Abstract
Proliferating murine C2C12 myoblasts can undergo either terminal differentiation or programmed cell death under conditions of mitogen deprivation. Unlike myoblasts, differentiated myotubes were resistant to apoptosis. During myogenesis the appearance of the apoptosis-resistant phenotype was correlated with the induction of the cyclin-dependent kinase (Cdk) inhibitor p21CIP1 but not with the appearance of myogenin, a marker expressed earlier in differentiation. Forced expression of the Cdk inhibitors p21CIP1 or p16INK4A blocked apoptosis during myocyte differentiation. These data indicate that induction of Cdk inhibitors may serve to protect differentiating myocytes from programmed cell death as well as play a role in establishing the postmitotic state.
Differentiation of C2C12 myocytes is induced when cultures are shifted to medium containing low concentrations of mitogens (differentiation medium). During this process myoblasts withdraw permanently from the cell cycle, express muscle-specific structural proteins, and fuse into multinucleated myotubes (I). The induction of the Cdk inhibitor p21CIP1 (2) and the hypophospho-rylation of the retinoblastoma protein (Rb) (3) are events that appear to be important in establishing the postmitotic state.
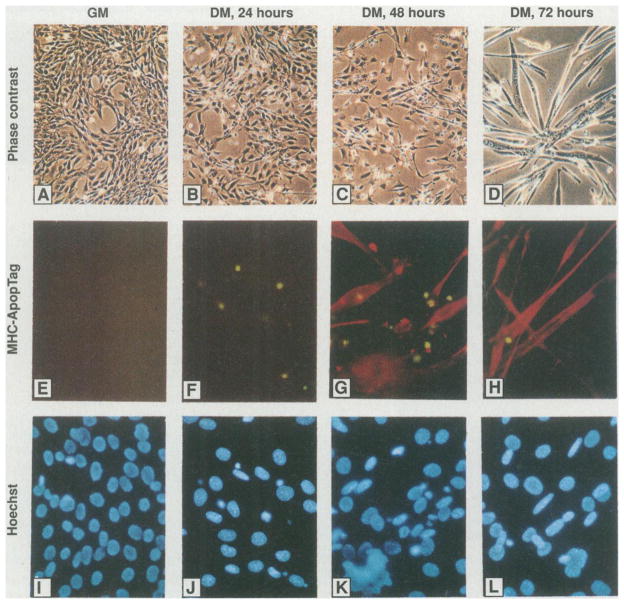
Growth factor withdrawal induces programmed cell death in various cell types (4). Extensive cell death was noted in cultures of C2C12 cells exposed to differentiation medium containing 2% horse serum (Fig. 1, A through D). Apoptosis was indicated by positive staining with the digoxi-genin–deoxyuridine 5′-triphosphate (dUTP) terminal dioxynucleotide transferase method (ApopTag, green stain in Fig. 1, E through H). These same cells also displayed cell shrinkage and condensed chromatin (Fig. 1, I through L), features characteristic of apoptosis. Cell death became evident 24 hours after the cells were changed to differentiation medium, but maximal cell death occurred after 48 hours. (Visual examinations revealed that about 20 to 30% of the cells appeared to be undergoing cell death after 48 hours.) After 72 to 96 hours, myotubes became abundant and cell death was diminished (Fig. 1, C, D, G, and H). DNA prepared from the floating C2C12 myocytes showed the typical nucleosome spacing ladder indicative of apoptosis upon agarose gel electrophoresis (Fig. 2). Differentiated C2C12 myotubes, which expressed skeletal myosin heavy chain (MHC) protein, were not stained with ApopTag (Fig. 1, G and H) and did not display DNA fragmentation (Fig. 2). C2C12 myotubes remained viable in differentiation medium for more than 2 weeks. Thus, under conditions of mitogen deprivation, a fraction of myoblasts proceed with their differentiation program and form myotubes, whereas other myoblasts undergo programmed cell death.
Fig. 1.
Induction of either apoptosis or terminal differentiation in C2C12 myocytes cultured in differentiation medium (DM). Proliferating C2C12 myoblasts in growth medium (GM) were shifted to differentiation medium for 24, 48, or 72 hours. (A through D) Phase contrast photomicroscopy revealed morphological changes. Floating cells were most evident in the DM 24- and 48-hour cultures. Multinucleated myotubes were detected in the DM 48-hour cultures and were predominant in the DM 72-hour cultures. (E through H) Double immunostaining (14) of C2C12 cells at different time points for apoptosis (ApopTag, green) and a muscle differentiation marker (MHC, red). (I through L) Hoechst dye staining of the same fields as in (E) through (H). Most of the ApopTag-positive cells [in (F) and (G)] also displayed condensed chromatin and cell shrinkage, which are characteristic of apoptosis. Magnification was × 150 for (A) through (D) and ×300 for (E) through (L).
Fig. 2.
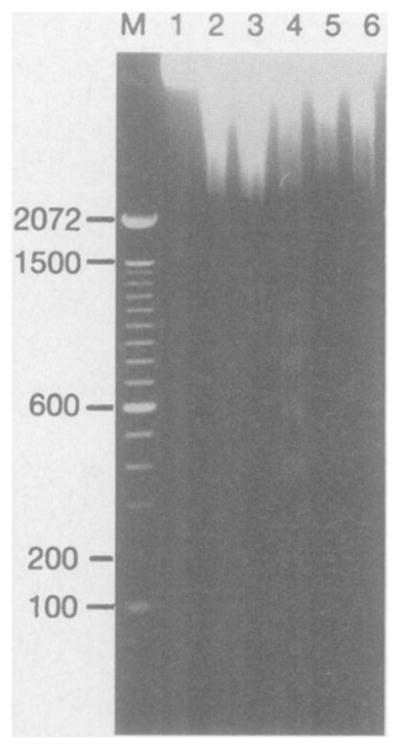
Electrophoresis of DNA isolated from C2C12 cells at different time points during differentiation. C2C12 myocytes at various time points in DM were collected, and genomic DNA was extracted and separated by electrophoresis on a 1.5% agarose gel. Lane 1, myoblasts grown in GM; lane 2, all cells (floating and attached) from cultures incubated for 24 hours in DM; lane 3, all cells after 48 hours in DM; lane 4, floating cells from cultures after 48 hours in DM; lane 5, adhesive cells from cultures after 48 hours in DM; lane 6, all cells at 72 hours in DM; M, molecular size marker lane with sizes indicated in base pairs.
Previous work showed induction of the Cdk inhibitor p21CIP1 during myocyte terminal differentiation (2). To investigate the relation between p21CIP1 induction and apoptosis during myogenic differentiation, we exposed C2C12 myocytes to differentiation medium for different times and then simultaneously immunostained these cells for p21CIP1 and for ApopTag. Throughout this time course, cells expressing p21CIP1 were largely unstained by ApopTag (Fig. 3, A through C). However, 16 ± 3.9% of the cells that did not express p21CIP1 were stained by ApopTag after 24 hours in differentiation medium, and the fraction of the p21-negative cells that stained positive for ApopTag increased with time (Fig. 3D). In contrast to p21CIP1, no correlation was found between cell viability and expression of the basic helix-loop-helix protein myogenin. Myogenin expression occurs early in myoblast differentiation, before the induction of p21CIP1 and cell cycle withdrawal (5). At 24 and 48 hours in differentiation medium, a substantial portion of myogenin-positive cells were also ApopTag-positive (Fig. 3, D through G). At 72 hours a smaller fraction of myogenin-expressing cells stained positive with ApopTag because many of these cells also become p21-positive and postmitotic as differentiation proceeds (5). These results indicate that p21CIP1 induction, but not an earlier step marking the commitment to terminal differentiation, is correlated with the acquisition of the apoptosis-resistant phenotype.
Fig. 3.
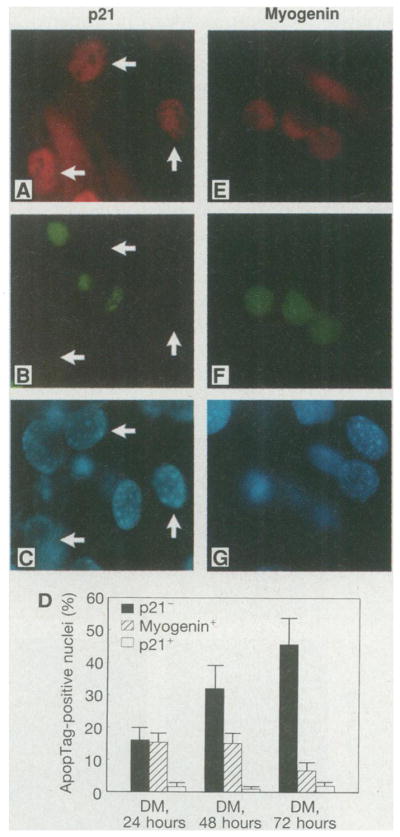
Correlation of myogenesis-induced p21CIP1 expression with the acquisition of the apoptosis-resistant phenotype. (A) Immunostaining for p21CIP1 of C2C12 cells cultured in DM for 48 hours. (B) ApopTag staining of the same field as in (A). (C) Hoechst dye staining of the same field as in (A). Arrows indicate cells that displayed elevated expression of endogenous p21CIP1. (D) Percentage of p21−, p21+, or myogenin+ cells that stained with ApopTag at different time points during C2C12 myocyte differentiation. In three separate experiments 200 cells were analyzed for each condition. The total percentages of cells that were p21 -positive at 24, 48, and 72 hours in DM were 8, 30, and 49%, respectively. (E) Immunostaining for myogenin in C2C12 cells cultured in DM for 48 hours. (F) ApopTag staining of the same field as in (E). (G) Hoechst dye staining of the same field as in (E). Myogenin-positive cells that are positive or negative for ApopTag are shown (15). Fields were chosen to illustrate phenotypic differences but not relative frequencies (magnification, ×800).
To test directly whether p21CIP1 expression can inhibit apoptosis, we transiently transfected C2C12 myoblasts with a mammalian expression vector containing p21CIP1 (pCDNA3-p21) or a mutant form of p21CIP1 (pCDNA3-p21C) that lacks the NH2-terminal Cdk-binding domain and is inactive as a Cdk inhibitor (6). Two days after transfection, cultures were exposed for 16 hours to medium containing 0.5% horse serum, which rapidly induces the onset of apoptosis. Endogenous p21CIP1 was not induced at this early time point (5, 7), but the ectopically expressed p21 or the mutant p21C was detected by immunostaining. Few cells expressing exogenous p21CIP1 stained positive for ApopTag (Fig. 4, A through C and G). In comparison, 24 ± 3.9% of cells expressing p21C were positive for ApopTag (Fig. 4, D through G). This frequency of apoptosis-positive cells was similar to that observed in nontransfected cultures. These data were corroborated by flow cytometric analyses on parallel cultures, which revealed 18% hypodiploid (apoptotic) cells in nontransfected cultures; cells positive for ectopically expressed p21 or p21C were 1.8 or 15.5% hypodiploid, respectively (8). The CDK4 inhibitor p16INK4A is expressed in low amounts in C2C12 cells and is not induced in differentiated myo-tubes (7). The forced expression of p16INK4A also decreased the frequency of apoptotic cells to 4.2 ± 3.1% as determined by ApopTag staining (Fig. 4G).
Fig. 4.
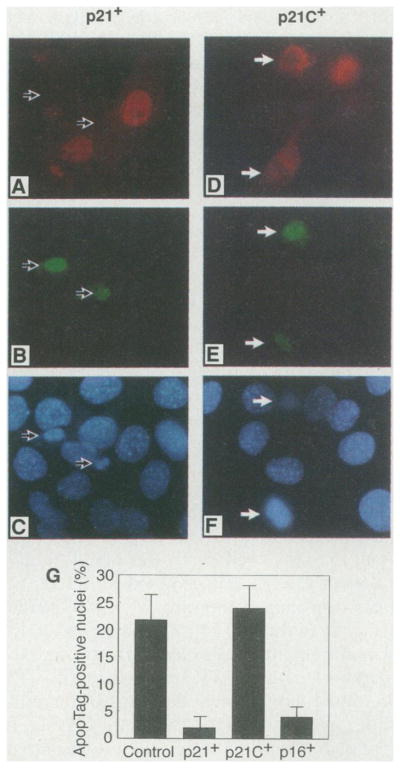
Inhibition of apoptosis in C2C12 myocytes expressing exogenous Cdk inhibitors. C2C12 myocytes grown on cover slips were transiently transfected with the wild-type p21 expression vector pCDNA3-p21 (A through C) or the mutant p21 expression vector pCDNA3-p21 C (D through F). Two days after transfection, cells were shifted to medium containing 0.5% serum for 16 hours to induce apoptosis and subsequently stained for p21 (A) or mutant p21 C (D), ApopTag [(B) and (E)], and Hoechst dye [(C) and (F)]. Medium containing 0.5% serum instead of 2% serum was used in these experiments because it induces apoptosis more rapidly in these cultures. Arrows indicate the ApopTag-positive cells. Fields were chosen to illustrate phenotypic differences but not relative frequencies (magnification ×800). (G) Percentage of cells expressing exogenous p21 (p21+), p21C (p21C+), or p16 (p16+) that stained positive for ApopTag. The percentage of ApopTag-positive cells in nontransfected, parallel cultures of C2C12 cells is also shown (control). For each condition four separate cover slips containing between 100 and 200 positive cells were analyzed.
These data show that Cdk inhibitors can also function as inhibitors of programmed cell death. A large fraction of cells are lost through apoptosis during in vitro myogen-esis. Mitotic cells committed to terminal differentiation (myogenin-positive and p21-negative) and noncommitted cells are susceptible to apoptosis. However, the subsequent induction of p21CIP1 is correlated with the acquisition of the apoptosis-resistant phenotype, and the ectopic expression of p21CIP1 protects differentiating myocytes from apoptosis. Similarly, ectopic expression of p16INK4A also protects differentiating myocytes from death. Because p16NK4A is specific for the CDK4 and CDK6 Rb kinases (9), these data also suggest that Rb may mediate the survival effects of the Cdk inhibitors. Consistent with this hypothesis is the observation that differentiated Rb−/− myotubes are highly susceptible to apop-totic cell death (7). Though the specific links between cell cycle control and apoptosis are currently unknown, the data presented here are consistent with observations that apoptosis in other cell types is associated with deregulated Cdk activity or can be inhibited by Rb overexpression (10). Cdk inhibitor expression may also influence myocyte survival in embryos, in which it is observed that cells in somites show patterns of death that depend on their location and stage of development (11). Because many nonmyocyte cell lines also induce Cdk inhibitors as they differentiate (12), the regulation of these molecules during development may be a general mechanism that influences whether a cell dies or continues with its differentiation program.
Acknowledgments
We thank E. Cosgrove for the CC42 myocytes, A. Dutta for pCDNA3-p21 and pCDNA3-p21C, G. Hannon for pCMV-p16INK4A, L. Zhu for pCMV-CD20, and W. Wright for antibody to myogenin. Supported by NIH grants HL50692 and AR40197.
Footnotes
Note added in proof: Recently, antisense oligonucleotides to p21CIP1 were shown to enhance cell death in differentiating neuroblastoma cells (13).
REFERENCES AND NOTES
- 1.Stockdale FE, Holtzer M. Exp Cell Res. 1961;24:508. doi: 10.1016/0014-4827(61)90450-5. [DOI] [PubMed] [Google Scholar]; Nadal-Ginard B. Cell. 1978;15:855. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- 2.Guo K, Wang J, Andres V, Smith R, Walsh K. Mol Cell Biol. 1995;15:3823. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]; Halevy O, et al. Science. 1995;267:1018. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]; Parker SB, et al. :1024. ibid. [Google Scholar]
- 3.Gu W, et al. Cell. 1993;72:309. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 4.Collins M, Lopez Rivas A. Trends Biochem Sci. 1993;18:307. doi: 10.1016/0968-0004(93)90042-l. [DOI] [PubMed] [Google Scholar]; Williams G, Smith C. Cell. 1993;74:777. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]; Evan G, et al. 1992;69:119. ibid. [Google Scholar]; Tomei LD, Shapiro JP, Cope FO. Proc Natl Acad Sci USA. 1993;90:853. doi: 10.1073/pnas.90.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres V, Walsh K. J Cell Biol. 1996;132:657. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Jackson PK, Kirschner MW, Dutta A. Nature. 1995;374:386. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Walsh K. unpublished data. [Google Scholar]
- 8.Flow cytometry analysis of apoptosis was performed as described [ Zhu L, et al. Genes Dev. 1993;7:1111. doi: 10.1101/gad.7.7a.1111. Subconfluent cultures of murine C2C12 myocytes were transfected with 1 μg of pCMV-CD20 DNA plus 20 μg of pCDNA-p21 by the Lipo-fectamine method BRL-Life Technologies. Thirty-six hours after transfection, the growth medium was changed to medium containing 0.5% horse serum for 16 hours and both floating and adhering cells were collected. Cells were subsequently stained with mouse antibody to CD20 and fluorescein isothiocya-nate-conjugated antibody to mouse immunoglobulins Dako. Cells were then fixed in 70% ethanol, stained with propidium iodide, and analyzed on a Becton-Dickinson FACScan. A gate was set to select CD20-positive cells that were more than 20 times as bright as the negative untransfected cells. Propidium iodide staining was simultaneously recorded on the CD20-positive and CD20-negative subpopulations.
- 9.Serrano M, Hannon GI, Beach D. Nature. 1993;366:704. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]; Serrano M, Gomez-Lahoz E, DePinho RA, Beach D, Bar-Sagi D. Science. 1995;267:249. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- 10.Haas-Kogan DA, et al. EMBO J. 1995;14:461. doi: 10.1002/j.1460-2075.1995.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Haupt Y, Rowan S, Oren M. Oncogene. 1995;10:1563. [PubMed] [Google Scholar]; Shi L, et al. Science. 1994;263:1143. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]; Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. 1995;268:429. doi: 10.1126/science.7716549. ibid. [DOI] [PubMed] [Google Scholar]
- 11.Glücksmann A. Biol Rev. 1951;26:59. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]; Jeffs P, Osmond M. Anat Embryol. 1992;185:589. doi: 10.1007/BF00185618. [DOI] [PubMed] [Google Scholar]
- 12.Jiang HP, et al. Oncogene. 1995;9:3397. [Google Scholar]; Steinman RA, et al. :3389. ibid. [Google Scholar]; Macleod KF, et al. Genes Dev. 1995;9:935. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]; Lois AF, Cooper LT, Geng Y, Nobori T, Carson D. Cancer Res. 1995;55:4010. [PubMed] [Google Scholar]
- 13.Poluha W, et al. Mol Cell Biol. 1996;16:1335. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.C2C12 myocytes were maintained in Dulbecco’s modified eagle’s medium (DMEM) supplemented with fetal bovine serum (20%, growth medium), and myogenic differentiation was initiated by shifting sub-confluent cultures into differentiation medium [DMEM supplemented with horse serum (2%)]. For immunofluorescence microscopy, cells grown on sterile glass cover slips were fixed for 10 min with 2% paraformaldehyde (in phosphate-buffered saline) and permeabilized with 0.5% NP-40. After they were blocked for 5 min with bovine serum albumin (2%), cells were stained with a monoclonal antibody to skeletal MHC proteins (MF20) and rhodamine-conjugated antibody to mouse immunoglobulin G. The cells were then incubated with digoxigenin-dUTP terminal dioxynucleotide transferase mixture and subsequently stained with fluorescein-conjugated antibody to digoxigenin (ApopTag, Oncor), counter-stained with Hoechst 33258, and mounted. Specimens were examined and photographed with a Nikon Diaphot fluorescence microscope.
- 15.Rabbit polyclonal antibody to p21 was from Santa Cruz Biotech. This antibody raised to the COOH-terminal peptide of p21CIP1 recognizes both full-length p21 and p21 proteins truncated at the COOH-terminus. Monoclonal antibody to myogenin antibody was a gift of W. Wright [ Wright WE, Binder M, Funk W. Mol Cell Biol. 1991;11:4104. doi: 10.1128/mcb.11.8.4104.