. This is a relatively new area of study, but a number of different immune cell types, innate and the adaptive, have been found to contribute to the progressive inflammatory changes that lead to PDA (Table 1). The activities of these cells, and their roles in immune regulation during tumor development, have not been completely elucidated. However, tumor cells appear to alter the activities of immune cells to promote tumor growth and progression; the presence of these immune cells in PDAs has been associated with poor prognosis. Interestingly, a few of the best-characterized cell types promote tumor development, however these cells have the ability to alter their polarization and inhibit tumor development.
Table 1.
Summary of immune cells in the tumor microenvironment of pancreatic adenocarcinoma
| Immune cell types | Infiltration and distribution | Associated cytokines | Functions | PDA-associated prognostic factors | ||||
|---|---|---|---|---|---|---|---|---|
| Pancreatitis | PanINs | PDA | ||||||
| Neutrophil | high | present | rare | IL-8, C5a, MMP | Proinflammatory, Procancer | Undifferentiated types of PDA | ||
| Mast Cell | evenly distributed | evenly distributed | present at infiltrating edges of tumor | GM-CSF, INFγ, TNFα, IL-4, IL-13 | Proinflammatory, Procancer | Poor survival | ||
| TAM | M1 | high | present | present | INFγ, IL-12, IL-23, TNFα | Proinflammatory, Procancer | ||
| M2 | low | low | high | IL-4, IL-10, IL-13, TGFβ | Immunosuppression | Poor survival | ||
| MDSC | present | low | high | GM-CSF, VEGF, TGFβ | Immunosuppression, Decreased CD8+ T cell infiltration | Poor survival | ||
| T cell | Treg | present | low | high | TGFβ, IL-6 | Immunosuppression, Decreased CD8+ T cell infiltration | Poor survival | |
| T helper | Th1 | present | low | low | IL-2, INFγ | Immune activation | Good survival | |
| Th2 | present | low | high | IL-4, IL-5, IL-6 IL-13 | Immunosuppression | Poor survival | ||
| Teff | present | low | low | INFγ, TNFα, IL-2 | Anti-tumor immunity | Good survival | ||
PanINs, pancreatic intraepithelial neoplasia; PDA, pancreatic ductal adenocarcinoma ; TAM, Tumor associated macrophages;
Treg, regulatory T cells.
Tumor-Associated Macrophages
PanINs and PDAs have significant increases in macrophage infiltration, compared to normal pancreatic tissue. Tumor-associated macrophages develop from peripheral blood monocytes in response to tumor-induced cytokines such as macrophage colony-stimulating factor (m-CSF), chemokines, VEGF, etc 67–69. These macrophages are functionally divided into M1 and M2 subtypes 69. The M1 subtype is characterized by production of high levels of IL12 and IL23, toxic intermediates, and inflammatory cytokines such as TNFα. The M2 subtype is characterized by production of transforming growth factor (TGF)β and IL10 and promotes adaptive Th2-mediated immunity 69. The M2 subtype has immunosuppressive activities and has been associated with a poor prognosis70. Although the M1 subtype is inflammatory, production of TNFα by these tumor-associated macrophages promotes tumor growth and epithelial to mesenchymal transition 71.
Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are a group of myeloid-derived cells that lack markers of mature myeloid cells72, 73. They have immunosuppressive activities and can inhibit T-cell functions. Increased numbers of MDSCs with suppressive activities have been found in PanINs and PDAs, compared with normal pancreatic tissue74. Tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) was recently shown to promote development of MDSCs and their recruitment to the tumor microenvironment of PDAs 75, 76.
Neutrophils
Neutrophils are often the first effector cells recruited to sites of acute inflammation. Although neutrophil infiltrates are rarely found in PDAs, they have been associated with undifferentiated types of PDAs and poor prognosis77. Neutrophils have been functionally characterized into N1 (anti-cancer) and N2 (oncogenic) subtypes78. The N2 subtype might be associated with early stages of PanIN progression (Keenan and Jaffee, unpublished data).
Mast cells
Mast cells are another type of cell in the innate immune response; they respond to tumor development by producing inflammatory cytokines79. However, these cytokines appear to promote PDA growth and development80–82.
Th and Treg Cells
Numbers of CD4+CD25highFoxp3+ Treg cells are significantly increased in mice with late-stage PanINs and PDA74. Their presence is associated with reduced numbers of CD4+ and CD8+ T cells74. Treg cells have been identified in human PDA tissues, in association with tumor stroma, in significantly higher quantities than in non-neoplastic, inflammed pancreatic stroma. Higher levels of Treg cells have been associated with more poorly differentiated tumors, with high-grade PanINs, and with invasive PDA, compared to low-grade PanINs, and are also associated with poor prognosis74, 83. In some cases, Treg cells are accompanied by effector CD4+ T cells with Th2 cell functions. The higher ratio of Th2 to Th1 cells in tumor infiltrating lymphocytes has been associated with shorter times of disease-free and overall survival84–86.
CD8+ T Cells and Natural Killer Cells
Immune effector cells such as CD8+ cytotoxic T and natural killer cells are usually decreased in PanINs and PDA74. In contrast to other inflammatory cells, increasing numbers of tumor antigen-specific CD8+ T and natural killer cells are associated with better prognosis87, 88. However, even if immune effector cells are present in PDAs, they are not functional or are poorly cytotoxic.
Stromal Fibroblasts
PDA-associated stromal fibroblasts, which are PSCs, are not considered to be an immune component of developing PanINs and PDA. However, PSCs are activated and desmoplastic stroma forms during development of CP. The role for PSCs in the pathogenesis PDA is supported by evidence showing that PSCs increase proliferation of PDA cells, as well as tumor cell migration and metastasis89, 90. Findings from Kraman et al. indicated that stromal cells support tumor growth through an immune mechanism. These investigators created transgenic mice with fibroblast activation protein (FAP)α, a protein expressed by human stromal fibroblasts, ablation to study the role of stromal cells in promoting tumor growth 91. Depletion of FAP-expressing stromal cells from transplanted lung tumors and subcutaneous, Kras-induced pancreatic tumors in mice caused immune-mediated, hypoxia-induced necrosis of tumor and stroma cells. It is therefore possible that the desmoplastic stroma formed during development of CP could help create an immunosuppressive microenvironment that causes immune failure and tumor evasion.
The Immunopathogenesis of PDA
Many of the immune components of the PDA microenvironment are inflammatory cells or factors. However, these do not support anti-tumor immunity. Instead, these inflammatory components (which include macrophages, neutrophils, and mast cells) promote tumor growth and invasion. These cell types have been observed in low-grade premalignant lesions, and their numbers increase when the low-grade premalignant lesions progress into high-grade lesions and invasive PDAs. Many of these cell types are also involved in the establishment of CP 13–17. It is common to see evidence for CP in resected PDA specimens. PDAs can develop in patients who have had CP for long periods of time, although pancreatic carcinoma is more frequently accompanied by subclinical, pathology findings of CP. One hypothesis for the development of a carcinogenic inflammatory response in patients with CP is that immune cells are initially recruited and activated in response to an acute stimulus, such as during AP, and are followed by a different group of immune cells, which attempt to clean up damage from the acute insult (Figure 1). In this model, the acute stimulus could be delivered by AP, but is on most occasions subclinical, because AP rarely precedes the development of PDAs. As the acute insult becomes chronic, genetic changes occur in the pancreatic tissue. Alternatively, the tumor initiation process (occurring via genetic alterations in early-stage PanINs and/or phenotypic changes in pancreatic ductal, acinar, and/or progenitor cells) induces an inflammatory response that mimics CP. Initially the inflammatory changes attempt to eliminate these early-stage, genetically altered pancreatic cells. However, with time, the anti-cancer response is insufficient to eliminate the genetically altered cells and alter their function to produce immunosuppressive signals. The inflammatory cytokines or toxic intermediates that are produced during this transition to a carcinogenic state can promote tumor growth. Once the tumor is established, the tumor microenvironment has a highly immunosuppressive composition (increased tumor-associated macrophages with an M2 phenotype, increased neutrophils with an N2 phenotype, Th2 cells, and Treg cells) that contributes further to immune evasion (Figure 2). There is evidence that the accumulation of genetic alterations during progression of CP to invasive PDA affects the inflammatory response, by providing additional factors such as IL-8 (via mutated Kras) and TGFβ (via SMAD4) that promote recruitment of carcinogenic inflammatory cells13–17.
Mouse Models to Study Inflammatory Responses and PDAs
Mouse models of PDA have accelerated our understanding of the inflammatory responses associated with their development and progression. The genetically engineered mouse model that most closely resembles the development of human PDAs was established via knock-in of a conditional KrasG12D allele under the control of the Pdx1 or Ptf1a/p48 promoters92. These mice spontaneously develop PanINs that progress to invasive PDAs, much like the human disease. Human PanINs associated with activating mutations in Kras are thought to progress to PDA following a series of alterations in the p16/CDKN2A and TP53, and in the TGFβ signaling pathway associated with the DPC4/SMAD4, TGFβRI, and TGFβRII. Transgenic expression of one or more of these proteins with KrasG12D speeds the development of PDA, and tumors form with distinct pathologic features66. These mouse models have been used to study the changes in the immune response during progression from PanINs to PDA74.
Mice that express KrasG12V under control of the elastase promoter in acinar cells are useful for studying the role of pancreatitis in PDA pathogenesis. The ductal-like cells observed in the PanINs that form in these mice do not arise through transformation of normal ductal cells93, 94. Instead, PanINs originate by abnormal differentiation of elastase-expressing acinar cells or their progenitor cells. These mice develop PanINs and PDAs with high penetrance following induction of AP or CP with caerulein. Studies with this mouse model have indicated that pancreatitis might promote initiation of PanIN lesions and PDAs by reprogramming adult acinar cells. The tissue damage that results from pancreatitis might induce a tumorigenic inflammatory response mediated by macrophages and T cells. This mouse model has therefore become the leading model for the study of the relationship between pancreatitis and PDA.
Immune-Based Therapeutic Strategies
For many years, researchers believed that human PDAs were poorly immunogenic. However, our current understanding of the role of inflammation in development of PanINs and progression to PDA contradicts this concept. PDAs evade the immune response by sending immunosuppressive signals to the microenvironment, and inflammation promotes formation of early, premalignant lesions and their progression. The inflammatory response in PanINs resembles that observed in patients with CP (Table 2). Nonetheless, they are not the same—they likely involve different pathways, and many patients with pancreatitis do not develop PDAs. Based on the gross similarities between cells that infiltrate CP and PDA tissues 95, regardless of the differences in etiologic or pathogenic mechanisms involved, the inflammatory outcomes (fibrosis and immune cell recruitment) could be similar. Studying the similarities and differences between CP and PDA, particularly using immune cell sub-type and functional analyses, could lead to immune-based anti-tumor therapies.
Table 2.
Comparison of pathological features between chronic pancreatitis and PanINs
| Chronic pancreatitis | PanINs |
|---|---|
|
| |
| Loss of acini | Cytological atypia and hyperchromatic nuclei with loss of polarity |
| Distorted and blocked ducts, terminal ductular proliferation | Adjacent parenchymal atrophy |
| Pancreatic fibrosis | Fibrotic foci |
| Chronic inflammation with mononuclear cell infiltrate | Inflammatory cell infiltration (mononuclear) |
Inflammation can also induce immunosuppressive signals that allow tumors to evade the immune response. So the good news is that the immune system does gain access to the pancreas during transformation and stages of pre-malignancy. However, in order for immunotherapies to have effects on tumors, their immunosuppressive mechanisms must be defined and antagonized. In addition to inhibiting the carcinogenic inflammatory signals, it is important to induce a strong anti-tumor inflammatory response for an optimal therapeutic effect. Otherwise, a weak inflammatory response, like that which occurs during the early stages of PanIN development, might not be sufficient to eradicate the tumor cells. Therefore, an ideal immunotherapeutic strategy would be to combine agents that induce strong anti-tumor immunity with those that inhibit immunosuppression.
Vaccine-based immunotherapy can induce tumor antigen-specific immune responses. Several vaccine-based approaches have been developed, including an allogeneic, GM-CSF–secreting tumor cell vaccine (GVAX) 96, 97 and a Muc1-pulsed dendritic cell vaccine98. Each vaccine has been shown in phase I and II studies to prolong survival of patients with resected PDA compared with patients who did not receive the vaccines 96–98. However, therapeutic agents that block immunosuppressive mechanisms were found to be ineffective as single agents in patients with metastatic pancreatic cancer 99, 100. Nonetheless, when ipilimumab, an antibody that inhibits the immune checkpoint CTLA-4, was given in combination with GVAX to patients with metastatic PDA, clinical and immune responses were observed that were associated with increased overall survival, compared to patients treated with only ipilimumab (Le et al. 2012 GI ASCO abstract - J Clin Oncol 30, 2012 (suppl 4; abstr 211)). These findings support the development of combination therapies, comprising vaccines and immune checkpoint inhibitors, for patients with PDA.
We are now realizing the complexity of the inflammatory changes associated with the development and progression of PDA. This complexity, however, provides numerous avenues for exploring targets for immunotherapy. All components of the inflammatory response are present in PDAs. However, there is an imbalance between these components and their respective functions, resulting in an immunosuppressive tumor microenvironment. The same inflammatory components that promote tumorigenesis might therefore be targeted to reestablish an anti-tumor microenvironment. This concept is supported by a recent study showing that an antibody agonist of CD40, given to patients with metastatic PDA in combination with gemictibine, activated infiltrating macrophages to kill tumor cells and also increased the cytotoxic effects of gemcitabine chemotherapy, possibly by depleting tumor stroma101.
Tumor-derived GM-CSF promotes development of PDAs by recruiting MDSCs, 75, 76 whereas secretion of a high intra-dermal concentration of GM-CSF, secreted by the tumor cell vaccine, facilitates anti-PDA immune response by activating dendritic cells96, 97. Therefore, targeting macrophages, neutrophils, and mast cells is a promising strategy along with traditional immunotherapy that activates T cells. Furthermore, the stromal fibroblasts provide a protective environment that not only supports and promotes PDA tumor growth and progression, but also likely suppresses the development and/or access of anti-tumor immune responses. Thus, strategies to deplete the desmoplastic stroma before immune therapy is instituted could promote robust response against tumor cells.
Acknowledgments
Funding: This work was supported in part by National Institutes of Health Grant R01 DK092421 (A.H.), K23 CA148964-01 (L.Z.), Digestive Disease Center grants DK56339 (Stanford University), The Robert Wood Johnson Foundation (A.H.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), The National Pancreas Foundation (L.Z.), Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924-14 (E.M.J. and L.Z.), Lustgarten Foundation (E.M.J. and L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (E.M.J. and L.Z), the Sol Goldman Pancreatic Cancer Center (L.Z.). Dr. Jaffee is the first recipient of the Dana and Albert “Cubby” Broccoli Endowed Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah AU, Sarwar A, Orabi AI, Gautam S, Grant WM, Park AJ, Liu J, Mistry PK, Jain D, Husain SZ. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. American journal of physiology Gastrointestinal and liver physiology. 2009;297:G967–73. doi: 10.1152/ajpgi.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. The American journal of physiology. 1999;276:G835–42. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 3.Mayerle J. A novel role for leucocytes in determining the severity of acute pancreatitis. Gut. 2009;58:1440–1. doi: 10.1136/gut.2009.186692. [DOI] [PubMed] [Google Scholar]
- 4.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184–97. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 5.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Pelli H, Sand J, Laippala P, Nordback I. Long-term follow-up after the first episode of acute alcoholic pancreatitis: time course and risk factors for recurrence. Scandinavian journal of gastroenterology. 2000;35:552–5. doi: 10.1080/003655200750023840. [DOI] [PubMed] [Google Scholar]
- 7.Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB, Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. The American journal of gastroenterology. 2009;104:2797–805. doi: 10.1038/ajg.2009.405. quiz 2806. [DOI] [PubMed] [Google Scholar]
- 8.Nojgaard C, Becker U, Matzen P, Andersen JR, Holst C, Bendtsen F. Progression from acute to chronic pancreatitis: prognostic factors, mortality, and natural course. Pancreas. 2011;40:1195–200. doi: 10.1097/MPA.0b013e318221f569. [DOI] [PubMed] [Google Scholar]
- 9.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:S15–7. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. The American journal of gastroenterology. 2012;107:1096–103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 11.Ammann RW, Muellhaupt B, Meyenberger C, Heitz PU. Alcoholic nonprogressive chronic pancreatitis: prospective long-term study of a large cohort with alcoholic acute pancreatitis (1976–1992) Pancreas. 1994;9:365–73. [PubMed] [Google Scholar]
- 12.Nojgaard C. Prognosis of acute and chronic pancreatitis - a 30-year follow-up of a Danish cohort. Danish medical bulletin. 2010;57:B4228. [PubMed] [Google Scholar]
- 13.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411–8. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. J Investig Med. 2012;60:643–63. doi: 10.231/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA. Stromal biology and therapy in pancreatic cancer. Gut. 2010;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 17.Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15:72–7. doi: 10.1038/sj.cr.7290269. [DOI] [PubMed] [Google Scholar]
- 18.McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22:65–73. doi: 10.1016/j.bpg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Vitone LJ, Greenhalf W, Howes NR, Neoptolemos JP. Hereditary pancreatitis and secondary screening for early pancreatic cancer. Rocz Akad Med Bialymst. 2005;50:73–84. [PubMed] [Google Scholar]
- 20.Goggins M, Kern SE, Offerhaus JA, Hruban RH. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol. 1999;10 (Suppl 4):4–8. [PubMed] [Google Scholar]
- 21.Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? International journal of pancreatology : official journal of the International Association of Pancreatology. 1988;3:105–12. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- 22.Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. American journal of physiology Gastrointestinal and liver physiology. 2000;279:G666–76. doi: 10.1152/ajpgi.2000.279.4.G666. [DOI] [PubMed] [Google Scholar]
- 23.Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–84. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 24.Abdulla A, Awla D, Thorlacius H, Regner S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. Journal of leukocyte biology. 2011;90:975–82. doi: 10.1189/jlb.0411195. [DOI] [PubMed] [Google Scholar]
- 25.Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- 26.Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. Journal of hepato-biliary-pancreatic surgery. 2002;9:401–10. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 27.McKay C, Imrie CW, Baxter JN. Mononuclear phagocyte activation and acute pancreatitis. Scandinavian journal of gastroenterology Supplement. 1996;219:32–6. doi: 10.3109/00365529609104997. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G1259–65. doi: 10.1152/ajpgi.00435.2004. [DOI] [PubMed] [Google Scholar]
- 29.Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, Takeda K, Hozawa S, Yoshimura A, Hibi T. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010–1020 e9. doi: 10.1053/j.gastro.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 30.Demols A, Le Moine O, Desalle F, Quertinmont E, Van Laethem JL, Deviere J. CD4(+ )T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118:582–90. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 31.Pezzilli R, Billi P, Gullo L, Beltrandi E, Maldini M, Mancini R, Incorvaia L, Miglioli M. Behavior of serum soluble interleukin-2 receptor, soluble CD8 and soluble CD4 in the early phases of acute pancreatitis. Digestion. 1994;55:268–73. doi: 10.1159/000201159. [DOI] [PubMed] [Google Scholar]
- 32.Xue J, Nguyen DT, Habtezion A. Aryl Hydrocarbon Receptor Regulates Pancreatic IL-22 Production and Protects Mice from Acute Pancreatitis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–52. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 34.Polyzogopoulou E, Bikas C, Danikas D, Koutras A, Kalfarentzos F, Gogos CA. Baseline hypoxemia as a prognostic marker for pulmonary complications and outcome in patients with acute pancreatitis. Digestive diseases and sciences. 2004;49:150–4. doi: 10.1023/b:ddas.0000011617.00308.e3. [DOI] [PubMed] [Google Scholar]
- 35.Closa D, Bardaji M, Hotter G, Prats N, Gelpi E, Fernandez-Cruz L, Rosello-Catafau J. Hepatic involvement in pancreatitis-induced lung damage. The American journal of physiology. 1996;270:G6–13. doi: 10.1152/ajpgi.1996.270.1.G6. [DOI] [PubMed] [Google Scholar]
- 36.Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58:813–9. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annual review of pathology. 2009;4:365–98. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 38.Akira S, Takeda K. Toll-like receptor signalling. Nature reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 39.Gao HK, Zhou ZG, Li Y, Chen YQ. Toll-like receptor 4 Asp299Gly polymorphism is associated with an increased risk of pancreatic necrotic infection in acute pancreatitis: a study in the Chinese population. Pancreas. 2007;34:295–8. doi: 10.1097/mpa.0b013e318032674a. [DOI] [PubMed] [Google Scholar]
- 40.Takagi Y, Masamune A, Kume K, Satoh A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene is associated with susceptibility to acute pancreatitis in Japan. Human immunology. 2009;70:200–4. doi: 10.1016/j.humimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Li HG, Zhou ZG, Li Y, Zheng XL, Lei S, Zhu L, Wang Y. Alterations of Toll-like receptor 4 expression on peripheral blood monocytes during the early stage of human acute pancreatitis. Digestive diseases and sciences. 2007;52:1973–8. doi: 10.1007/s10620-006-9211-4. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji Y, Watanabe T, Kudo M, Arai H, Strober W, Chiba T. Sensing of Commensal Organisms by the Intracellular Sensor NOD1 Mediates Experimental Pancreatitis. Immunity. 2012;37:326–38. doi: 10.1016/j.immuni.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, Zambirinis CP, Fallon NC, Rehman A, Pylayeva-Gupta Y, Badar S, Hajdu CH, Frey AB, Bar-Sagi D, Miller G. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. The Journal of experimental medicine. 2012;209:1671–87. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–7. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 45.Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. TNF-alpha as a therapeutic target in acute pancreatitis--lessons from experimental models. TheScientificWorldJournal. 2007;7:431–48. doi: 10.1100/tsw.2007.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumot JA, Conwell DL, Zuccaro G, Jr, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. The American journal of gastroenterology. 2001;96:2098–102. doi: 10.1111/j.1572-0241.2001.04092.x. [DOI] [PubMed] [Google Scholar]
- 47.Deviere J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498–505. doi: 10.1053/gast.2001.21172. [DOI] [PubMed] [Google Scholar]
- 48.Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P, Larvin M, Curtis LD. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–9. doi: 10.1136/gut.48.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Triantafillidis JK, Cheracakis P, Hereti IA, Argyros N, Karra E. Acute idiopathic pancreatitis complicating active Crohn’s disease: favorable response to infliximab treatment. The American journal of gastroenterology. 2000;95:3334–6. doi: 10.1111/j.1572-0241.2000.03332.x. [DOI] [PubMed] [Google Scholar]
- 50.Gloor B, Blinman TA, Rigberg DA, Todd KE, Lane JS, Hines OJ, Reber HA. Kupffer cell blockade reduces hepatic and systemic cytokine levels and lung injury in hemorrhagic pancreatitis in rats. Pancreas. 2000;21:414–20. doi: 10.1097/00006676-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Gea-Sorli S, Closa D. In vitro, but not in vivo, reversibility of peritoneal macrophages activation during experimental acute pancreatitis. BMC immunology. 2009;10:42. doi: 10.1186/1471-2172-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. The Journal of clinical investigation. 2005;115:3007–14. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, Stevenson DK, Butcher EC, Omary MB. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60:671–9. doi: 10.1136/gut.2010.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gea-Sorli S, Closa D. Role of macrophages in the progression of acute pancreatitis. World journal of gastrointestinal pharmacology and therapeutics. 2010;1:107–11. doi: 10.4292/wjgpt.v1.i5.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 56.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. The Journal of clinical investigation. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Apte MV, Pirola RC, Wilson JS. Molecular mechanisms of alcoholic pancreatitis. Digestive diseases. 2005;23:232–40. doi: 10.1159/000090170. [DOI] [PubMed] [Google Scholar]
- 58.Schmid-Kotsas A, Gross HJ, Menke A, Weidenbach H, Adler G, Siech M, Beger H, Grunert A, Bachem MG. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. The American journal of pathology. 1999;155:1749–58. doi: 10.1016/S0002-9440(10)65490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treiber M, Neuhofer P, Anetsberger E, Einwachter H, Lesina M, Rickmann M, Liang S, Kehl T, Nakhai H, Schmid RM, Algul H. Myeloid, but not pancreatic, RelA/p65 is required for fibrosis in a mouse model of chronic pancreatitis. Gastroenterology. 2011;141:1473–85. 1485 e1–7. doi: 10.1053/j.gastro.2011.06.087. [DOI] [PubMed] [Google Scholar]
- 60.Hunger RE, Mueller C, Z’Graggen K, Friess H, Buchler MW. Cytotoxic cells are activated in cellular infiltrates of alcoholic chronic pancreatitis. Gastroenterology. 1997;112:1656–63. doi: 10.1016/s0016-5085(97)70048-9. [DOI] [PubMed] [Google Scholar]
- 61.Grundsten M, Liu GZ, Permert J, Hjelmstrom P, Tsai JA. Increased central memory T cells in patients with chronic pancreatitis. Pancreatology : official journal of the International Association of Pancreatology. 2005;5:177–82. doi: 10.1159/000085269. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz-Winnenthal H, Pietsch DH, Schimmack S, Bonertz A, Udonta F, Ge Y, Galindo L, Specht S, Volk C, Zgraggen K, Koch M, Buchler MW, Weitz J, Beckhove P. Chronic pancreatitis is associated with disease-specific regulatory T-cell responses. Gastroenterology. 2010;138:1178–88. doi: 10.1053/j.gastro.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1–14. doi: 10.1097/01.mpa.0000246658.38375.04. [DOI] [PubMed] [Google Scholar]
- 64.Aghdassi AA, Mayerle J, Christochowitz S, Weiss FU, Sendler M, Lerch MM. Animal models for investigating chronic pancreatitis. Fibrogenesis & tissue repair. 2011;4:26. doi: 10.1186/1755-1536-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrache F, Tu SP, Bhagat G, Pendyala S, Osterreicher CH, Gordon S, Ramanathan V, Penz-Osterreicher M, Betz KS, Song Z, Wang TC. Overexpression of interleukin-1beta in the murine pancreas results in chronic pancreatitis. Gastroenterology. 2008;135:1277–87. doi: 10.1053/j.gastro.2008.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079–92. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 68.Mantovani A, Ming WJ, Balotta C, Abdeljalil B, Bottazzi B. Origin and regulation of tumor-associated macrophages: the role of tumor-derived chemotactic factor. Biochim Biophys Acta. 1986;865:59–67. doi: 10.1016/0304-419x(86)90013-2. [DOI] [PubMed] [Google Scholar]
- 69.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–9. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 71.Baran B, Bechyne I, Siedlar M, Szpak K, Mytar B, Sroka J, Laczna E, Madeja Z, Zembala M, Czyz J. Blood monocytes stimulate migration of human pancreatic carcinoma cells in vitro: the role of tumour necrosis factor - alpha. Eur J Cell Biol. 2009;88:743–52. doi: 10.1016/j.ejcb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 75.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–9. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–55. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 79.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, Abbruzzese JL, Liu YJ, Logsdon CD, Hwu P. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:7015–23. doi: 10.1158/1078-0432.CCR-11-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai SW, Yang SZ, Gao J, Pan K, Chen JY, Wang YL, Wei LX, Dong JH. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149:576–84. doi: 10.1016/j.surg.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–6. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 84.Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999;155:537–47. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, Doglioni C, Braga M, Di Carlo V, Protti MP. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. 2008;181:6595–603. doi: 10.4049/jimmunol.181.9.6595. [DOI] [PubMed] [Google Scholar]
- 86.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 88.Degrate L, Nobili C, Franciosi C, Caprotti R, Brivio F, Romano F, Leone BE, Trezzi R, Uggeri F. Interleukin-2 immunotherapy action on innate immunity cells in peripheral blood and tumoral tissue of pancreatic adenocarcinoma patients. Langenbecks Arch Surg. 2009;394:115–21. doi: 10.1007/s00423-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 89.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149:319–28. doi: 10.1016/j.jss.2007.12.757. [DOI] [PubMed] [Google Scholar]
- 91.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 92.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 93.Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 94.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emmrich J, Weber I, Nausch M, Sparmann G, Koch K, Seyfarth M, Lohr M, Liebe S. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion. 1998;59:192–8. doi: 10.1159/000007488. [DOI] [PubMed] [Google Scholar]
- 96.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 97.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, Finn OJ, Ramanathan RK. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 99.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]