Abstract
Context
Attention-deficit/hyperactivity disorder (ADHD) in adulthood is a prevalent, distressing, and impairing condition that is not fully treated by pharmacotherapy alone and lacks evidence-based psychosocial treatments.
Objective
To test cognitive behavioral therapy for ADHD in adults treated with medication but who still have clinically significant symptoms.
Design, Setting, and Patients
Randomized controlled trial assessing the efficacy of cognitivebehavioraltherapyfor86symptomaticadultswithADHDwhowerealreadybeing treated with medication. The study was conducted at a US hospital between November 2004 and June 2008 (follow-up was conducted through July 2009). Of the 86 patients randomized, 79 completed treatment and 70 completed the follow-up assessments.
Interventions
Patients were randomized to 12 individual sessions of either cognitive behavioral therapy or relaxation with educational support (which is an attention-matched comparison).
Main Outcome Measures
The primary measures were ADHD symptoms rated by an assessor (ADHD rating scale and Clinical Global Impression scale) at baseline, posttreatment, and at 6- and 12-month follow-up. The assessor was blinded to treatment condition assignment. The secondary outcome measure was self-report of ADHD symptoms.
Results
Cognitive behavioral therapy achieved lower posttreatment scores on both the Clinical Global Impression scale (magnitude −0.0531; 95% confidence interval [CI], −1.01 to −0.05; P=.03) and the ADHD rating scale (magnitude −4.631; 95% CI, −8.30 to −0.963; P=.02) compared with relaxation with educational support. Throughout treatment, self-reported symptoms were also significantly more improved for cognitive behavioral therapy (β=−0.41; 95% CI, −0.64 to −0.17; P<001), and there were more treatment responders in cognitive behavioral therapy for both the Clinical Global Impression scale (53% vs 23%; odds ratio [OR], 3.80; 95% CI, 1.50 to 9.59; P=.01) and the ADHD rating scale (67% vs 33%; OR, 4.29; 95% CI, 1.74 to 10.58; P=.002). Responders and partial responders in the cognitive behavioral therapy condition maintained their gains over 6 and 12 months.
Conclusion
Among adults with persistent ADHD symptoms treated with medication, the use of cognitive behavioral therapy compared with relaxation with educational support resulted in improved ADHD symptoms, which were maintained at 12 months.
Approximately 4.4% of adults in the United States have attention-deficit/hyperactivity disorder (ADHD),1 which is a disorder characterized by impairing levels of inattention, hyperactivity, and impulsivity.2 Medications have been the primary treatment; however, many adults with ADHD cannot or will not take medications while others show a poor medication response.3 Furthermore, those considered responders to medications (ie, 30% symptom reduction4) may continue to experience significant and impairing symptoms. Thus, there is a need for alternative and next-step strategies.
Despite this need, our recent review5 found only 3 randomized controlled trials of psychosocial interventions for adult ADHD. These studies showed beneficial acute effects for cognitive behavioral approaches, but they had small sample sizes, used wait-list controls, and did not examine whether gains were maintained. Subsequent to our review, one randomized controlled trial found better outcomes for a group-based cognitive behavioral treatment compared with a time-matched control group treatment.6
We report the first, to our knowledge, randomized controlled trial with an active control condition of an individual-based nonmedication treatment for adult ADHD designed to target those symptoms not ameliorated by pharmacotherapy. Based on our prior work,7 we conducted a randomized controlled trial with a treatment condition and a time-matched control condition and follow-up assessments. We hypothesized that cognitive behavioral therapy would outperform the comparison treatment for ADHD outcomes.
METHODS
Eighty-six adults meeting Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria for ADHD and taking medications but still reporting clinically significant symptoms were included. Medication stability was defined as no change in medication and no more than a 10% change in dose in the 2 months prior to initial evaluation. To maximize generalizability, any medication prescribed by a psychiatrist for ADHD was permitted (Table 1 and the eTable at http://www.jama.com). If the medicines were not prescribed by a psychiatrist and were not typically used for ADHD, patients had a consultation with a study psychiatrist, were referred back to their prescribing physician, and could enter the study after 2 months of taking the new regimen. Groups were not stratified by medication type or dose. Groups were stratified by sex and Clinical Global Impression scale score for severity (score ≥5), in blocks of 2 (determined from a table, constructed by coin flip), and were randomly assigned by the research assistant to either cognitive behavioral therapy or a time-matched comparison treatment of relaxation with educational support. Enrollment occurred between November 2004 and June 2008. The last follow-up occurred in July 2009.
Table 1.
Baseline Demographics and Medication Informationa
| Cognitive Behavioral Therapy (n = 43) | Relaxation With Education (n = 43) | |
|---|---|---|
| Sex | ||
| Male | 24 (55.8) | 24 (55.8) |
|
| ||
| Female | 19 (44.2) | 19 (44.2) |
|
| ||
| Age, mean (SD), y | 42.3 (10.3) | 44 (12.2) |
|
| ||
| Race | ||
| White | 39 (90.7) | 39 (90.7) |
|
| ||
| Black | 2 (4.7) | 3 (7) |
|
| ||
| Asian | 0 | 1 (2.3) |
|
| ||
| Middle Eastern | 1 (2.3) | 0 |
|
| ||
| Otherb | 1 (2.3) | 0 |
|
| ||
| Ethnicity | ||
| Not Hispanic or Latino | 38 (88.4) | 41 (95.3) |
|
| ||
| Hispanic or Latino | 3 (7) | 0 |
|
| ||
| Did not specify | 2 (4.7) | 2 (4.7) |
|
| ||
| Stimulant-based treatment | ||
| Monotherapy | 27 (62.8) | 25 (58.1)c |
|
| ||
| Duotherapy | 6 (14) | 7 (16.3) |
|
| ||
| Bupropion plus stimulant | 6 (14) | 5 (11.6) |
|
| ||
| Atomoxetine plus stimulant | 1 (2.3) | 1 (2.3) |
|
| ||
| Nonstimulant treatment | ||
| Bupropion only | 2 (4.7) | 3 (7) |
|
| ||
| Bupropion plus modafanil | 0 | 1 (2.3) |
|
| ||
| Atomoxetine only | 0 | 1 (2.3) |
|
| ||
| Atomoxetine plus bupropion | 1 (2.3) | 0 |
Values are expressed as number (percentage) unless otherwise indicated.
Individuals did not specify race.
Two patients received stimulant-based therapy via a transdermal patch.
Major study assessments were at baseline, posttreatment acute outcome, 6 months (3 months after posttreatment), and 12 months (9 months after posttreatment). The protocol was ap proved by the Massachusetts General Hospital institutional review board. Signed informed consent was obtained from all participants by study therapists. This consent included patients' agreeing to not change medications during acute treatment.
Power was based on our prior study,7 which had very large between-group effect sizes (d=1.19 for ADHD symptoms and d=1.43 for Clinical Global Impression scale scores). However, that study did not have a time-matched control. Therefore, we estimated an adjustment for a less than large effect (d of approximately 0.8), which would yield a total patient requirement of 60 and an 80% power at a .05 level of significance, targeting a randomized sample of 80 participants, allowing for attrition over follow-up.
Inclusion criteria were (1) principal diagnosis of ADHD (with childhood onset) and a Clinical Global Impression scale score for severity of 3 (mildly ill) or greater, (2) between the ages of 18 and 65 years, (3) able to provide informed consent and comply with study procedures, and (4) stabilized on psychotropic medications. Exclusion criteria were (1) moderate to severe major depression, clinically significant (ie, Clinical Global Impression scale score for severity>4) panic disorder, organic mental disorders, psychotic spectrum disorders, bipolar disorders, active substance abuse or dependence, mental retardation, or pervasive developmental disorder, (2) active suicidality, (3) history of cognitive behavioral therapy, and (4) antisocial personality disorder or a learning disability that would interfere with treatment.
Patients were seen at Massachusetts General Hospital after being recruited through clinics affiliated with the hospital, local radio advertisements, advertisements posted throughout the hospital, as well as through referrals from other mental health professionals.
For both study conditions, treatment sessions (12 sessions for each study condition) were approximately 50 minutes. Therapists were clinical psychologists and postdoctoral-level clinical psychology fellows. Therapists had prior experience with cognitive behavioral therapy, and were supervised weekly in group meetings. Both study treatments included rehearsal, repetition, and review of previously learned skills. Because the same therapists were used for both study conditions, sessions were audio recorded and an outside consultant rated at least 10% for contamination and adherence (14% of sessions rated). All patients continued taking their medications for ADHD as prescribed outside of the study. At the end of acute treatment, those who did not partially or fully respond to treatment (defined by a difference of ≥1 point on the Clinical Global Impression scale for severity) were referred to next-step clinical care inclusive of obtaining the treatment that they did not receive as part of the study.
Cognitive behavioral therapy for ADHD was delivered consistent with our manuals.8,9 It consisted of 3 core modules and 2 optional modules. The first module (4 sessions) focused on psycho-education about ADHD and training in organizing and planning (use of calendar and task list system), including problem-solving training (generating alternatives and picking the best solution, breaking down overwhelming tasks into steps). The second module (2 sessions) involved learning skills to reduce distractibility, such as techniques to time the length of one's attention span, and, when doing a task, write down distractions vs acting on them. The third module (3 sessions) was cognitive restructuring, which involved learning to think more adaptively in situations that cause distress. Optional modules were one session of application of skills to procrastination and one session including the patient's family member for support. Patients for whom the optional sessions were not relevant had booster sessions on prior material. The final session was focused on review and relapse prevention.
Patients in the relaxation condition received training in progressive muscle relaxation and other relaxation techniques as applied to ADHD symptoms, as well as education about ADHD and supportive psychotherapy. The first module involved psychoeducation (1 session). The second module trained patients in progressive muscle relaxation (6 sessions). The third module involved training in application of relaxation to ADHD symptoms (4 sessions). The final session involved review and planning for continued use of these skills (ie, when feeling distracted or overwhelmed, use cued relaxation to calm down and decide what to do next).
Assessments had 3 components: initial diagnosis, an assessment by an interviewer blinded to treatment assignment, and self-report measures. Blinding was maintained by (1) having a single independent assessor who would not participate in meetings when cases were discussed, (2) all participants regardless of assignment have the same number of visits, and by (3) reminding the participants not to discuss which treatment condition they were in with the assessor. The major outcome assessments were repeated at posttreatment (approximately 15 weeks), and at 6-month and 12-month follow-ups. The primary outcomes were blinded Clinical Global Impression scale for severity score and ADHD rating scale scores at the acute outcome assessment. Race and ethnicity were assessed via self-report to comply with the National Institutes of Health reporting requirements.
Initial diagnoses were performed by study therapists, trained on using structured assessments of Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) psychiatric disorders, including the Structured Clinical Interview10 supplemented by questions from the Kiddie Schedule for Affective Disorders and Schizophrenia-Epidemiologic Version11 to assess ADHD. For ADHD symptoms, questions were worded in the past tense, and patients were asked if similar problems were current. Patients who met inclusion criteria then completed the baseline blinded assessment prior to randomization.
The blinded assessments were conducted by a doctoral-level clinician with specific training from the Massachusetts General Hospital ADHD program. The rater and another blinded assessor listened to, rated, and discussed 28% of audio recordings of the ADHD ratings to ensure continued reliability. The assessor first administered the ADHD rating scale,12 which assesses each symptom of ADHD using a 4-point severity grid (score of 0 indicates ADHD not present and a score of 3 indicates severe ADHD). This scale has been shown to be sensitive to medication treatment effects in pediatric13 and adult samples.14,15 Lastly, the assessor rated patients' ADHD using the Clinical Global Impression scale16 for severity (a score of 1 indicates not ill and a score of 7 indicates extremely ill). At each assessment, patients rated their ADHD symptom severity using the Current Symptoms Scale.17
Analysis of covariance models (adjusting for baseline values) with multiple imputation (PROCMI using SAS version 9.2, SAS Institute Inc, Cary, North Carolina) were used throughout the analyses. The models also calculate parameter estimates of between-group differences with 95% confidence intervals (CIs). At the post-treatment assessment, to complement traditional significance testing, we computed between-group effect sizes (d=Meanchange score for cognitive behavioral therapy condition − Meanchange score for relaxation condition/SDpooled) for these measures.
For categorical analyses, we used SPSS version 17.0 (SPSS Inc, Chicago, Illinois) crosstabs feature to calculate Fisher exact tests, odds ratios (ORs) and 95% CIs. For the Clinical Global Impression scale, we considered those who made a 2-point reduction or those who were rated either as 1 or 2 (no longer meeting current criteria for ADHD) as responders. For the ADHD rating scale, we used at least a 30% reduction in symptoms to classify responders.4,6,18
Following intent-to-treat principles, data were analyzed for all participants regardless of whether they changed their medications postrandomization, despite the consent and inclusion criteria that specified that only those with a stable regimen of medications with no plans to change should enroll and agree not to do this during the acute treatment period of approximately 15 weeks. A total of 6 participants (4 in the cognitive behavioral therapy condition and 2 in the relaxation with educational support condition) reported that they changed their medications during the acute treatment period. Two of these participants (both in the cognitive behavioral therapy condition) reported they had stopped taking their medications completely.
We used self-report total scores on the ADHD Current Symptoms Scale that had 14 potential data points (baseline, 12 treatment visits, and posttreatment). Mixed-effect modeling automatically imputes missing data using the slope up to the point of discontinuation. Maintenance of gains was modeled with mixed-effects analyses. We restricted the primary follow-up analyses to those in the cognitive behavioral therapy condition who had at least a 1-point reduction on the Clinical Global Impression scale from baseline to posttreatment a priori because those who did not respond to treatment were generally referred to additional care, potentially confounding between-group comparisons.
Exploratory follow-up analyses were conducted by treatment condition with mixed-effects analysis over the post-treatment, 6-month, and 12-month assessments using baseline variables as a covariate and by modeling the interaction of condition by time.
RESULTS
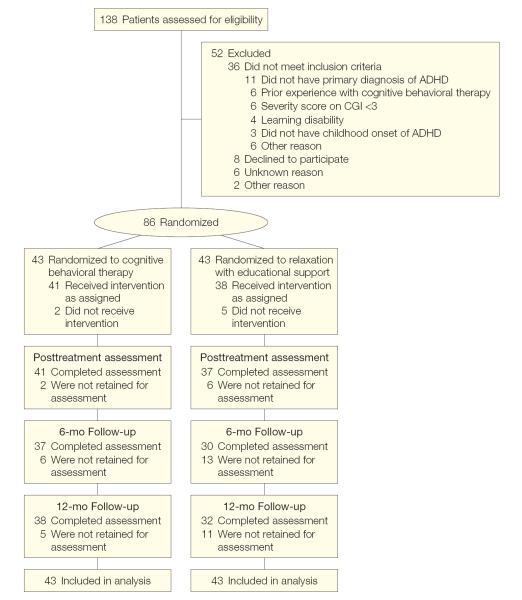
The flow of patients throughout the trial is depicted in Figure 1, which provides information on screening and randomization. Table 1 summarizes the baseline demographic information for those randomized. None of the demographic or outcome data differed by treatment condition.
Figure 1.
Flow of Patients From Randomization Through Analysis
ADHD indicates attention-deficit/hyperactivity disorder; CGI, Clinical Global Impression scale.
At posttreatment, patients who received cognitive behavioral therapy had significantly better ADHD rating scale scores (estimated parameter for treatment effect, −4.63 [95% CI, −8.30 to −0.96]; t23.73=−2.36, P=.02; d=0.60) and Clinical Global Impression scale scores (estimated parameter for treatment effect, −0.53 [95% CI, −1.01 to −0.05]; t24.31=−2.29, P=.03; d=0.53) than those who were assigned to relaxation with educational support. For the weekly ADHD current symptom scores obtained during treatment, the slope of improvement in the cognitive behavioral therapy condition was greater than that for the relaxation condition (β=−0.41 [95% CI, −0.64 to −0.17]; P<.001) (Figure 2). Unadjusted means and SDs for these outcomes appear in Table 2.
Figure 2.

Mixed-Effects Analysis of Self-report Current Symptoms Scale Score for Baseline to Posttreatment
CBT indicates cognitive behavioral therapy. On the x-axis, the 0 time point indicates baseline and 13 weeks indicates posttreatment.
Table 2.
Unadjusted Baseline and Posttreatment Outcome Scoresa
| Cognitive Behavioral Therapy |
Relaxation With Education |
|||
|---|---|---|---|---|
| Baseline | Posttreatment | Baseline | Posttreatment | |
| ADHD rating scale | (n = 43) | (n = 41) | (n = 43) | (n = 37) |
| 26.44 (8.48) | 14.46 (8.46) | 25.33 (8.21) | 19.19 (9.71) | |
|
| ||||
| CGI for severity | (n = 43) | (n = 41) | (n = 43) | (n = 37) |
| 4.74 (0.85) | 3.20 (1.19) | 4.63 (0.85) | 3.73 (1.31) | |
|
| ||||
| Self-report on CSS | (n = 41) | (n = 38) | (n = 40) | (n = 34) |
| 24.73 (8.72) | 11.84 (7.16) | 26.40 (9.48) | 19.12 (11.21) | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CGI, Clinical Global Impression; CSS, Current Symptoms Scale.
Values are expressed as mean (SD).
There was a greater proportion of responders in the cognitive behavioral therapy condition compared with the relaxation condition, respectively, using criteria from both the Clinical Global Impression scale (53% vs 23%; OR, 3.80 [95% CI, 1.50 to 9.59]; P=.01) and the ADHD rating scale (67% vs 33%; OR, 4.29 [95% CI, 1.74 to 10.58]; P=.002). With respect to the optional treatment modules in the cognitive behavioral therapy condition, 40 of 43 patients used the procrastination module and 27 of 43 patients used the module on involving a significant other.
Of those who were assigned to the cognitive behavioral therapy condition and responded or had a partial response, the slope of scores for the ADHD rating scale (β=−0.12; 95% CI, −0.41 to 0.18; P=.41), the Clinical Global Impression scale (β=0.01 [95% CI, −0.03 to 0.05]; P=.59) and the self-report Current Symptoms Scale (β=0.05 [95% CI, −0.04 to 0.15]; P=.26) did not significantly differ from zero when examining the posttreatment, 6-month follow-up, and 12-month follow-up assessment time points, indicating maintenance of gains.
For both blinded assessor-rated scales, there was evidence of overall stability for posttreatment scores over time as evaluated by the slopes of scores across the posttreatment, 6-month, and 12-month assessment time points. The slopes did not change statistically over these time points (ADHD rating scale, β=−0.17 [95% CI, −0.47 to 0.13], P=.27; Clinical Global Impression scale, β=0.01 [95% CI, −0.03 to 0.05], P=.73) and the slopes did not differ by condition (ADHD rating scale score, β=0.08 [95% CI, −0.33 to 0.49], P=.69; Clinical Global Impression scale, β=0 [95% CI, −0.05 to 0.06], P=.97). Therefore, the cognitive behavioral therapy condition maintained gains and the relaxation with educational support condition did not improve during the follow-up.
For the self-report Current Symptoms Scale, there was a significant main effect for treatment condition, with the cognitive behavioral therapy condition having lower (better) scores (β=−8.18 [95% CI, −12.41 to −3.96]; P<.001). However, this was qualified by an interaction of treatment condition by time (β=−0.15 [95% CI, 0.04 to 0.27]; P=.01). Analysis of slopes for each treatment condition separately indicated an increasing slope for the cognitive behavioral therapy condition (β=0.08 [95% CI, 0 to 0.15]; P=.04). However, the small magnitude of these effects reveal changes of limited clinical significance. The means and SDs appear in Table 2 and Table 3.
Table 3.
Unadjusted 6- and 12-Month Follow-up Scoresa
| Cognitive Behavioral Therapy |
Relaxation With Education |
|||
|---|---|---|---|---|
| 6-mo Follow-up | 12-mo Follow-up | 6-mo Follow-up | 12-mo Follow-up | |
| ADHD rating scale | (n = 37) | (n = 38) | (n = 30) | (n = 32) |
| 13.51 (7.70) | 13.39 (8.49) | 16.20 (9.81) | 16.97 (1.72) | |
|
| ||||
| CGI for severity | (n = 37) | (n = 38) | (n = 30) | (n = 32) |
| 3.05 (1.31) | 3.21 (1.24) | 3.43 (1.19) | 3.69 (1.26) | |
|
| ||||
| Self-report on CSS | (n = 34) | (n = 36) | (n = 27) | (n = 29) |
| 11.97 (6.05) | 13.58 (9.14) | 15.52 (11.75) | 16.10 (9.18) | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CGI, Clinical Global Impression Scale; CSS, Current Symptoms Scale.
Values are expressed as mean (SD).
COMMENT
The primary goal of the present study was to test the efficacy of cognitive behavioral therapy for continued symptoms of ADHD in adults treated with medication compared with time-matched treatment of relaxation with educational support. Across ADHD outcomes, those who were randomized to cognitive behavioral therapy showed significantly better outcomes than those randomized to relaxation with educational support. Additionally, among those who showed at least a partial response to cognitive behavioral therapy, improvements were maintained at the follow-up assessments, up to 9 months posttreatment. For the 2 categorical definitions of responder status, the cognitive behavioral therapy condition had significantly more responders than relaxation with educational support. These results demonstrate that the type of cognitive behavioral therapy studied has effects over and above time and attention with a therapist, and that gains were sustained over follow-up. This trial successfully documents the usefulness of this this type of cognitive behavioral therapy as a next-step strategy for patients with ADHD who are treated with medications but continue to have residual symptoms.
Data from the a priori within-group follow-up analyses of those who were at least partial responders to cognitive behavioral therapy showed that gains were maintained. For ethical reasons, those who did not show a response to treatment were encouraged to receive the next-step treatment outside of the study. Consideration was given to dropping or covarying out data from those who received additional treatment outside of the study; however, this was not appropriate because these data are not missing at random, and it would not be following the principles of an intent-to-treat study. Hence, between-group differences over the follow-up periods are confounded by potential subsequent treatment among nonresponders and conclusions regarding these analyses are limited and subject to multiple interpretations. Nonetheless,at follow-up the slopes for change for the primary outcomes were not significant for these exploratory between-group analyses, suggesting that the greater improvements in cognitive behavioral therapy were stable compared with relaxation with educational support. For the self-report measure, however, there was a statistically significant interaction of time × study condition. For this measure, individuals originally assigned to relaxation and able to receive open treatment outside of the study, had slightly decreasing symptom scores and those who had been assigned to cognitive behavioral therapy had increasing scores of similarly small magnitude. Controlled long-term studies of treatment outcome are needed.
In addition to the open follow-up period, our study is limited by a small proportion of missing data. The multiple imputation technique, like all techniques for missing data, does not account for the possibility that those who did not come for a posttreatment assessment may be more likely to be those who were not benefitting from or did not tolerate the treatment. In this scenario, the treatment effect seen in the primary analyses in this study may be artificially attenuated compared with the actual potential treatment effect. The categorical analyses of responders, however, account for this because those who dropped out were considered nonresponders. Also, we are unable to judge motivational effects for patients entering a clinical trial vs clinical treatment, and subsequent samples may differ in motivation, education, or other factors that may influence the generalizability of treatment effects.
The treatment evaluated in this study targeted patients who were taking medications for ADHD but still had residual symptoms. Further study is required to examine whether this cognitive behavioral therapy intervention may be useful for individuals who may be unwilling or unable, for medical reasons, to take medication for ADHD. Additionally, because the only other tested treatment6is a group intervention, further investigation is needed to examine whether different patients or settings may be more receptive or conducive to an individual vs a group approach.
This study suggests that cognitive behavioral therapy for ADHD in adults appears to be a useful and efficacious next-step strategy for adults who show continued symptoms despite treatment with medication. Generally, the treatment was well tolerated, with very low drop-out rates, and had positive and sustained effects on ADHD symptoms. Clinical application of these strategies to patients in need is encouraged.
Acknowledgments
Funding/Support: This study was funded by National Institutes of Health grant 5R01MH69812.
Role of the Sponsors: The National Institutes of Health had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Additional Contributions: We thank Joseph Biederman, MD, Thomas Spencer, MD, and Timothy Wilens, MD, for their support for this project. Each works for Massachusetts General Hospital and they did not receive any compensation for their contributions. We also thank Petra V. Duran, Christine Cooper-Vince, Jennifer Burbridge, PhD (Massachusetts General Hospital), Jonathan Lerner, PhD (Massachusetts General Hospital), Robert Knauz, PhD (Massachusetts General Hospital), Robert Dolye, MD (Massachusetts General Hospital), Carol Perlman, PhD (Massachusetts General Hospital during initial time of study, then private practice), and Tracey Rogers, PhD (private practice and Suffolk University) for assisting in carrying out the study. These individuals received financial contribution for their institutional or consulting (Perlman and Rogers) efforts. We also thank David Schoenfeld, PhD, for consultation about and assistance with biostatistical analysis during the time of grant submission and on the revision for this article. He received compensation for his institutional salary for this work through the Massachusetts General Hospital Clinical Research Program, which provides biostatistical support to investigators at Massachusetts General Hospital.
Trial Registration clinicaltrials.gov Identifier: NCT00118911
Footnotes
Author Contributions: Dr Safren had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Safren, Sprich, Otto.
Acquisition of data: Safren, Sprich, Mimiaga, Surman, Groves.
Analysis and interpretation of data: Safren, Mimiaga, Surman, Knouse, Otto.
Drafting of the manuscript: Safren, Sprich, Groves.
Critical revision of the manuscript for important intellectual content: Sprich, Mimiaga, Surman, Knouse, Otto.
Statistical analysis: Mimiaga, Otto.
Obtained funding: Safren, Sprich, Otto.
Administrative, technical, or material support: Safren, Sprich, Surman, Knouse, Groves.
Study supervision: Safren, Sprich, Surman.
Financial Disclosures: Drs Safren, Sprich, and Otto reported receiving royalty payments from Oxford University Press. Dr Surman reported receiving research support from Abbott, Alza, Cephalon, Eli Lilly, ElMinda the Hilda and Preston Davis Foundation, McNeil, Merck, New River, National Institutes of Health, Organon, Pfizer, Shire, and Takeda; being a speaker for Janssen-Ortho, McNeil, Novartis, Shire, and MGH Academy/Reed Medical Education (which receives funding from multiple pharmaceutical companies); and being a consultant or advisor for McNeil, Shire, and Takeda. Dr Knouse reported receiving consulting income from Eli Lilly. Dr Otto reported receiving consulting income from Jazz Pharmaceuticals, Organon (Schering-Plough), Pfizer, and Sanofi-Aventis; research support from Organon (Schering-Plough); and royalty payments for use of the SIGH-A from Lilly.
Previous Presentations: Initial (acute outcome) results were presented at the World Congress of Behavioral and Cognitive Therapies; June 2–5, 2010; Boston, Massachusetts.
Online-Only Material: The eTable is available at http://www.jama.com.
REFERENCES
- 1.Kessler RC, Adler L, Barkley RA, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 3.Prince J, Wilens T, Spencer T, Biederman J. Pharmacotherapy of ADHD in adults. In: Barkley RA, editor. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 3rd ed Guilford; New York, NY: 2006. pp. 704–736. [Google Scholar]
- 4.Steele M, Jensen PS, Quinn DMP. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clin Ther. 2006;28(11):1892–1908. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Knouse LE, Cooper-Vince C, Sprich S, Safren SA. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537–1548. doi: 10.1586/14737175.8.10.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solanto MV, Marks DJ, Wasserstein J, et al. Efficacy of metacognitive therapy for adult ADHD. Am J Psychiatry. doi: 10.1176/appi.ajp.2009.09081123. published ahead of print March 15, 2010. doi:10.1176/appi.ajp.2009.09081123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safren SA, Otto MW, Sprich S, Winett CL, Wilens TE, Biederman J. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther. 2005;43(7):831–842. doi: 10.1016/j.brat.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Safren SA, Perlman CA, Sprich S, Otto MW. Mastering Your Adult ADHD: A Cognitive-Behavioral Therapy Approach. Oxford Univeristy Press; New York, NY: 2005. [Google Scholar]
- 9.Safren SA, Sprich S, Perlman CA, Otto MW. Mastering Your Adult ADHD: A Cognitive-Behavioral Treatment Program Client Workbook. Oxford Univeristy Press; New York, NY: 2005. [Google Scholar]
- 10.First M, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- 11.Orvaschel H. Psychiatric interviews suitable for use in research with children and adolescents. Psychopharmacol Bull. 1985;21(4):737–745. [PubMed] [Google Scholar]
- 12.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. Guilford Publications; New York, NY: 1998. [Google Scholar]
- 13.Faries DE, Yalcin I, Harder D, Heiligenstein JH. Validation of the ADHD rating scale as a clinician administered and scored instrument. J Atten Disord. 2001;5(2):107–115. [Google Scholar]
- 14.Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1995;52(6):434–443. doi: 10.1001/archpsyc.1995.03950180020004. [DOI] [PubMed] [Google Scholar]
- 15.Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1147–1153. doi: 10.1176/ajp.153.9.1147. [DOI] [PubMed] [Google Scholar]
- 16.NIMH CGI Clinical Global Impression Scale. Psychopharmacol Bull. 1985;21:839–844. [Google Scholar]
- 17.Barkley RA, Murphy KR. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. 3rd ed Guilford Press; New York, NY: 2006. [Google Scholar]
- 18.Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737–746. doi: 10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]