Abstract
The immortalized human cerebral microvascular endothelial cell line HCMEC/D3 presents a less expensive and more logistically feasible alternative to primary human brain microvascular endothelial cells (HBMEC’s) for use in constructing in vitro models of the blood brain barrier (BBB). However, the fidelity of the HCMEC/D3 cell line to primary HBMEC’s in studies of immune transmigration has yet to be established. Flow cytometric analysis of primary human leukocyte migration across in vitro BBB’s generated with either HCMEC/D3 or primary HBMEC’s revealed that HCMEC/D3 maintains the immune barrier properties of primary HBMEC’s. Leukocyte migration responses and inflammatory cytokine production were statistically indistinguishable between both endothelial cell types, and both cell types responded similarly to astrocyte coculture, stimulation of leukocytes with phorbol myristate acetate (PMA) and ionomycin, and inflammatory cytokine treatment. This report is the first to validate the HCMEC/D3 cell line in a neuroimmunological experimental system via direct comparison to primary HBMEC’s, demonstrating remarkable fidelity in terms of barrier resistance, immune migration profiles, and responsiveness to inflammatory cytokines. Moreover, we report novel findings demonstrating that interaction effects between immune cells and resident CNS cells are preserved in HCMEC/D3, suggesting that important characteristics of neuroimmune interactions during CNS inflammation are preserved in systems utilizing this cell line. Together, these findings demonstrate that HCMEC/D3 is a valid and powerful tool for less expensive and higher throughput in vitro investigations of immune migration at the BBB.
1. Introduction
Central nervous system (CNS) homeostasis is largely dependent on the presence of the blood brain barrier (BBB), a highly specialized arrangement of vascular endothelial cells joined by tight and adherens junctions, and associated with pericytes and astrocytic endfeet (Abbott et al.2006 ). This complex endothelial barrier exerts tight regulation over solutes and cells in the systemic circulation, restricting access to parenchymal CNS tissue. Importantly, the capacity of the BBB to control immune cell entry into the CNS during episodes of inflammation is critical, both for effective pathogen clearance during an infection and for protection from immunopathology during injury and disease (Pachteret al. 2003). Our group and others have demonstrated that immune-mediated structural and functional changes to the BBB during disease can orchestrate both protective and pathological physiological processes in the CNS (McCandless et al. 2008; Cruz-Orengo et al. 2011)
In vitro models of the BBB are a powerful tool for specific mechanistic studies of BBB physiology (Lundquist and Renftel 2002). Most commonly, these models consist of transwell culture systems in which brain microvascular endothelial cells (BMEC’s) are grown on a porous filter membrane, and the integrity of the in vitro barrier is assessed by measurements of transendothelial electrical resistance (TEER) or the diffusive capacity of fluorescently labeled solutes. While such models have traditionally been composed of endothelial cells derived from animal origins, the growing availability of both primary and immortalized human endothelial cells and astrocytes promises to further increase the usefulness of in vitro BBB models in basic and clinical investigations (Cucullo et al. 2008; Hatherell et al. 2011).
In particular, the development of the immortalized human cerebral microvascular endothelial cell line HCMEC/D3 (Weksler et al. 2005) represents a significant advance for in vitro BBB models. This well characterized cell line maintains fundamental properties of primary human BMEC’s(HBMEC’s), including tight junction/transporter protein expression and contact growth inhibition, for up to 35 passages. While studies using primary HBMEC’s are typically costly and low-throughput due to the scarcity of these cells and the challenges of isolating them from fresh tissue (discussed in Naik and Cucullo 2012), the HCMEC/D3 cell line allows much more efficient and high-throughput in vitro investigations of the human BBB. However, while several reports have asserted that this cell line preserves the properties of primary HBMEC’s in various experimental systems, there have been few direct experimental comparisons of HCMEC/D3 to primary HBMEC’s.
Here, we report that in vitro BBB’s generated using HCMEC/D3 exhibit remarkable similarity to those constructed with primary cells in a model of trans-BBB immune migration. Using this experimental system, we were able to observe key BBB features, such as increased barrier integrity after coculture of endothelial cells with astrocytes, dysregulation of barrier integrity after inflammatory cytokine treatment, and enhanced trafficking of primary human T lymphocytes after stimulation of total peripheral blood mononuclear cells (PBMC’s) with the standard experimental leukocyte stimulators phorbol myristate acetate (PMA) and ionomycin. Importantly, both primary HBMEC’s and HCMEC/D3 exhibited statistically indistinguishable responses to every experimental manipulation, both in terms of changes to barrier properties and differential leukocyte migration. This report is the first to validate that in vitro BBB’s created with the HCMEC/D3 cell line can reproduce the barrier phenotype of primary HBMEC’s for in vitro investigations of immune migration across the BBB. While a growing list of studies have characterized HCMEC/D3 in various experimental systems, our report is the first extensive validation of the HCMEC/D3 cell line in a neuroimmunological setting and the first to explicitly demonstrate that in vitro BBB’s generated with HCMEC/D3 maintain interaction effects between experimentally relevant neurobiological and immunological manipulations, including astrocyte coculture, leukocyte stimulation, and inflammatory cytokine treatment. In light of this validation, the significant advantages of using an immortalized cell line for BBB investigations in vitro promises to enhance the usefulness of transmigration experiments for addressing important neuroimmunological questions.
2. Materials and methods
2.1 Cell culture
In vitro human BBB cultures were generated using either primary HBMEC’s or HCMEC/D3, cultured alone or cocultured with primary human astrocytes. HBMEC’s and primary human astrocytes were obtained from ScienCell (Carlsbad, CA). HBMEC’s available from ScienCell have been broadly characterized by groups performing in vitro BBB investigations in a number of experimental systems (Cucullo et al. 2007; Simard et al. 2007; Bachmeier et al. 2010), including immune transmigration (Gobel et al. 2011), and have been specifically shown to form more robust barriers and develop specific BBB characteristics in culture when compared to non-CNS endothelial cells (Cucullo et al. 2008). HCMEC/D3, first described by Weksler et al. (2005), is a human brain endothelial cell line, immortalized with human telomerase reverse transcriptase and Simian vacuolating virus 40 (hTERT/SV40) large T antigen. All cells were grown in endothelial basal medium-2 (EBM-2; Lonza Group, Ltd.), supplemented with 5% Fetal Bovine Serum “Gold” (PAA, The Cell Culture Company), 5μg/ml ascorbic acid (Sigma), 1% chemically defined lipid concentrate (Invitrogen, Gibco), 10mM HEPES buffer (PAA, The Cell Culture Company), and 1% penicillin-streptomycin (Invitrogen, Gibco). HCMEC/D3 cells were used between passages 25 and 35, while primary endothelial cells and primary astrocytes were used only in their second passage. All cells were maintained under 5% carbon dioxide at 37°C.
2.2 In vitro BBB’s
In vitro BBB’s were generated using a well-established transwell system (schematized in Fig. 1A). Briefly, HCMEC/D3 or primary HBMEC’s were seeded at 1×105 cells/cm2 on the apical side of a 0.9 cm2 polyethylene terephthalate filter insert with 3.0 μm porosity (BD Falcon), coated with 150 μg/ml Cultrex® Rat Collagen I (R&D Systems). For coculture chambers, 105 primary human astrocytes were seeded in 12-well plates and cultured for 2 days (to reach confluence) prior to addition of the endothelial cell inserts. TEER values (ohm/cm2) were measured via chopstick electrode recording with an EVOM apparatus (World Precision Instruments). Resistance values are reported as recorded values for each replicate minus the resistance of cell-free inserts (~90 ohm) measured alone. Measurements were taken 24 hours after initial seeding of endothelial cells (day 1); subsequent measurements were taken daily for 10 days, at which point cultures were used for PBMC migration studies.
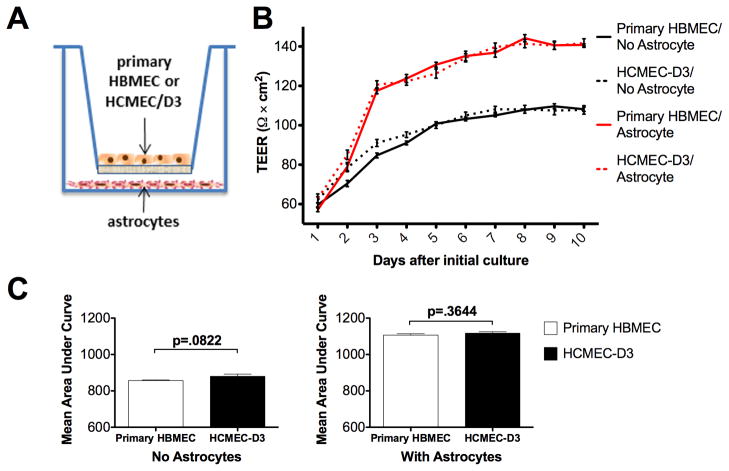
Fig. 1.
HCMEC/D3 maintain the barrier formation characteristics of primary HBMEC’s as assessed by measurement of transendothelial electrical resistance (TEER). (A) Schematic depiction of in vitro BBB’s; see text for details. (B) HCMEC/D3 exhibited virtually identical TEER values for each day of culture in both monoculture and astrocyte coculture systems (p>0.05). (C) Area under the curve (AUC) analysis revealed no significant difference in overall barrier formation kinetics between HCMEC/D3 and HBMEC cultures, with or without astrocyte coculture. Data are mean values of 15 (HCMEC/D3) or 9 (HMBEC) replicates +/− SEM, taken from 3 independent experiments.
2.3 Isolation and stimulation of primary human PBMC’s
Fresh whole blood was taken from healthy volunteers and PBMC’s were isolated by density centrifugation in 10 ml of Histopaque 1077 over 10 ml of Histopaque 1119 (Sigma) at 700g (4°C) for 30 min. Buffy coats were then rinsed, once in 45 ml of sterile Dulbecco’s phosphate buffered saline (DPBS, Sigma) and twice more in C5 media (1% glutamine, 1% penicillin-streptomycin, 5% FBS in Dulbecco’s Modified Eagle Medium [Sigma]; 10 min at 250g [4°C] between rinses). After the final rinse, PBMC’s were resuspended at 2 X 106 cells/ml in C5 media and then treated for 6h at 37°C with either stimulation media (5ng/ml PMA [Sigma] and 1ug/ml ionomycin [Sigma] in C5 media) or a matched non-stimulating vehicle (0.1% DMSO in C5 media).
2.4 Migration Assay
After stimulation, 5×105 PBMC’s in 200ul of C5 media were added to the top chamber of each in vitro BBB (~ 5:1 ratio, PBMC to endothelial cell) and allowed to incubate for 12h at 37°C. Medium was then harvested from top and bottom chambers and PBMC’s in each fraction were collected by centrifugation (1500, 10 min, 4°C). Cell pellets were next resuspended in 200ul of FACS buffer (2% FBS, 0.5mM EDTA, 0.02% sodium azide in sterile DPBS), and leukocyte subsets were identified and counted with an LSRII (BD) flow cytometer using FCS Express™ Diva 4.0 from De Novo Software™. Data were analyzed with FlowJo™: version 9 (Tree Star, Inc.). The following antibodies were utilized to identify leukocyte subsets: CD3-FITC (BD), CD4-Brilliant Violet (Biolegend), CD8-PerCP-Cy5.5 (Biolegend), CD19-APC-Cy7 (Biolegend), CD11b-PE (BD), CD11c-PE-Cy5 (Biolegend), and GFAP-Alexa 647 (BD). Leukocytes isolated from the bottom chamber were counted after prior gating for live cells and GFAP− cells (in case of contamination from astrocytes in the bottom chamber). CD4+ and CD8+ lymphocytes were counted after prior gating for CD3+ cells. For each leukocyte subset, migration was quantified as the number of cells present in the bottom chamber after 12h divided by the total number of that cell type added to the top chamber (proportion migrating).
2.4 Cytokine analysis
Supernatants from both top and bottom chambers were saved after the collection of PBMC’s at 12h in the migration experiments. Samples from both chambers of each culture replicate were added to separate wells of a BioPlex™ human cytokine bead array plate (Biorad) customized to detect IL-1β, IL-17, TNF-α, and IFN-γ. Assay was performed according to manufacturer’s instructions. Cytokine concentrations are reported as pg/ml.
2.5 Cytokine treatment assays
On day 10 of culture, either TNF-α or IFN-γ (100ng/ml) was added to both chambers of in vitro BBB cultures. TEER values were recorded both before and 6h after addition of cytokine to culture media. TEER changes after cytokine treatment are expressed as % change at 6h. Alternatively, 5×104 cells of either primary HBMEC or HCMEC/D3 were seeded into 8-well BioCoat™ chamber slides (BD), coated with human fibronectin (by manufacturer). Chamber slides were additionally coated with 150 μg/ml Cultrex® Rat Collagen I (R&D Systems). On day 2–3 of culture, when cultures were confluent, cells were treated with either TNF-α or IFN-γ (100ng/ml) for 6 h. Cells were then fixed in 4% paraformaldehyde for 30 min, followed by blocking and permeabilization in 0.1% Triton X-100 and 10% goat serum for 30 min at room temperature. Cells were then incubated in primary antibody (mouse anti-human CD144/VE-cadherin, BD, 1:200) in blocking buffer for 1h at room temperature. Cells were then washed three times in PBS, followed by incubation in fluorescently conjugated goat anti–mouse Alexa Fluor 555 (Invitrogen, 1:1000) secondary antibody in blocking buffer for 15 min at room temperature. Cells were then washed in PBS, counterstained with ToPro3 (1:500), and coverslipped before being visualized via scanning confocal microscopy.
2.5 Statistical Analysis
TEER measurements were compared via repeated measures two-way ANOVA. Bonferroni’s posthoc test was used to compare individual time points. Area under the curve statistics (AUC) were calculated individually for each experimental replicate, then averaged to generate a mean AUC. AUC’s were compared with a two-tailed student’s t-test. Migration and cytokine response statistics were compared via two-way ANOVA (non-repeated measures) and individual groups were compared via Bonferroni’s posthoc test. For all graphs, error bars represent +/− 1 standard error of the mean. For TEER measurements (Figures 1B and 1C), data are the average of 3 independent experiments (n=15, total for each group of HCMEC/D3; n=9, total for each group of HBMEC’s). For migration results (Figure 2) and cytokine responses (Figures 3 and 4) data are the average of 2 independent experiments (n=6 per group). All statistical analysis was performed with GraphPad Prism software, version 5.0. P values <0.05 were considered significant for all comparisons.
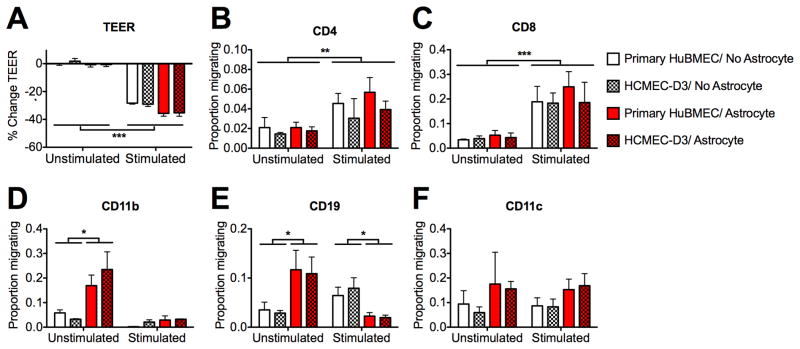
Fig. 2.
Leukocyte migration patterns are preserved for in vitro BBB’s constructed with HCMEC/D3 compared to primary HBMEC’s. Stimulated (PMA/ionomycin) or unstimulated primary human PBMC’s were used in model BBB transmigration assays as described in the text. TEER values were recorded before and after transmigration experiments (reported as percent change) and cells that migrated to the bottom chamber were identified via flow cytometry (reported as the proportion of total cells added to top chamber). (A) Only stimulated PBMC’s caused a change in barrier resistance; this response was identical for barriers composed of HCMEC/D3 and HBMEC. (B – F) The migration of (B) CD4+, (C) CD8+, (D) CD11b+, (E) CD19+, and (F) CD11c+ leukocytes was statistically indistinguishable (p>.05) between cultures of HCMEC/D3 (patterned bars) and HBMEC (open bars). These barriers influenced migration identically in every condition tested, including those where significant differences were induced by PBMC stimulation (B, C) or the presence of astrocytes (D, E). (*=p<.05; **=p<.01; ***=p<.001). Data are mean values +/− SEM of 6 replicates taken from 2 independent experiments.
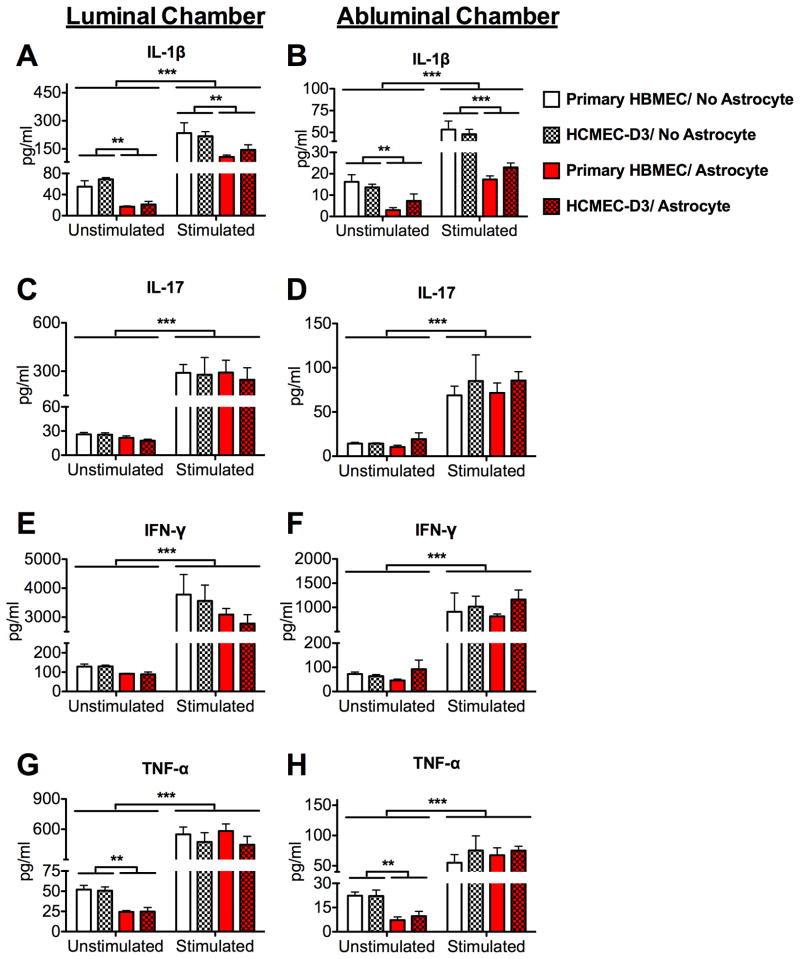
Fig. 3.
Inflammatory cytokine production is similar for in vitro BBB’s constructed with HCMEC/D3 compared to primary HBMEC’s. At the end of the 12h PBMC migration experiments, supernatants in both luminal (top) and abluminal (bottom) chambers of each BBB culture were examined for the presence of inflammatory cytokines via BioPlex™ human cytokine bead array. The expression of (A–B) IL-1β, (C–D) IL-17, (E–F) IFN-γ, and (G–H) TNF-α was statistically indistinguishable (p>.05) between cultures of HCMEC/D3 (patterned bars) and HBMEC (open bars). In both luminal and abluminal chambers, significant changes to cytokine expression due to PBMC stimulation (A–H, p<0.001) or astrocyte coculture (A–B; G–H, p<.01) were similar in HCMEC/D3 and HBMEC cultures. (*=p<.05; **=p<.01; ***=p<.001). Data are mean values of 6 replicates +/− SEM taken from 2 independent experiments.
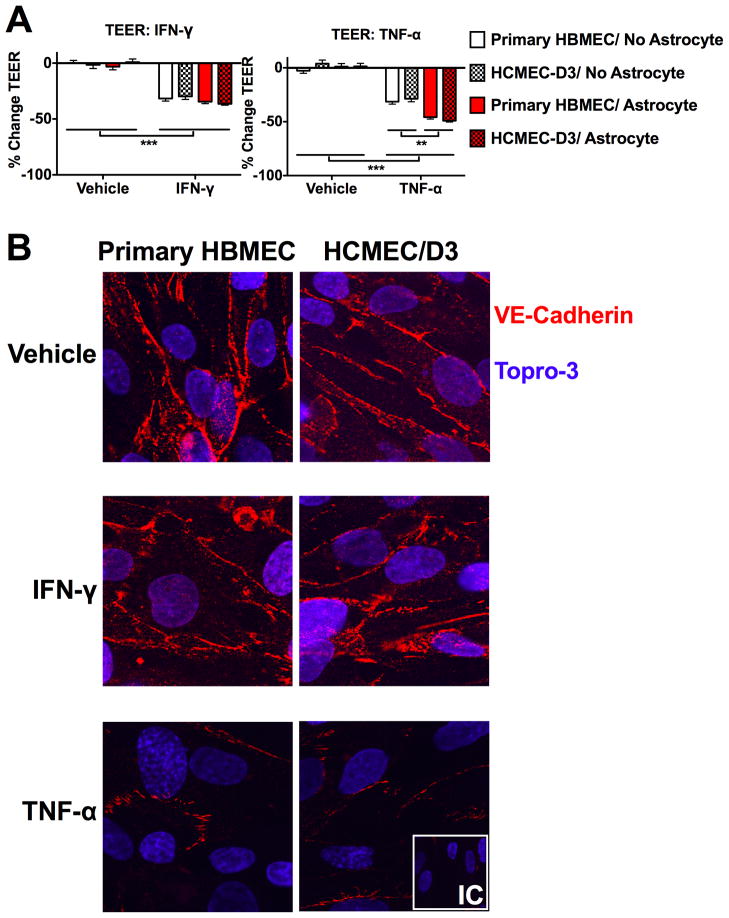
Fig. 4.
Barrier responses to inflammatory cytokine treatment are similar in HCMEC/D3 compared to HBMEC cultures. (A) BBB cultures of both cell types were treated for 6h with either 100ng/ml IFN-γ, TNF-α, or a PBS vehicle. TEER values were taken before and after cytokine treatment (reported as percent change). Both IFN-γ and TNF-α significantly reduced TEER in BBB cultures (p<0.001), while astrocyte coculture enhanced the effect of TNF-α treated cultures only (p<0.01). These effects were statistically indistinguishable between HCMEC/D3 and HBMEC cultures (p>0.05). (B) Confluent monolayers of either HBMEC or HCMEC/D3 were treated for 6h with either 100ng/ml IFN-γ, TNF-α, or a PBS vehicle, then probed via immunocytochemistry for expression of VE-cadherin. Both HBMEC and HCMEC/D3 exhibited discrete staining of VE-cadherin at intercellular junctions, which was diminished by TNF-α but not IFN-γ treatment. (*=p<.05; **=p<.01; ***=p<.001). TEER data are mean values +/− SEM of 6 replicates taken from 2 independent experiments. Immunocytochemistry pictures are representative 63× images of 6 images taken per condition from 2 independent experiments. IC= isotype control.
3. Results
3.1 HCMEC/D3 barrier formation is similar to that of primary HBMEC’s
To compare in vitro BBB’s composed of either HCMEC/D3 or primary HBMEC’s alone, we first tested them in the absence of astrocytes. BBB’s of the two cell types exhibited extremely similar barrier formation kinetics (Fig. 1B, solid and dashed black lines). Strikingly, barrier resistance was indistinguishable between the primary and immortalized cells for days 3–10 of culture (p>0.05), with only day 2 showing a significant difference between them (p<0.05). For both cell types, astrocyte coculture yielded more robust in vitro barriers (Fig. 1B, solid and dashed red lines), characterized by higher TEER values (p<0.001) and exhibiting statistically indistinguishable TEER values over each of the 10 days of culture (p>0.05). Comparisons of mean area under the curve (Fig. 1C) for cultures with and without astrocytes revealed nearly identical values for primary and cell line cultures in both conditions (p=0.3644 and 0.0822, respectively).
3.2 Astrocyte coculture and PMA/ionomycin stimulation impact PBMC trafficking across in vitro BBB’s
Flow cytometric analysis revealed that several leukocyte subsets migrated across the in vitro BBB after their addition to the top culture chamber, and that PMA/ionomycin stimulation of PBMC’s affected both leukocyte migration and endothelial barrier integrity. Transmigration of unstimulated PBMC’s did not impact barrier resistance in cultures with or without astrocytes (Fig. 2A). However, prior stimulation of PBMC’s resulted in a significant reduction in endothelial barrier resistance after 12h (p<0.001). This decrease in resistance was further augmented in barriers cocultured with astrocytes (p<0.001; interaction effect: p<0.05). PMA/ionomycin stimulation also significantly enhanced the migration of CD4+ (Fig. 2B) and CD8+ (Fig. 2C) T-lymphocytes (p<0.01, <0.001, respectively), regardless of whether astrocytes were present (interaction effect: p>.05).
In contrast to results observed with T lymphocytes, the presence of astrocytes had a significant interaction effect with PMA/ionomycin stimulation on the migration of both CD11b+ (Fig. 2D) and CD19+ (Fig. 2E) leukocyte subsets. Astrocytes significantly enhanced the migration of CD11b+cells when PBMC’s were unstimulated (p< 0.05), but exerted no effect on the migration of CD11b+cells when PBMC’s were stimulated (p>0.05; interaction effect: p<0.05). Astrocytes similarly enhanced the migration of CD19+cells when PBMC’s were unstimulated (p<0.05), but decreased the migration of those cells when PBMC’s were stimulated (p<0.05; interaction effect: p<0.05). Neither astrocyte coculture nor PBMC stimulation had a significant effect on the migration of CD11c+ (Fig. 2F) cells (p>0.05 for all comparisons).
3.3 HCMEC/D3 maintains the immune barrier properties of primary HBMEC’s
Although stimulation of PBMC’s and coculture with astrocytes had significant effects on both PBMC migration and TEER changes after leukocyte trafficking across the in vitro BBB, our results indicate that the HCMEC/D3 cell line maintains the properties of primary HBMEC’s in this system. For every culture condition (with/without astrocytes, stimulated/unstimulated PBMC’s), there were no significant differences in migration between cultures with HCMEC/D3 and primary HBMEC’s for any of the leukocyte subsets analyzed (p>0.05 for all comparisons). Moreover, while stimulation of PBMC’s and coculture with astrocytes significantly affected the change in TEER occurring after PBMC migration (effect of astrocytes: p<0.05; effect of stimulation: p<0.001; interaction effect: p>0.05), these changes were also virtually identical between HCMEC/D3 and primary HBMEC cultures (p>0.05 for all comparisons).
3.4 Inflammatory cytokine production and regulation of barrier integrity is comparable in HCMEC/D3 vs HBMEC cultures
In order to assess characteristics of the inflammatory response in our model, we measured inflammatory cytokine production in both the top (luminal) and bottom bottom (abluminal) chamber of each BBB culture after 12h of PBMC migration via cytokine bead array detection of IL-1β, IL-17, TNF-α, and IFN-γ (Fig. 3). Stimulation of PBMC’s significantly enhanced expression of each of these cytokines in both luminal and abluminal chambers (p<.001 for both). In addition, coculture with astrocytes suppressed upregulation of IL-1β in both chambers (Fig. 3A–B, p<0.01 for both), whether or not PBMC’s were stimulated (interaction effect: p>0.05). Astrocyte coculture suppressed TNF-α expression in both chambers if PBMC’s were unstimulated (Fig. 3G–H, p<.01 for both), but had no effect when PBMC’s were stimulated (p>0.05; interaction effect: p<0.05). Although astrocyte coculture and PBMC stimulation significantly regulated the production of inflammatory cytokines, each of these changes was identical between HCMEC/D3 and primary HBMEC cultures. Moreover, the presence of an endothelial barrier was necessary to elicit the inflammatory cytokine responses we observed, as measurements of media in transwell systems with no endothelial cells or astrocytes (only PBMC’s added to empty filter inserts) revealed cytokine levels that were largely below limits of detection (data not shown).
In light of these findings, we next evaluated the impact of inflammatory cytokine expression on endothelial barrier integrity. Treatment with either IFN-γ or TNF-α (100ng/ml, 6h) resulted in barrier disruption (Fig. 4A), indicated by significant reductions in TEER (p<.001 for both). Moreover, astrocyte coculture enhanced barrier disruption by TNF-α (p<.01) but not IFN-γ (p>.05). Ultimately, while inflammatory cytokine treatment resulted in substantial decreases in TEER, this effect was equivalent in HCMEC/D3 and primary HBMEC cultures (p>0.05 for all comparisons).
In order to further compare inflammatory cytokine regulation of barrier integrity in both endothelial cell types, we performed fluorescent immunocytochemical staining for the adherens junction marker VE-cadherin in either HCMEC/D3 or primary HBMEC monolayers. Both HCMEC/D3 and primary HMBEC cells exhibited similar fluorescence intensities for VE-cadherin, characterized by discrete localization of VE-cadherin to intercellular borders where adherens junctions form between adjacent endothelial cells and contribute to proper barrier function (Fig. 4B). TNF-α, but not IFN-γ, treatment (100ng/ml, 6h) substantially reduced the staining intensity of VE-cadherin in both cell types, suggesting that inflammatory cytokine regulation of molecular barrier properties is similar in HCMEC/D3 and primary HBMEC cultures.
4. Discussion
We report that the immortalized HCMEC/D3 cell line can be used to construct in vitro BBB’s for use in immune-CNS transmigration experiments. In terms of barrier formation kinetics, immune migration profiles, and inflammatory cytokine responses, HCMEC/D3 maintained all properties of primary HBMEC’s, demonstrating that the HCMEC/D3 cell line will be useful for less expensive and higher-throughput studies of immune migration across the BBB. Although the utility of HCMEC/D3 for investigations of drug transport across the BBB has been established (Cucullo et al. 2008; Poller et al. 2008), there are a number of important experimental systems, including neuroimmunological and neuroinfectious disease models, for which the usefulness of HCMEC/D3 has not yet been thoroughly addressed. While the effects of astrocyte coculture and leukocyte migration in HCMEC/D3 BBB’s have been addressed separately in previous reports, our report additionally performs direct comparisons of HCMEC/D3 to primary HBMEC’s in these systems in order to validate the fidelity of this cell line to primary cells. Moreover, our report is the first to demonstrate that interaction effects between immunologically relevant experimental manipulations (astrocytes, leukocyte stimulation, cytokine treatment) are preserved in HCMEC/D3, as prior reports have primarily investigated these facets of the model in isolation. Such comparisons are critical to ensure that investigations using HCMEC/D3 produce results that actually model the behavior of primary cells in a given experimental system.
The fidelity of HCMEC/D3 to primary HBMEC’s was evident across a number of parameters in this study, including both the magnitude and time course of their responsiveness to astrocyte coculture (Figure 1B). Prior reports have demonstrated that coculture with astrocytes enhances the barrier integrity of endothelial monolayers in transwell cultures (Garcia et al. 2004; Deli et al. 2005; Hatherell et al. 2011). Our data support this observation, with equivalent results from HCMEC/D3 and primary HBMEC’s. It should be noted, however, that previous reports on permeability and the responsiveness of HCMEC/D3 to astrocytes have been mixed. While we report TEER values of >1000 ohm/cm2, TEER values for HCMEC/D3 have varied in previous reports, with reported TEER values of between ~30–65 ohm/cm2 (Weksler et al. 2005; Hatherell et al. 2011) in static transwell cultures, and TEER values of >1000 ohm/cm2 in “dynamic,” flow-based BBB models in which HCMEC/D3 are grown in the lumen of hollow microporous fibers and exposed to shear stress from pulsatile flow of culture media. Additionally, while Weksler et al. (2005) and Cucullo et al. (2008) reported that HCMEC/D3 were relatively unresponsive to stimulation by astrocytes, Hatherell et al. (2011) observed a significant increase in TEER values of HCMEC/D3 cocultured with several human astrocyte cell lines (although primary HBMEC’s were not included in this analysis).
Variations in TEER and responsiveness to astrocytes among reports using static transwell cultures may arise from a number of experimental factors, including media formulation, type/condition of astrocytes, and the porosity and coating substrate of the transwell filters used. For example, Hatherell et al. (2011) used fibronectin coated filters with 8 μm porosity, while our study utilizes collagen I coated filters with 3 μm porosity. The timing of measurements is likely also important, as Weksler et al. (2005) report TEER values after only 2 days of culture, while our maximum TEER values were observed on ~day 10 after culture. The substantially higher TEER values obtained by Cucullo et al. (2008) arise from the incorporation of physiological sheer stress and dynamic flow into the BBB culture system; however, while these dynamic flow systems have obvious and important advantages, they also pose significant technical and logistical challenges when compared to static transwell models, which are less expensive, easier to manipulate and maintain, and allow for much higher throughput investigations of relatively simple experimental manipulations (Naik and Cucullo 2012), which is the focus of this report. While each of these distinctions may be important depending on the experimental system one uses, our data suggest that TEER values, alone, do not substantially influence leukocyte migration, as many leukocyte subsets had identical migration through barriers with and without astrocyte coculture (which had significant differences in TEER).
In addition to comparing effects on permeability, we have further demonstrated that HCMEC/D3 maintains the properties of primary HBMEC’s in the context of immune migration. Like primary cells, HCMEC/D3 allowed the migration of unstimulated leukocytes without a significant reduction in TEER (Fig. 2A). However, the addition of PBMC’s stimulated with PMA/ionomycin significantly reduced barrier resistance, most likely as a result of the production of large concentrations of inflammatory cytokines (Fig. 3), which have been shown to enhance BBB permeability (Oshima et al. 2001). In addition, enhanced migration of stimulated leukocytes may result in physical disruption of the endothelial barrier, with a resultant reduction in TEER. While stimulation enhanced the migration of T-lymphocytes (Figs. 2B and 2C), it seemed to decrease the migration of CD11b+ (Fig. 2D) and CD19+ (Fig. 2E) leukocytes, an effect modulated by the presence of astrocytes in culture. Remarkably, each of these findings in BBB’s generated with primary HBMEC’s was preserved in those constructed with HCMEC/D3. Moreover, the production of inflammatory cytokines by PBMC’s during transmigration (Fig. 3) and the effects of inflammatory cytokine treatment on TEER and VE-cadherin expression were indistinguishable in cultures of both cell types (Fig. 4). Together, these data demonstrate that the mechanisms that regulate immune cell transmigration across the vascular endothelium at the BBB are preserved in the HCMEC/D3 cell line in this in vitro system. In particular, the novel findings demonstrating that interaction effects between astrocyte coculture and PBMC stimulation/inflammatory cytokine treatment are preserved in HCMEC/D3 in this system are strong indication that important interactions between immune and resident CNS cells exist in the setting of the in vitro BBB, and that these more nuanced neuroimmunological phenomena are equally well-modeled by HCMEC/D3 and primary cells.
5. Conclusion
Key studies have used an in vitro BBB model to study immune migration across the BBB during inflammatory episodes in the CNS (Kebir et al. 2007; Man et al. 2012). Unfortunately, the significant experimental challenges presented by working with primary HBMEC’s (Naik and Cucullo 2012) limit the application of this powerful experimental tool. Here, we report that immortalized HCMEC/D3 maintain the properties of primary HBMEC’s in an in vitro model of trans-BBB immune migration. Using this cell line in model BBB’s will significantly increase the feasibility of investigating important neuroimmunological processes, including inflammation during CNS autoimmunity or infectious disease.
Highlights.
We validate the use of an immortalized blood brain barrier cell line for use in studies of immune migration.
The HCMEC/D3 endothelial cell line maintains the properties of primary cells in immune transmigration assays.
HCMEC/D3 will be a powerful tool for high-throughput studies of immune trafficking at the blood brain barrier.
Acknowledgments
This work was supported by NIH grant R01 NS052632 and grants from the Multiple Sclerosis Society, all to RSK. BPD was supported by a National Science Foundation Graduate Research Fellowship (DGE-1143954), LCO was supported by a Ruth L. Kirschstein Postdoctoral NRSA award (1F32NS0748424-01), and TLD was partially supported by NIH grant R01 AI078795. The funders had no role in the design, performance, analysis, or decision to publish this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Bachmeier C, Mullan M, Paris D. Characterization and use of human brain microvascular endothelial cells to examine beta-amyloid exchange in the blood-brain barrier. Cytotechnology. 2010;62(6):519–529. doi: 10.1007/s10616-010-9313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208(2):327–339. doi: 10.1084/jem.20102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28(2):312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48(3):505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25(1):59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CM, Darland DC, Massingham LJ, D’Amore PA. Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res. 2004;152(1):25–38. doi: 10.1016/j.devbrainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gobel K, Pankratz S, Schneider-Hohendorf T, Bittner S, Schuhmann MK, Langer HF, Stoll G, Wiendl H, Kleinschnitz C, Meuth SG. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. J Autoimmun. 2011;36(2):106–114. doi: 10.1016/j.jaut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199(2):223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist S, Renftel M. The use of in vitro cell culture models for mechanistic studies and as permeability screens for the blood-brain barrier in the pharmaceutical industry--background and current status in the drug discovery process. Vascul Pharmacol. 2002;38(6):355–364. doi: 10.1016/s1537-1891(02)00203-3. [DOI] [PubMed] [Google Scholar]
- Man S, Tucky B, Cotleur A, Drazba J, Takeshita Y, Ransohoff RM. CXCL12-induced monocyte-endothelial interactions promote lymphocyte transmigration across an in vitro blood-brain barrier. Sci Transl Med. 2012;4(119):119ra114. doi: 10.1126/scitranslmed.3003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008;105(32):11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci. 2012;101(4):1337–1354. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61(1):130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol. 2003;62(6):593–604. doi: 10.1093/jnen/62.6.593. [DOI] [PubMed] [Google Scholar]
- Poller B, Gutmann H, Krahenbuhl S, Weksler B, Romero I, Couraud PO, Tuffin G, Drewe J, Huwyler J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem. 2008;107(5):1358–1368. doi: 10.1111/j.1471-4159.2008.05730.x. [DOI] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117(8):2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19(13):1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]