Abstract
Background
Overexpression of adiponectin receptor 1 in macrophages can physiologically modulate metabolic activities in vivo by enhancing adiponectin actions in distal metabolically active tissues. To investigate the effects of enhanced adiponectin actions in TALLYHO (TH) diabetic mouse model, we crossed the adiponectin receptor 1 macrophage-specific transgenic mice (AdR1-TG) with the TALLYHO diabetic mice (TH) to examine the changes of lipid accumulation and insulin sensitivity in these mice.
Methods
AdR1-TG/TH and the control WT/TH mice were fed either normal diet or high fat diet for twenty-eight weeks. Whole body weights of these mice were measured and mouse sera were analyzed for the levels of cholesterol, triglyceride, and free fatty acids. Glucose tolerance testing (GTT) and insulin tolerance testing (ITT) in these mice were performed to investigate systemic insulin sensitivity in vivo. Molecular markers for insulin signaling pathway in mouse skeletal muscle tissues, IRS-1 and AKT, were examined. Mouse serum insulin levels were measured and Sirt1 gene expression in mouse pancreatic tissues was also quantified related to the insulin secretion. The Caspase 3 protein levels were analyzed by Western blot methods.
Results
Compared to the control WT/TH mice, AdR1-TG/TH mice showed significantly lower body weights under either normal diet or high fat diet and the mouse serum levels of cholesterol, triglyceride and free fatty acids were significantly decreased in the transgenic crossed mice when compared to those from the control mice. Improved GTT and ITT tests indicating increased systemic insulin sensitivity in the transgenic crossed mice demonstrated the enhanced adiponectin actions on the systemic metabolism in vivo. The increases of insulin secretion and its related gene expression were also detected in the transgenic crossed mice. In contrast, the control mice showed hypertrophy pancreases companying with high apoptosis gene expression. These results suggest that enhanced adiponectin actions by overexpressing adiponectin receptor 1 in macrophages can provide unique interactions with the metabolic tissues/cells, improving lipid accumulation and insulin sensitivity in TALLYHO diabetic mice.
Keywords: adiponectin receptor, TALLYHO mouse model, diabetes
1. Introduction
Animal models have been valuable resources and broadly used for diabetes research, including type 1 diabetes and type 2 diabetes [1]. The TALLYHO/JngJ (TH) mouse strain is one of the commercial available polygenic models for diabetes characterized by obesity, impaired glucose tolerance and uptake, insulin resistance, and hyperglycemia [2, 3]. Although recent studies have used this model to study diabetes [4–8], the TALLYHO mouse model has not yet been completely characterized for diabetes related other metabolic disorders [9, 10].
Adiponectin is one of several important, metabolically active cytokines secreted from adipose tissue [11–13]. Epidemiological evidence has indicated that plasma adiponectin levels are reduced in patients with insulin resistance, diabetes, obesity, or cardiovascular disease [14–16]. In the circulation, adiponectin exerts bioeffects on multiple cell types and has insulin-sensitizing, anti-inflammatory, and anti-atherosclerotic properties. Two cell-surface trans-membrane receptors have been identified for adiponectin, AdipoR1 and AdipoR2 [17], and adiponectin action is known to signal through these receptors and the docking protein APPL1 [18]. AdipoR1 is abundantly expressed in skeletal muscle and macrophages, whereas AdipoR2 is predominantly expressed in the liver [17].
Macrophages are a heterogeneous and plastic population of phagocytic cells, which arise from circulating myeloid-derived blood monocytes, enter target tissues, and gain phenotypic and functional attributes partly determined by their tissue of residence [19], however, adiponectin expression is undetectable in macrophages. To beneficially and systemically influence other active metabolic tissues/cells, we recently developed a novel approach to enhance adiponectin action at the level of the macrophage by genetic manipulation of the major receptor for adiponectin in macrophages, AdipoR1. As our data demonstrated, overexpression of AdipoR1 can alter macrophage biology and impact systemic metabolism in vivo [20].
Our current studies used the adiponectin receptor 1 macrophage-specific transgenic mice (AdR1-TG) to cross with the TALLYHO diabetic mice (TH) for generating AdR1-TG/TH transgenic crossed mice to investigate the effects of enhanced adiponectin actions in TALLYHO diabetic mice. The results suggest that enhanced adiponectin actions by overexpressing adiponectin receptor 1 in macrophages can provide unique interactions with the metabolic tissues/cells, improving lipid accumulation and insulin sensitivity in TALLYHO diabetic mice.
2. Methods
2.1. Experimental animals
To generate AdR1-TG/TH transgenic crossed mice, the macrophage AdipoR1 transgenic mice (AdR1-TG) and TALLYHO (TH) diabetic mice were inbred at our Transgenic Animal/Embryonic Stem Cell (TA/ESC) Core Facility at the University of Alabama at Birmingham. All of the confirmed transgenic crossed mice were confirmed by the skin color and genotyping. As the control mice, we also generated the WT/TH mice by crossing wild-type mice with the TALLYHO diabetic mice for our experiments. All of these animals were housed in a specific pathogen-free facility with 12-hours light/dark cycles and received a standard laboratory chow diet except for the high fat diet experiments. Only male mice were used for the experiments. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Animal Resources Program (ARP) at the University of Alabama at Birmingham.
2.2. Determination of cholesterol, triglyceride, and free fatty acid concentrations in mouse plasma
Control and transgenic crossed mice were fed with a high fat diet (60% kcal% fat) from the Research Diets Company (New Brunswick, NJ) for twenty weeks before measuring and analyzing the concentrations of cholesterol, triglyceride, and free fatty acids in mouse plasma. The levels of cholesterol, triglyceride, and free fatty acids were determined using enzymatic colorimetric assays (Wako, Richmond, VA) according to the manufacturer’s protocols.
2.3. Gene expression in pancreatic tissues and plasma concentrations of insulin
To determine the expression levels of Sirt1 gene related to insulin secretion in mouse pancreatic tissues, total RNA was extracted from control WH/TH and AdR1-TG/TH transgenic crossed mouse pancreatic tissues using a commercially available TRIzol reagent from Invitrogen (Carlsbad, CA) according to the manufacturer’s instructions. The quantitative real-time PCR analysis was using an ABI StepOnePlus Real-Time PCR System. Reactions were carried out in triplicate in a total volume of 20µl using and a SYBR Green QPCR Master Mix (Applied Biosystems). Quantification was calculated using the starting quantity of the cDNA of interest relative to that of 18S ribosomal cDNA in the same sample.
The insulin levels in mouse plasma were measured using an ultra sensitive mouse insulin ELISA kit from Crystal Chem INC (Downers Grove, IL) according to the protocols from the manufacturer.
2.4. Glucose and insulin tolerance testing
Glucose tolerance testing (GTT) and insulin tolerance testing (ITT) in AdR1-TG/TH transgenic crossed and control WT/TH mice were performed as described previously [21]. Mice were injected with glucose or insulin at 16 or 20 weeks of age after consuming the high fat diet. To determine glucose tolerance, animals were first fasted overnight and then given an intraperitoneal injection of glucose solution (100g glucose/L; 10µl/g body weight) and glucose concentration was determined in mouse tail blood collected at baseline (prior to injection), and at 30, 60, 90, 120 and 150 minutes post-injection using a HemoCue glucose 201 glucometer (HemoCue USA). To determine insulin tolerance, mice were fasted for 6 hours in the morning of the measurement and then administered an intraperitoneal injection of insulin solution (1.5U insulin/kg body weight). Glucose levels were measured in blood samples collected as above described for the glucose tolerance testing.
2.5. Western blot analysis
For Western blot analysis, mouse tissues, including pancreas and skeletal muscle, were homogenized in tissue lysis buffer containing freshly added protease inhibitor cocktail (Sigma). Cell lysate proteins (25 µg protein) were separated by SDS-polyacrylamide gel electrophoresis and then electrophoretically transferred onto nitrocellulose membranes and incubated overnight at 4°C with blocking solution (5% nonfat milk in TBS). The blocked membranes were separately incubated with the specific antibodies of Caspase 3 (Abcam), AKT or P-AKT (Santa Cruz), and IRS-1 or P-IRS-1 (Cell Signaling) (1:5000 dilutions with 1% nonfat milk in TBS) for 1 hour at room temperature, and washed three times with TBS buffer containing 0.1% Tween 20 for 15 min at room temperature with shaking. The secondary antibody conjugated to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA) against to the primary antibody was added, incubated, and washed as described above for the first antibody. Immunoreactive protein bands were detected using the Enhance Chemiluminescence Kit (New England Nuclear Life Science Products, Boston, MA).
2.6. Statistics
Experimental results are reported as the mean ± SEM. Statistical analyses were conducted using the unpaired Students’s t-test assuming unequal variance unless otherwise indicated. Significance was defined as the p < 0.05.
3. Results
3.1. Weight reduction of whole body in AdR1-TG/TH mice
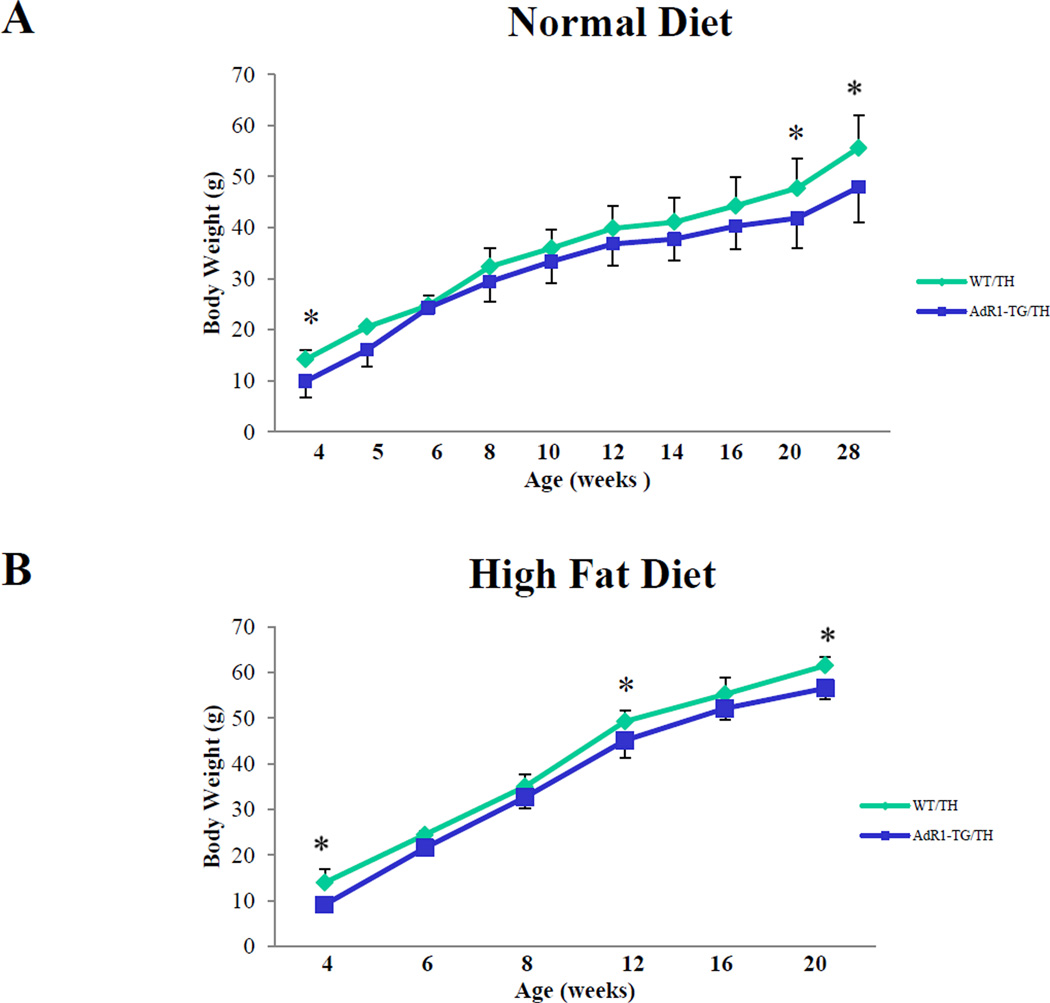
We assessed whether growth and development were affected in AdR1-TG/TH transgenic crossed mice compared to the control WT/TH mice. There were no significant differences in reproduction, food consumption, or development between the control and transgenic crossed mice when these animals were fed normal chow or the high fat diet. However, compared to WT/TH mice, AdR1-TG/TH mice had significantly lower body weights when these mice fed with either normal diet or high fat diet (Figure 1A and B).
Figure 1. AdR1-TG/TH mice have reduced body weights.
The weights of mouse bodies of control wild-type/TALLYHO (WT/TH) and AdipoR1 transgenic/TALLYHO (AdR1-TG/TH) mice fed a normal diet (A) or a high fat diet (60% kcal% fat) (B) for 20 to 28 weeks; n=12 for each mouse group, *p < 0.05.
3.2. Decreased lipid levels in AdR1-TG/TH mouse plasma
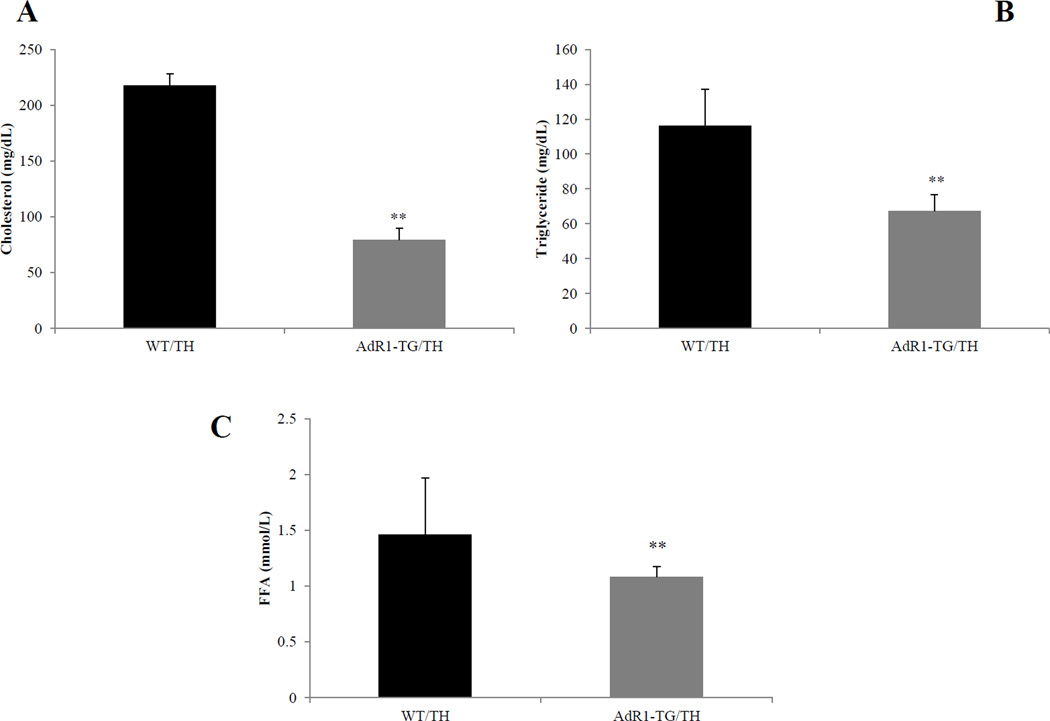
Considering that the metabolic changes in cells/tissues in the AdR1-TG/TH mice would probably cause plasma lipid level changes in these mice, we next decided to measure the lipid levels in these mice. To quantitatively measure the concentrations of cholesterol, triglyceride, and free fatty acids in plasma of control WT/TH and AdR1-TG/TH mice, enzymatic colorimetric assays were performed with plasma from control WT/TH and AdR1-TG/TH mice. As shown in Figure 2A, B and C, the data indicated that cholesterol levels in AdR1-TG/TH mice were on average 68% (p < 0.01) lower than those from WT/TH mice; triglyceride levels in AdR1-TG/TH mice were on average 42% (p < 0.01) and free fatty acids in AdR1-TG/TH mice were on average 21% (p < 0.01) lower than those from the control WT/TH mice.
Figure 2. Lipid accumulation in mouse plasma.
Plasma from 20 weeks of age control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice fed with the high fat diet was analyzed for the contents of cholesterol (A), triglyceride (B), and free fatty acids (C) using enzymatic colorimetric kits from Wako Company. Mean ± SEM from three separate experiments with triplicate samples (n=10 for each group) were presented, **p < 0.01.
3.3. Improved glucose tolerance and insulin sensitivity in AdR1-TG/TH mice
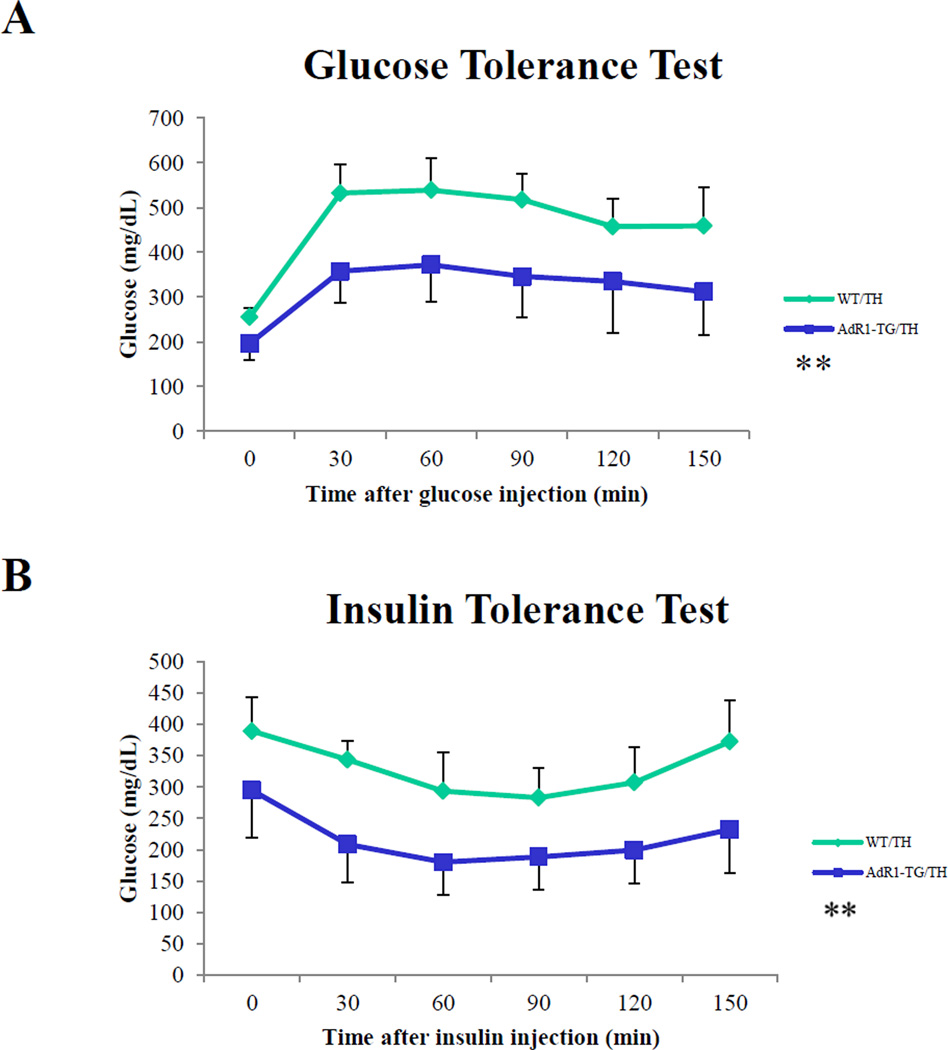
To investigate systemic insulin sensitivity in vivo, we performed glucose tolerance tests (GTT) and insulin tolerance tests (ITT) in the control and transgenic mice fed with high fat diet for 16 to 20 weeks. Plasma glucose levels were consistently higher (p < 0.05) during the glucose tolerance tests in the control WT/TH animals compared to the levels in AdR1-TG/TH transgenic crossed mice (Figure 3A). As shown in Figure 3B, in the control WT/TH mice, the plasma glucose levels were also consistently higher (p < 0.05) when insulin was injected for the insulin tolerance tests than the levels in the AdR1-TG/TH transgenic crossed mice.
Figure 3. Mouse glucose tolerance tests (GTT) and insulin tolerance tests (ITT).
(A) Glucose tolerance tests were performed on control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice fed with high fat diet for 16 weeks. Experiments were performed in fasting overnight male mice. Glucose solutions were injected into peritoneal cavity at the dose of 1.0 ml/kg (1M solution). Blood was collected via tail vein at the indicated time points. Glucose concentration in plasma was measured using a glucometer (Precision); n = 10 in each group of mice. (B) Insulin tolerance tests were performed on the control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH mice under high fat diet condition for 20 weeks. Experiments were conducted similar as the described above for the glucose tolerance tests but fasting 6 hours before the injections and insulin solutions were injected into peritoneal cavity at the dose of 0.5 U/kg. Blood was collected via tail vein at the indicated time points, and glucose levels were measured; n = 10 in each group of animals, **p < 0.01.
3.4. Increased insulin signaling pathway in AdR1-TG/TH mouse skeletal muscle
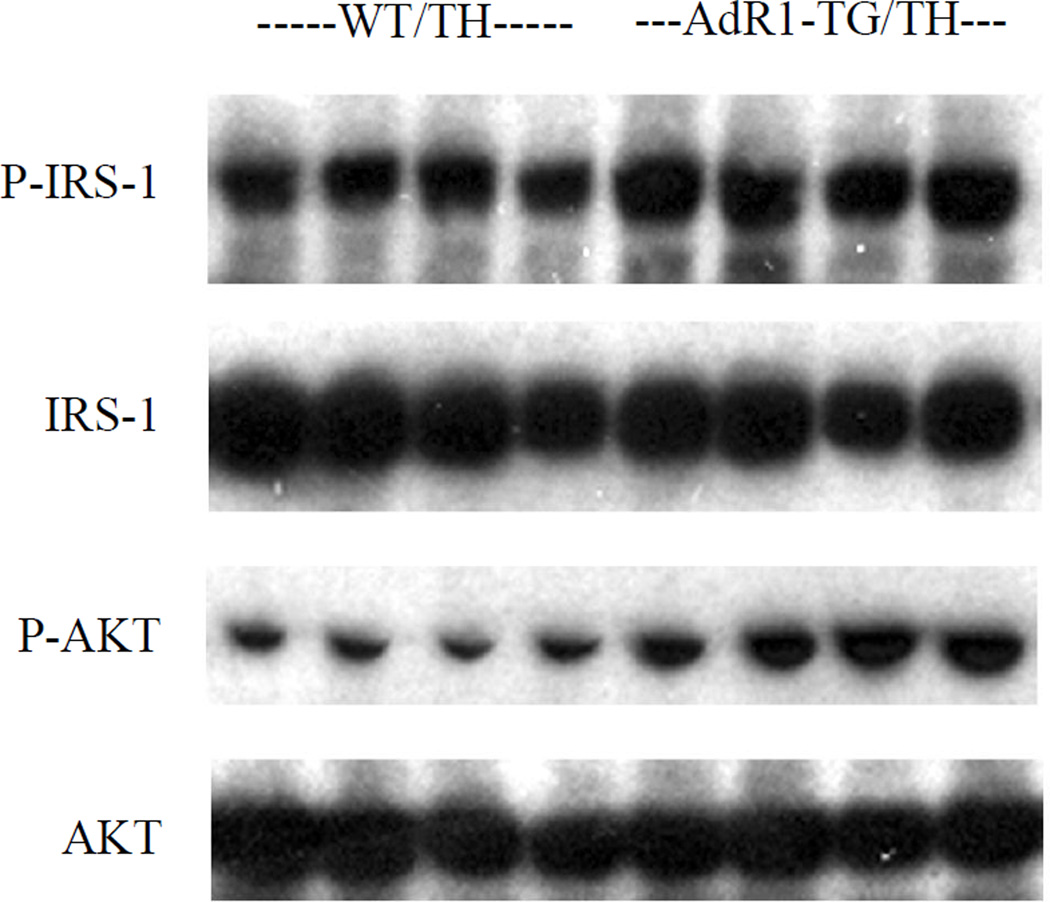
To further investigate the role of AdipoR1 in glucose homeostasis, we assessed two molecular markers for insulin signaling pathway in the mouse skeletal muscle tissues. IRS-1 and Akt phosphorylations were assessed under insulin stimulated conditions in skeletal muscle tissues from control WT/TH and AdR1-TG/TH transgenic crossed mice fed the high fat diet for 20 weeks (Figure 4). Serine phosphorylations of IRS-1 (Ser302) and Akt (Ser473) were enhanced with insulin-stimulation in the AdR1-TG/TH transgenic crossed mouse skeletal muscles when compared to those from the control WT/TH animals.
Figure 4. Insulin signaling pathways in mouse skeletal muscle.
Insulin-stimulated muscle tissue strips which sampled from control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice at 20 weeks of age under the high fat diet were performed in vitro. Dissected skeletal muscle strips from control (WT/TH) or AdipoR1 transgenic crossed (AdR1-TG/TH) were treated with 100 nM insulin for 30 min and the total proteins were extracted for immunoblot analyses using antibodies against phosphorylated IRS-1 (Ser302) or total IRS-1 (Cell Signaling), and phosphorylated Akt (Ser473) or total Akt (Santa Cruz). Results representd one of the three separate experiments.
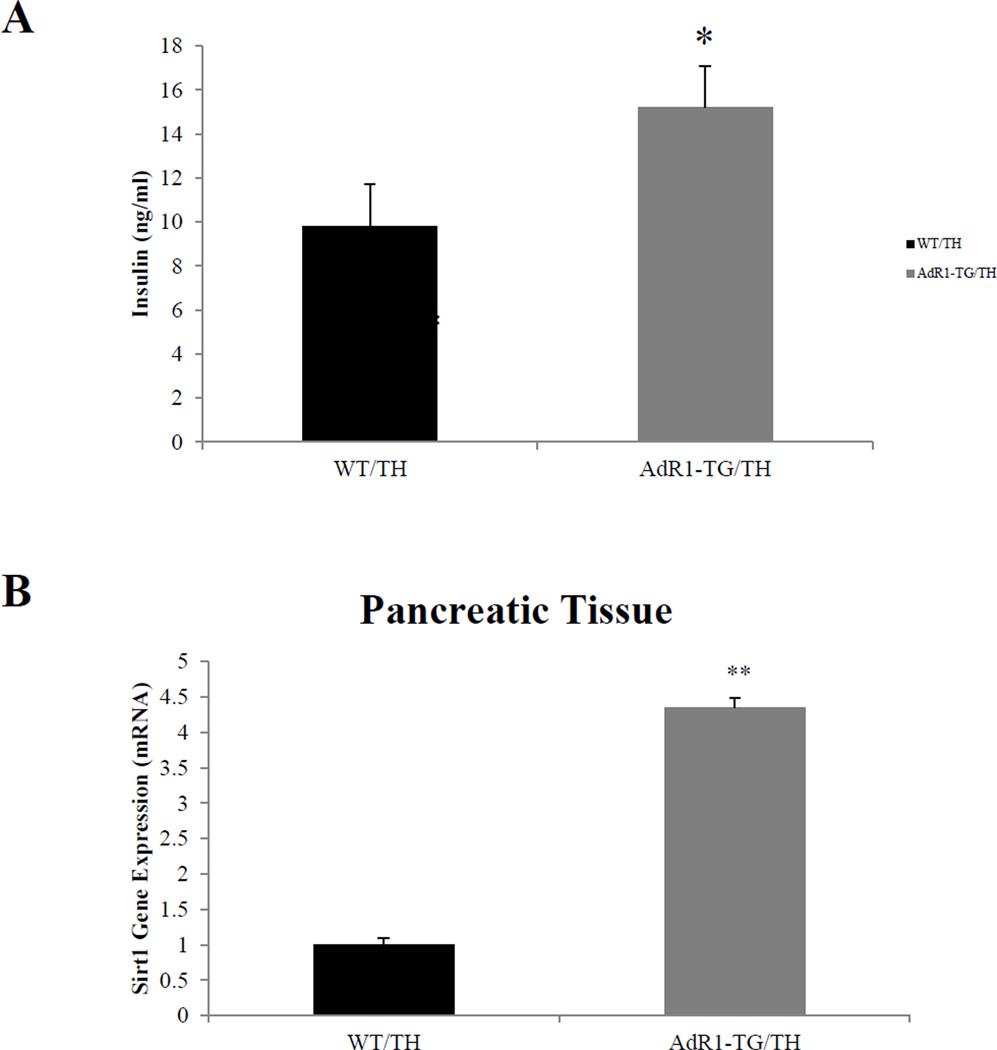
3.5. Mouse plasma insulin concentrations and gene expression in pancreatic tissues
Plasma insulin levels at baseline in AdR1-TG/TH transgenic crossed mice fed with the high fat diet were significantly higher (average 38%, p < 0.05) than the insulin levels in control WT/TH mice (Figure 5A). We further examined the gene expression levels of Sirt1 which is related to insulin secretion in mouse pancreatic tissues. In mouse pancreas tissues, the data (Figure 5B) showed that expression of Sirt1 gene in AdR1-TG/TH transgenic crossed mice was significantly higher (average 3.5 fold, p < 0.01) than those observed in the control WT/TH mice.
Figure 5. Measurement of plasma insulin levels and analysis of gene expression in pancreatic tissues.
(A) Insulin levels in plasmas from control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice under the high fat diet condition for 20 weeks were measured using an ultra sensitive mouse insulin ELISA kit. Error bars represented the ± SEM, *p < 0.05. (B) Sirt1 gene expression in pancreatic tissues/cells from the control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice were examined by using QPCR analysis. Mean ± SEM from three separate experiments with triplicate samples (n=12 for each group) were examined, **p < 0.01.
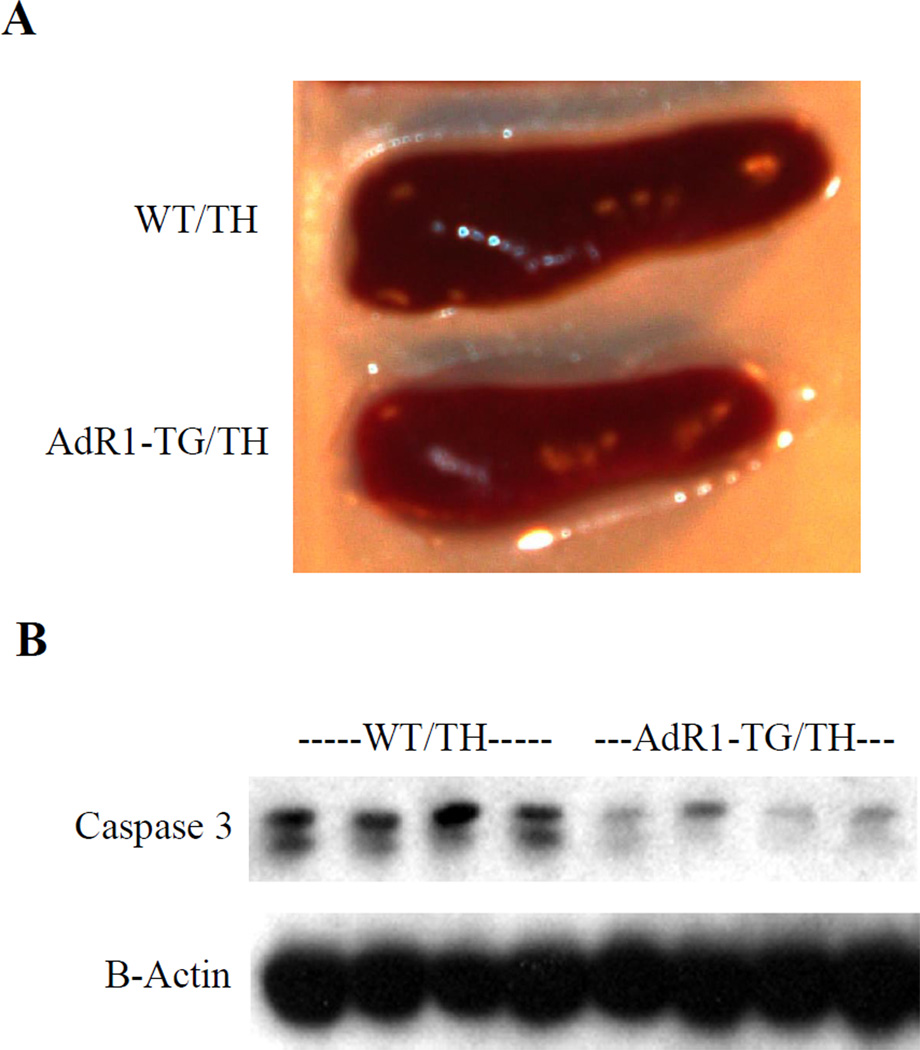
3.6. Decreased pancreatic hypertrophy and apoptotic gene expression in AdR1—TG/TH mice
To investigate morphological changes of pancreases in mice, we next examined mouse pancreases by dissecting these organs from control WT/TH and AdR1-TG/TH mice. We observed a much larger pancreas in WT/TH mice than AdR1-TG/TH transgenic crossed mice (Figure 6A). Tissue sections detected with insulin antibody showed a morphological hypertrophy structure in WT/TH mice (data not shown). When we analyzed the molecular marker of apoptosis, Caspase 3, for these mouse pancreases, the results of Caspase 3 protein levels revealed significantly increased in the control WT/TH mouse pancreases than those from AdR1-TG/TH transgenic crossed mice (Figure 6B). These data suggest that more functional pancreatic cells in AdR1-TG/TH transgenic crossed mice than those from the control WT/TH animals.
Figure 6. Analysis of mouse pancreas and apoptotic gene expression in in pancreatic tissues.
(A) Pancreases from the Control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mice (n=12 for each group) fed with the high fat diet were dissected and examined for the organ sizes under microscope. (B) Total proteins were extracted from control (WT/TH) and AdipoR1 transgenic crossed (AdR1-TG/TH) mouse pancreatic cells/tissues for immunoblot analyses using antibodies against Caspase 3 (Abcam) and β-actin (Santa Cruz). Results representd one of the three separate experiments.
4. Discussion
The TALLYHO/JngJ (TH) mouse model is an inbred polygenic model for type 2 diabetes characterized by moderate obesity, impaired glucose tolerance and uptake, insulin resistance, and male limited hyperglycemia. Since scientists in the Jackson Laboratory established a research colony of the TH mice by selective inbreeding based on the hyperglycemia phenotype of the TH male mice [2, 3], this inbred polygenic mouse model has been commercially available for diabetes and other metabolic disease research [4–8]. The TH mouse is a naturally occurring model of obesity and type 2 diabetes derived from selective breeding of mice that spontaneously developed hyperglycaemia and hyperinsulinaemia in an outbred colony of Theiler original mice [3]. In these mice, adiposity is increased, and plasma triglycerides, cholesterol and free fatty acid levels are elevated. However, hyperglycaemia is only limited to male mice, which develops as early as between 10 and 14 weeks of age. The TH mouse has not yet been completely characterized for diabetic complications [9], although a recent study has used this model to study diabetic wound healing [8].
Adiponectin is naturally expressed and secreted exclusively from adipocytes, and adiponectin levels in circulation have recently been reported to highly associate with human metabolic diseases [22–25]. For adiponectin actions, adiponectin receptors play pivotal roles in many metabolic tissues [26]. Although adiponectin expression is undetectable in macrophages, adiponectin has been demonstrated to have effects on inhibiting both the inflammatory process and lipid accumulation in macrophages/foam cells [27–29]. Therefore, adiponectin receptors play key roles for adiponectin actions on macrophages. To investigate the mechanisms of adiponectin receptor-mediated alterations of systemic metabolism in vivo, we recently developed a mouse model in which the AdipoR1 gene was specifically overexpressed in macrophages based on that macrophages can actively circulate or infiltrate into other tissues/cells to influence the systemic metabolism in vivo [20].
Our studies from these AdR1-TG mice have shown that the adiponectin receptor “modified macrophages” circulate or infiltrate into other metabolically active tissues such as adipose and skeletal muscle tissues, as well as liver, and there they can enhance local adiponectin actins on these metabolically active tissues and influence favorable changes to multiple metabolic pathways in these tissues, contributing to increased glucose tolerance and improved insulin sensitivity in systemic metabolism in vivo.
To investigate whether the AdR1-TG mice can influence or rescue the diabetic symptoms in other diabetic mouse models, in the current studies, we crossed the AdR1-TG mice with TH diabetic mice to generate AdR1-TG/TH transgenic crossed mice. Our results showed that AdR1-TG/TH mice had significantly lower body weights when fed with either normal diet or high fat diet; and the mouse serum levels of cholesterol, triglyceride and free fatty acids were significantly decreased in the AdR1-TG/TH transgenic crossed mice when compared to those from the control WT/TH mice. Improved GTT and ITT data from the AdR1-TG/TH transgenic crossed mice also indicated the increased systemic insulin sensitivity in these mice.
Interestingly, AdR1-TG/TH mice also showed increased serum levels of insulin when compared to those of control WT/TH mice, probably resulted from the improved metabolic functions of those insulin targeting tissues/cells affected by the AdR1 modified macrophages, supporting evidence for this proposal including enhanced insulin signaling pathway, increased IRS-1 and AKT phosphorylations in skeletal muscle, and reduced pancreatic hypertrophy and apoptotic gene expression in the pancreatic tissues/cells. Thus, using macrophages as the carriers for in vivo enhanced actions of adiponectin can reduce the diabetic phenotypes or metabolic abnormality of the TALLYHO diabetic mice. Although additional studies are required and the potential for other cellular or molecular physiological effects among these metabolically active tissues should be further pursed in the future for these mouse models, our current results from these unique mouse models probably provide a new insight into the prevention and therapy of metabolic disorders in humans.
Highlights.
AdR1-TG/TH mouse model shows improved metabolism in insulin targeting tissues.
We examine lipid metabolism and insulin sensitivity under high fat diet condition.
AdR1-TG/TH mice are a unique animal model for studying metabolic disorders in vivo.
Acknowledgments
We are grateful to the UAB Diabetes Research and Training Center for providing outstanding core services (NIH P30 DK-56336). This work was supported by grants from American Diabetes Association (1-07-RA-49 and 1-13-IN-19) to YF, and grant from NIH (DK-083562) to TG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Sen S, Avery CS, Simpson E, Chandler P, Nishina PM, Churchill GA, Naggert JK. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74:273–286. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Stewart TP, Zhang W, Kim HY, Nishina PM, Naggert JK. Type 2 diabetes mouse model TallyHo carries an obesity gene on chromosome 6 that exaggerates dietary obesity. Physiol Genomics. 2005;22:171–181. doi: 10.1152/physiolgenomics.00197.2004. [DOI] [PubMed] [Google Scholar]
- 4.Sung YY, Lee YS, Jung WH, Kim HY, Cheon HG, Yang SD, Rhee SD. Glucose intolerance in young TallyHo mice is induced by leptin-mediated inhibition of insulin secretion. Biochem Biophys Res Commun. 2005;338:1779–1787. doi: 10.1016/j.bbrc.2005.10.160. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Stewart TP, Soltani-Bejnood M, Wang L, Fortuna JM, Mostafa OA, Moustaid-Moussa N, Shoieb AM, McEntee MF, Wang Y, Bechtel L, Naggert JK. Phenotypic characterization of polygenic type 2 diabetes in TALLYHO/JngJ mice. J Endocrinol. 2006;191:437–446. doi: 10.1677/joe.1.06647. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Nishina PM, Naggert JK. Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J Endocrinol. 2009;203:65–74. doi: 10.1677/JOE-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart TP, Kim HY, Saxton AM, Kim JH. Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice. BMC Genomics. 2010;11:713. doi: 10.1186/1471-2164-11-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck DW, Jin DP, Geringer M, Jong HS, Galiano RD, Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg. 2011;128:427–437. doi: 10.1097/PRS.0b013e31822b7333. [DOI] [PubMed] [Google Scholar]
- 9.Leiter EH. Selecting the ‘right’ mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol. 2009;560:1–17. doi: 10.1007/978-1-59745-448-3_1. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Saxton AM. The TALLYHO mouse as a model of human type 2 diabetes. Methods Mol Biol. 2012;933:75–87. doi: 10.1007/978-1-62703-068-7_6. [DOI] [PubMed] [Google Scholar]
- 11.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedionemediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 13.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating the risk of metabolic and cardiovascular disease. Curr Opinion Lipidology. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 14.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay RS, Funahashi T, Krakoff J, Matsuzawa Y, Tanaka S, Kobes S, Bennett PH, Tataranni PA, Knowler WC, Hanson R. Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes. 2003;52:2419–2425. doi: 10.2337/diabetes.52.9.2419. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 18.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 bonds to adiponectin receptors and mediates adiponectin signaling and function. Nat Cell Biology. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Luo N, Chung BH, Wang X, Klein RL, Tang C, Garvey WT, Fu Y. Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis. 2013 doi: 10.1016/j.atherosclerosis.2013.02.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhash M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker PA, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metabolism. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Oka T, Horiguchi J, Awai K, Kihara Y. Association between plasma high-molecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687–1696. doi: 10.1253/circj.cj-11-1442. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi T, Shibata R, Morimoto T, Kanashiro M, Ishii H, Ichimiya S, Hiro T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura T, Daida H, Murohara T, Matsuzaki M. Correlation between circulating adiponectin levels and coronary plaque regression during aggressive lipidlowering therapy in patients with acute coronary syndrome: subgroup analysis of JAPANACS study. Atherosclerosis. 2010;212:237–242. doi: 10.1016/j.atherosclerosis.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Broedl UC, Lebherz C, Lehrke M, Stark R, Greif M, Becker A, von Ziegler F, Tittus J, Reiser M, Becker C, Göke B, Parhofer KG, Leber AW. Low adiponectin levels are an independent predictor of mixed and non-calcified coronary atherosclerotic plaques. PLoS One. 2009;4:e4733. doi: 10.1371/journal.pone.0004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizza S, Gigli F, Galli A, Micchelini B, Lauro D, Lauro R, Federici M. Adiponectin isoforms in elderly patients with or without coronary artery disease. J Am Geriatr Soc. 2010;58:702–706. doi: 10.1111/j.1532-5415.2010.02773.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metabolism. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa K, Hori M, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Miyazaki A, Nakayama H, Horiuchi S. Adiponectin down-regulates acyl-coenzyme A: cholesterol acyltransferase-1 in cultured human monocyte-derived macrophages. Biochemical and Biophysical Research Communications. 2004;317:831–836. doi: 10.1016/j.bbrc.2004.03.123. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152–161. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]