Abstract
Background
It is unknown if cardiac ischemia has any deleterious effect on the contractile properties of nonischemic, peripheral vascular beds. Thus, the objective of the present study was to determine whether acute myocardial ischemia results in peripheral vascular dysfunction.
Methods and Results
This study characterized force maintenance and the sensitivity to ACh mediated smooth muscle relaxation of tertiary (3rd) mesenteric arteries from Sprague-Dawley rats following 30 min of myocardial ischemia. Both the phosphorylation of nonmuscle (NM) light chain (LC) and smooth muscle (SM) light chains (LCs) as well as the expression of myosin phosphatase targeting subunit 1 (MYPT1) were also determined. Our data demonstrate that acute myocardial ischemia resulted in vascular dysfunction of 3rd mesenteric vessels, characterized by decreases in force maintenance, ACh and cGMP mediated smooth muscle relaxation, the phosphorylation of NM-LCs and SM-LCs, and MYPT1 expression. Ischemia was also associated with an increase in protein poly-ubiquitination, suggesting that during ischemia the MYPT1 is targeted for degradation or proteolysis.
Conclusion
Acute myocardial ischemia produces peripheral vascular dysfunction; the changes in LC phosphorylation and MYPT1 expression result in a decrease in both tone and in the sensitivity to NO mediated smooth muscle relaxation of the peripheral vasculature.
Keywords: mesenteric artery, ACh, 8-Br-cGMP, MYPT1, nonmuscle myosin
INTRODUCTION
The effects of acute ischemia on the ischemic vascular bed include decreases in vascular tone and the sensitivity of smooth muscle to nitric oxide (NO) mediated vasodilatation [1, 2]. These changes in vascular reactivity occur within 30 min of ischemia and are thought to be mediated by an increase in the production of free radicals as well as interleukin (IL)-1 and tumor necrosis factor (TNF)-α. Recurrent ischemia often leads to heart failure (HF), which has been documented to be associated with an increase in peripheral vascular tone as well as a decrease in the sensitivity to NO mediated vasodilatation [1–4]. However, the effect of acute myocardial ischemia on the vasoreactivity of non-ischemic peripheral vascular smooth muscle has not been investigated.
Contraction of smooth muscle is dependent on the level of phosphorylation of the 20 kDa regulatory smooth muscle myosin light chain (SM-LC), which is determined by the relative activities of myosin light chain (MLC) kinase and MLC phosphatase [5–7]. However, recent evidence suggests that nonmuscle (NM) myosin also participates in the force maintenance phase of smooth muscle contraction, and hence the regulation of vascular tone [5–8]. In addition, NO mediated vasodilatation is considered a fundamental response of the vasculature [9], and changes in the sensitivity of NO mediated smooth muscle relaxation are produced, in part, by an alteration in the expression of myosin targeting regulatory subunit (MYPT1) of MLC phosphatase [10, 11], specifically by a decrease in the expression of the leucine zipper positive (LZ+) MYPT1 isoform [11–13].
This study was designed to determine the effect of 30 min of myocardial ischemia on the reactivity of tertiary (3rd) mesenteric arteries. Our data suggest that acute ischemia causes a dysregulation of SM and NM myosin, which results in a decrease in vascular tone, and a reduction in the expression of MYPT1, which attenuates the sensitivity of NO mediated vasodilatation.
MATERIALS AND METHODS
Rat acute ischemia model and mesenteric artery preparation
The Institutional Animal Care and Use Committee of the Mayo Clinic approved all experimental protocols and animal care, and the study conforms to the guidelines of the NIH. Our model of cardiac ischemia has been previously described in detail [14–16]; briefly, adult male Sprague-Dawley rats (250–350 g) were injected intramuscularly with ketamine (60mg/kg) and xylazine (12mg/kg) and ventilated with room air. Animals were closely monitored for pain by testing the blink reflex. The heart was exposed by a midline thoracotomy, and a ligature was placed near the bifurcation of the left coronary artery, restricting flow through the left anterior descending and circumflex artery. Animals were divided into two experimental groups; 30 min of perfusion (sham) and 30 min of coronary occlusion (ischemia). Following either 30 min of perfusion or ischemia, mesenteric vessels were exteriorized, and mesenteric arteries were gently excised and transferred to a vessel chamber containing Ca2+-free Tyrode’s solution (in mM: 135 NaCl, 4 KCl, 1 MgCl2, 0.33 Na2HPO4, 0.03 EDTA, 10 Glucose, 10 HEPES, pH 7.4). Following careful removal of connective and adipose tissue, tertiary (3rd) branches of the mesenteric artery were isolated.
Mechanical Studies
For force recordings, isolated 3rd mesenteric preparations (100–200 µm in diameter; ~2 mm in length) with an intact endothelium were mounted using wires (40 µm in diameter) on a DMT (Mulvany) 4-channel myograph system [17–19] and stretched to Lo (the length for maximal force) in the myograph chamber containing continuously oxygenated physiological saline solution (PSS in mM: 140 NaCl, 3.7 KCl, 2.5 CaCl2, 0.81 MgSO4, 1.19 KH2PO4, 0.03 EDTA, 5.5 Glucose, 25 HEPES, pH 7.4). The muscle preparations were stimulated to contract with 80 mM KCl depolarization (in mM: 64.5 NaCl, 80 KCl, 2.5 CaCl2, 0.81 MgSO4, 1.19 KH2PO4, 0.03 EDTA, 5.5 Glucose, 25 HEPES, pH 7.4) or 10 µM phenylephrine (PE). For one group of vessels, the force response to either KCl depolarization or to PE was recorded for 10–15 min, before the vessel was relaxed. In a separate series of experiments, the vessels were depolarized and after the force reached a steady state, the dose-response relationship of force relaxation produced by either acetylcholine (ACh) or the cell permeable cGMP analog (8-Br-cGMP) was determined.
SM and NM myosin LC phosphorylation
The time course of SM-LC and NM-LC phosphorylation in perfused versus ischemic mesenteric preparations was determined using 2-D gel electrophoresis [20]. We have demonstrated that this technique resolves the phosphorylated and nonphosphorylated NM-LCs, and the phosphorylated and nonphosphorylated SM-LCs as four distinct spots [20]. The phosphorylation of NM-LC and SM-LC can then be determined using densitometry [20]. For these experiments, the vessels were activated with 80 mM KCl depolarization, and LC phosphorylation was determined at 0 (rest), 2, and 10 min of KCl depolarization. At the specified time, mesenteric microvessels were frozen in liquid nitrogen. As previously described [15, 20], frozen tissues were homogenized in 2-D gel extraction buffer (7M Urea, 2 M thiourea, 4% CHAPS, 1% 3–5.6 immobilized pH gradient (IPG) buffer), and EDTA-free Complete Protease Inhibitor (Roche, Indianapolis, IN). The homogenates were cleared of lipids and extraneous salts using the 2-D gel clean-up kit (Amersham Biosciences, Piscataway, NJ). The acidic halves of 13 cm IPG drystrip gels (pH 3–5.6 NL) were rehydrated in the presence of suitable amounts of sample in rehydration buffer solution (7 M urea, 2 M thiourea, 2% CHAPS, 0.5% pH 3.5–5 IPG buffer, 0.002% bromophenol blue and 12 µl/ml Destreak Reagent) for at least 10 hrs in the “face-down” mode on the Ettan IPG rehydration tray and then resolved by isoelectric focusing (IEF) in the “face-up” mode on an Ettan IPGhor III (GE Healthcare). Following IEF, the gel strips were equilibrated in 6M urea, 50mM Tris-HCL, pH 6.4, 30% glycerol, 2% (w/v) SDS, 0.002% bromophenol blue, first containing 130 mM DTT for 15 min and then containing 135 mM iodoacetamide for 15 min before undergoing SDS-PAGE for protein separation by molecular weight. Subsequently, resolved 2-D SDS-PAGE gels were silver stained. To measure the extent of SM-LC or NM-LC phosphorylation, stained gels were scanned using a Personal Densitometer SI, and the spots were quantified using ImageQuant TL software. The two spots closest to the anode represent the phosphorylated and nonphosphorylated NM-LC, and the two spots nearest the cathode represent the phosphorylated and nonphosphorylated SM-LC [20]. NM-LC phosphorylation level is determined by the ratio of phosphorylated NM-LC to total NM-LC, and SM-LC phosphorylation level is similarly calculated as the ratio of the phosphorylated SM-LC to total SM-LC [20].
Immunoblotting
Western blots were used to determine protein expression in perfused versus ischemic mesenteric preparations as previously described [10, 11, 13, 21]. Briefly, frozen total protein homogenates were suspended in SDS sample buffer. Proteins were resolved by SDS-PAGE using 8% gels with an acrylamide/bisacrylamide ratio of 29:1. Following protein separation by SDS-PAGE, the proteins were transferred onto a PVDF membrane. To detect MYPT1, LZ+ MYPT1, desmin and actin, a rabbit polyclonal anti-MYPT1 (Upstate Biotechnology), a mouse monoclonal anti-LZ+ MYPT1 isoform [10, 11], a mouse monoclonal anti-desmin (D1033, Sigma) and an anti-actin (A2066, Sigma) antibodies were used. Following washing, the blots were incubated with Cy3-labeled anti-mouse IgG (Jackson Immunoresearch) and Cy5-labeled anti-rabbit IgG (GE Lifesciences), respectively. Blots were scanned on a Typhoon 9410 imager, and the scanned images were analyzed using ImageQuant TL software. For all sample preparations, the quantified signal for the protein of interest was divided by the β-actin signal to provide an internally controlled value of the relative expression. To normalize values across different blots, one of the sample preparations was chosen as a standard sample and loaded on all Western blots.
Protein poly-ubiquitination was assessed using immunoblotting with a mouse monoclonal antibody (Ub (P4D1), Santa Cruz Biotechnology), and ubiquitination was quantified with densitometry and normalized to actin, as described above.
Statistical Analysis
All data are presented as means ± SEM, and n represents the number of animals in each group. The Student’s t-test was performed to evaluate for significant differences between the two groups, and considered to be significant at P < 0.05 (n indicates the total number of animals in each group). When multiple comparisons between groups were necessary, a Bonferroni correction was performed.
RESULTS
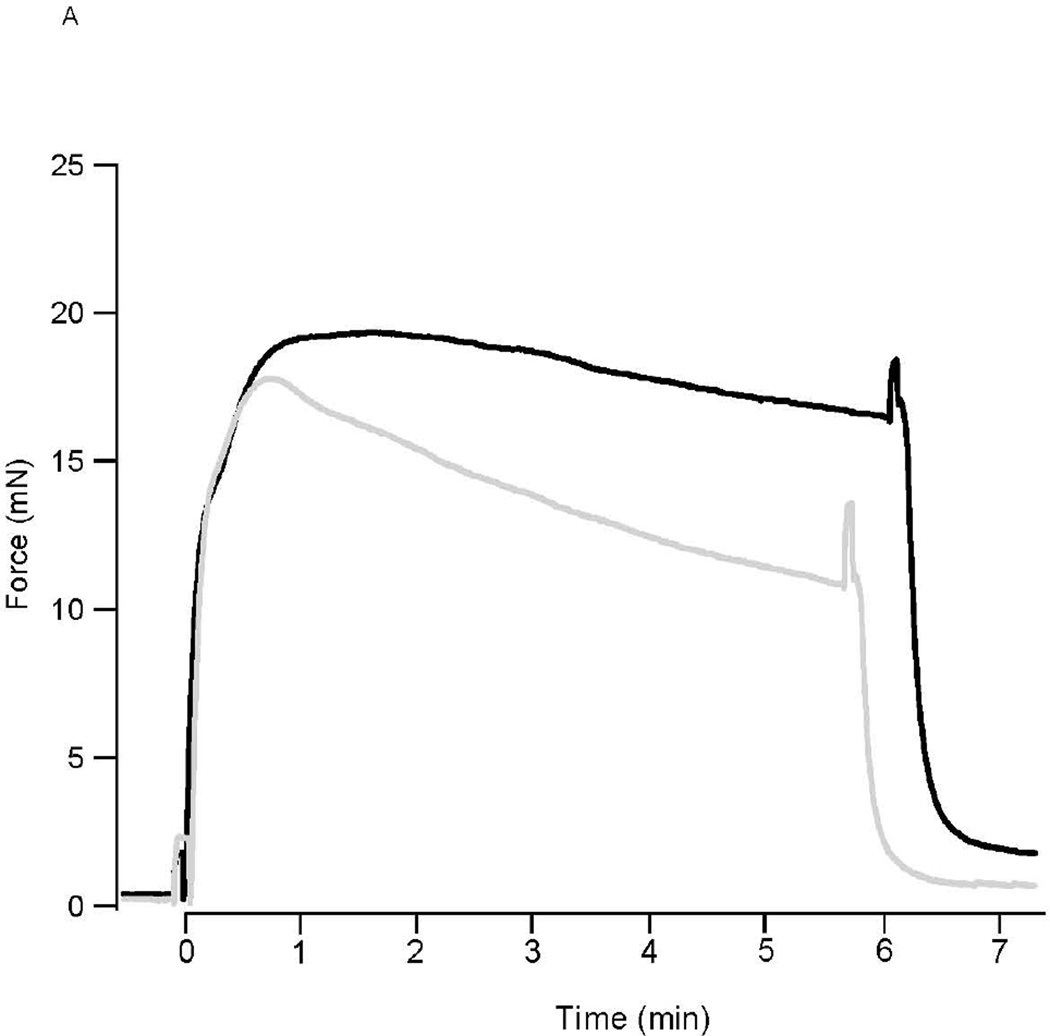
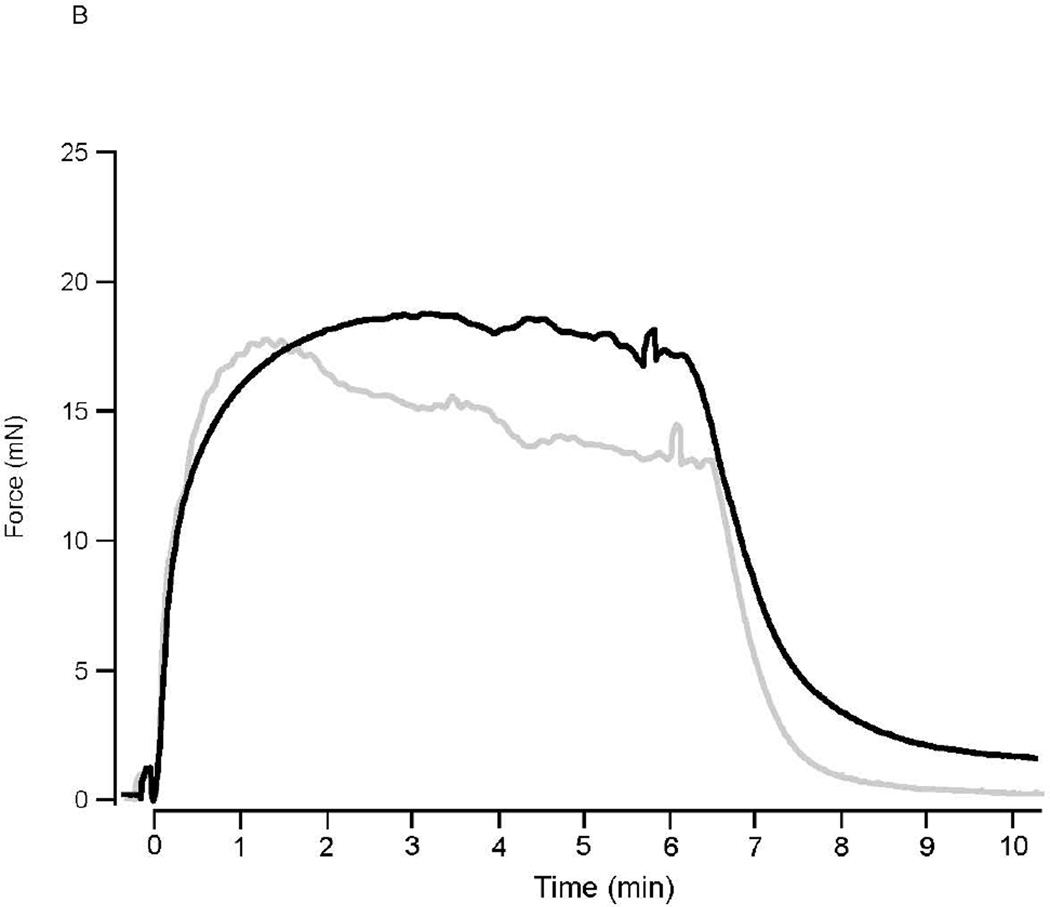
Following KCl depolarization, force rapidly rose to a peak in both perfused and ischemic preparations (16.8±2.2mN vs 16.3±2.1mN, n=6; P>0.05). In perfused preparations, force slowly declined to 78.6±3.1% (n=6) of the peak force during force maintenance (Fig. 1A). In contrast for the ischemic 3rd mesenteric arterial vessels, the ability to maintain force was significantly compromised (Fig. 1); force maintenance was 70.6±1.1% of peak force (n=6, P<0.05 vs perfused). For PE stimulation, force also rose to a peak in perfused and ischemic preparations (13.8±1.1mN (n=5) vs 12.5±1.5mN (n=4); P>0.05). In perfused preparations, force remained at a steady state (97.8±1.2% of peak, P>0.05) while in ischemic preparations, force fell by ~30% to 72.3±6.5% of peak force (p<0.05) during force maintenance (Fig. 1B).
Figure 1. Ischemia Decreases Force Maintenance.
(A) Representative force tracings of perfused (solid line) and ischemic (grey line) mesenteric microvessels during 80mM KCl depolarization. Following KCl depolarization, force rapidly rises to a peak in both perfused and ischemic preparations. However, force maintenance was reduced in the ischemic mesenteric vessels, compared to that in perfused vessels (P<0.05). (B) Representative force tracings of perfused (solid line) and ischemic (grey line) mesenteric microvessels during PE activation. Force rapidly rises to a peak in both perfused and ischemic preparations. However, force maintenance was reduced in the ischemic mesenteric vessels, as compared to that in perfused vessel (P<0.05).
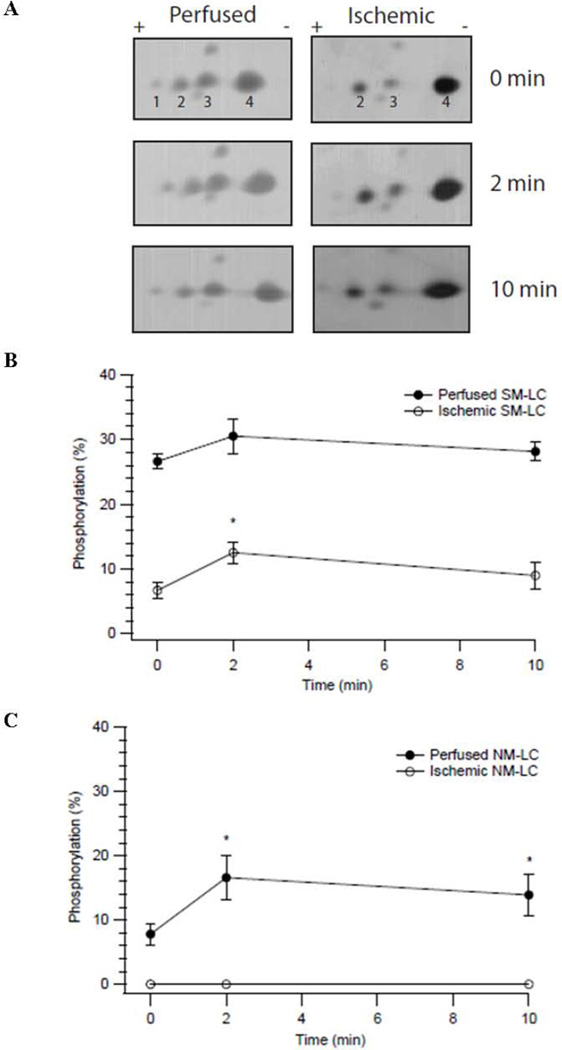
The expression of NM myosin was similar in perfused and ischemic 3rd mesenteric vessels (7.8±1.9% vs 10.8±2.4%, n=6; P>0.05). Thus, to explore the mechanism underlying the ischemia-induced decline in force maintenance, we determined the time course of SM-LC and NM-LC phosphorylation. For depolarization of perfused preparations, SM-LC phosphorylation did not change, but there was a significant increase in NM-LC phosphorylation (7.8±1.9% at rest vs. 16.6±3.5% at 2 min, P<0.05, n=5; Fig. 2), suggesting that in 3rd mesenteric vessels both force activation and maintenance is regulated by the activation of NM myosin. For depolarization of ischemic preparations, there was no detectable NM-LC phosphorylation. Resting SM-LC phosphorylation compared to perfused, was significantly lower, and depolarization resulted in a significant increase in SM-LC phosphorylation (6.7±1.4% at rest vs. 12.5±1.7% at 2 min, P<0.05, n=5; Fig. 2). These data suggest that following myocardial ischemia, force activation and maintenance are dependent on the activation of SM myosin, and ischemia results in a change in the activation (phosphorylation) of both SM and NM myosin.
Figure 2. SM-LC and NM-LC Phosphorylation in Ischemic and Perfused Vessels.
(A). Representative silver stained 2-D gel of rat mesenteric artery tissue lysates. Spots 1 & 2 represent the phosphorylated and nonphosphorylated of NM-LC, respectively, while spots 3 & 4 represent the phosphorylated and nonphosphorylated SM-LC. NM-LC phosphorylation is computed as the density of (1/(1+2)) × 100% and SM-LC phosphorylation is (3/(3+4))x100%, while the expression of NM myosin is ((1+2)/(1+2+3+4))x100%. Time course of SM-LC (B) and NM-LC (C) phosphorylation in perfused (●) and ischemic (○) 3rd mesenteric vessels during KCl depolarization. A significant increase in LC phosphorylation vs LC phosphorylation at 0 min is indicated by the * (P < 0.05, n=5).
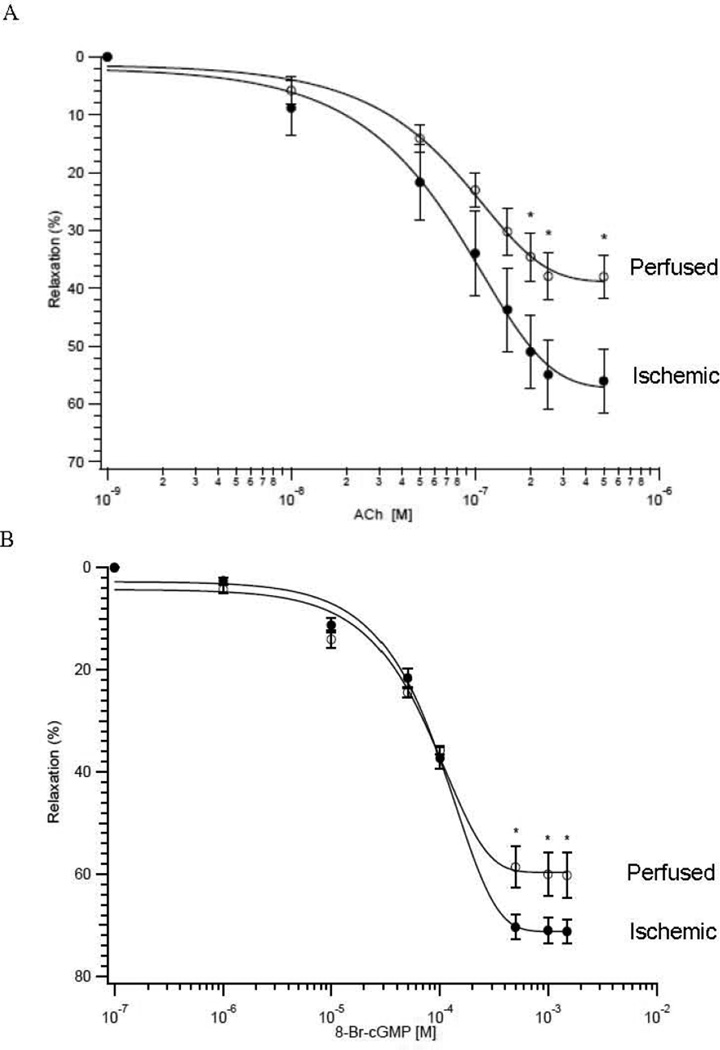
We investigated NO mediated signaling by determining the dose-response relationship of ACh induced relaxation of 3rd mesenteric arteries. For the perfused and ischemic preparations, ACh produced a dose dependent relaxation (Fig. 3A). For perfused vs ischemic vessels, there was no significant difference in the sensitivity to ACh (ED50, 100±8nM vs 97±4nM; P>0.05; n=5). However, ACh produced a significantly larger maximal relaxation in the perfused 3rd mesenteric vessels (56±6% vs 38±4%; P<0.05, n=5; Fig. 3A). To determine whether the impairment in ACh mediated smooth muscle relaxation in ischemic mesenteric vessels was due to a defect at the level of the smooth muscle contractile apparatus, similar experiments were performed using 8-Br-cGMP (Fig. 3B); similar to ACh mediated relaxation, there was no difference in the sensitivity to 8-Br-cGMP (ED50, 96±4 µM vs 77±11 µM, P>0.05, n=5), but the maximum relaxation was significantly reduced in the ischemic preparations as compared to perfused (60±4% vs 71±2%, P<0.05, n=5).
Figure 3. Ischemia Decreases ACh and 8-Br-cGMP Mediated Smooth Muscle Relaxation.
(A) The dose response relationship of smooth muscle relaxation produced by ACh. The magnitude of relaxation produced by ACh is blunted (*P<0.05, n=5) in the 3rd mesenteric vessels from ischemic (○) compared to perfused (●) preparations. (B) The dose response relationship of smooth muscle relaxation produced by 8-Br-cGMP. The magnitude of relaxation produced by 8-Br-cGMP is blunted (*P<0.05, n=5) in the 3rd mesenteric vessels from ischemic (○) compared to perfused (●) preparations.
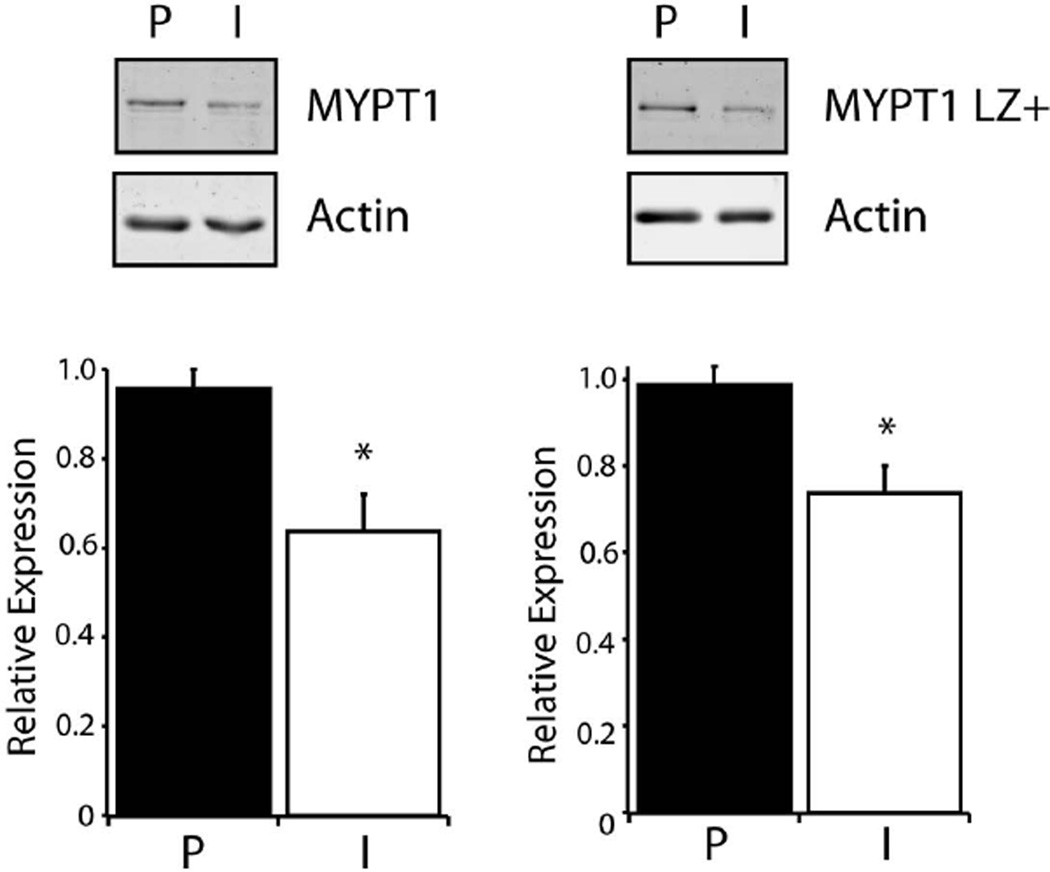
We have previously demonstrated that a decrease in the sensitivity to cGMP mediated smooth muscle relaxation is produced by a decrease in the expression of the LZ+ MYPT1 isoform [10, 11]. Thus, Western blots were used to evaluate MYPT1 and LZ+ MYPT1 expression in perfused and ischemic mesenteric vessels (Fig. 4), and the expression of both MYPT1 and LZ+ MYPT1 in ischemic mesenteric vessels was significantly depressed when compared to perfused vessels (MYPT1; 64±8%, P<0.05, n=4, LZ+ MYPT1; 74±6%, P<0.05, n=4; Fig. 4).
Figure 4. MYPT1 and LZ+ MYPT1 Expression.
Western blots of MYPT1 and LZ+ MYPT1 expression in perfused (P) vs ischemic (I) preparations. Bar graph demonstrates that MYPT1 and LZ+ MYPT1 expression is significantly reduced (*P<0.05, n=4) in ischemic compared to perfused 3rd mesenteric vessels.
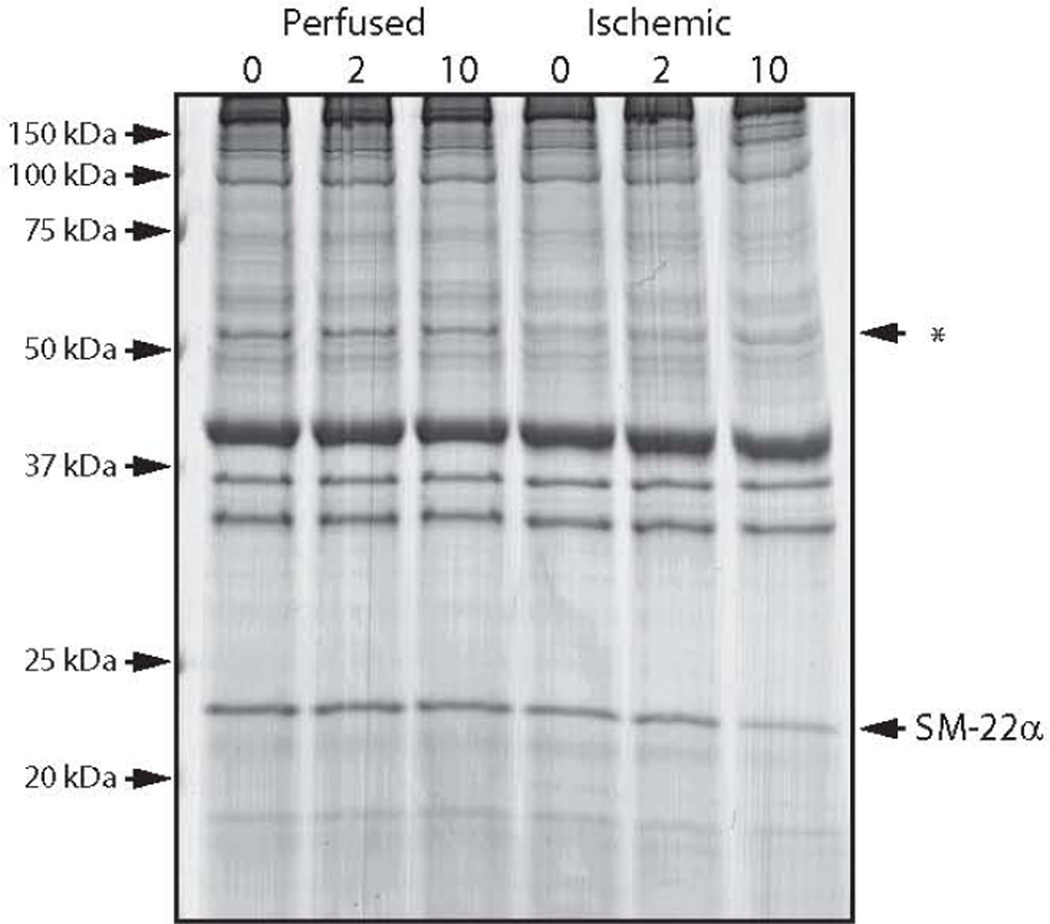
We also examined Coomassie staining of SDS gels, and noted that there was a significant difference in the density of two protein bands comparing perfused and ischemic vessels (Fig. 5); at ~25kDa and ~60kDa. We identified these proteins using mass spectrometry; the 25kDa protein is SM-22α, while the 60 kDa protein(s) was either ATP synthase, adenylyl cyclase, glutamate dehydrogenase and/or desmin. The expression of SM-22α decreased during depolarization (66±3% of perfused at 10 min, P<0.05, n=4). Desmin expression was similar in perfused and ischemic vessels (see Supplemental Figure 1), and thus, the identity and significance of the protein(s) at ~60kDa with lower expression in the ischemic preparations will require further study.
Figure 5. Ischemia Decreases Protein Expression in Ischemic Mesenteric Vessels.
Representative Coomassie stained SDS-PAGE of tissue lysates during KCl depolarization (0, 2 and 10 min). The expression of SM-22α decreases during depolarization (P<0.05, n=4), while the expression of the protein(s) representing the band (←*) at 60 kDa is lower in the ischemic vessels (P<0.05, n=4).
To investigate if the decline in protein expression could be due to degradation by an ubiquitin-proteasome system, we examined the extent of protein poly-ubiquitination with immunoblotting. A significant increase in protein poly-ubiquitination was observed in the ischemic compared to perfused mesenteric vessels (P<0.05; see Supplemental Figure 2).
DISCUSSION
The effect of acute myocardial ischemia on the vasoreactivity of the coronary vessels has been documented [1, 2], but the effect of myocardial ischemia on the reactivity of noncardiac resistance microvessels has not been investigated. Therefore, the main objective of this study was to investigate the effect of acute ischemia on vascular function of nonischemic, resistance vessels. In the present study, we found that acute myocardial ischemia produced dysfunction in 3rd mesenteric vessels, which was characterized by a decrease in force maintenance for both KCl and PE stimulation, and also, a decrease in NO mediated smooth muscle relaxation.
Force Maintenance
Recent evidence suggests that NM myosin participates in the force maintenance phase of smooth muscle contraction, and the regulation of vascular tone [20, 22–25]; we have reported that a 50% reduction in the expression of NM myosin in the rat aorta was associated with a 25% decrease in force maintenance [20]. In 3rd mesenteric vessels, NM myosin expression is ~10% of total MHC, similar to NM myosin expression in rat aorta [20]. In perfused preparations, KCl depolarization resulted in a sustained increase in phosphorylation of NM-LC, but not of SM-LC (Fig. 2). These data suggest that NM myosin participates in the regulation of force and vascular tone in 3rd mesenteric vessels. Thus a change in the phosphorylation of NM myosin in ischemic vessels would be expected to decrease force maintenance. Following 30 min of myocardial ischemia, there was no detectable phosphorylation of NM-LC. However, KCl depolarization produced a significant increase in SM-LC phosphorylation (Fig. 2). These results demonstrate that ischemia changes the mechanism for force regulation in 3rd mesenteric arteries; ischemia produces a shift from a dependence on the activation of NM myosin to SM myosin. Thus as proposed by others [8, 26, 27], our data demonstrates that the activation of NM myosin is important for force maintenance.
The magnitude of the change in the phosphorylation of the LCs in the mesenteric vessels was ~6–8% (Fig. 2). In resistance vessels, pressurization has been demonstrated to be important for the absolute magnitude of LC phosphorylation [28], and in the present study, it is possible that the changes in both NM-LC and SM-LC phosphorylation would be larger if the vessels were pressurized. Further following depolarization, force peaks at 30–60 sec, which would suggest that LC phosphorylation would be at a maximum earlier than the 2 min time point measured in our study. However, force activation during PE stimulation and depolarization of rabbit urethral smooth muscle is regulated by Rho kinase dependent pathway, but with no detectable change in LC phosphorylation [29] The NM-LC and SM-LC differ by only 9 amino acids, and the anti-SM-LC antibody detects both SM-LC and NM-LC [20]. A Rho kinase mediated pathway has been demonstrated to activate NM myosin [20], and thus for urethral smooth muscle, an increase in NM-LC phosphorylation may have been detected using two-dimensional SDS-PAGE.
NO mediated Smooth Muscle Relaxation
Others have demonstrated that ischemia produces endothelial dysfunction of the ischemic vascular bed [1, 2]. NO signaling causes smooth muscle relaxation using both Ca2+ dependent and Ca2+ independent (Ca2+ desensitization) mechanisms [30–32]. During NO stimulation, NO diffuses into the smooth muscle cell to activate guanylate cyclase, which increases cGMP, subsequently activating protein kinase G (PKG). PKG mediates phosphorylation of a number of targets to produce a reduction in intracellular Ca2+, leading to a Ca2+ dependent relaxation, but also activates MLC phosphatase to produce Ca2+ desensitization. Our data demonstrate that myocardial ischemia produces a decrease in the relaxation of 3rd mesenteric vessels produced by both ACh and 8-Br-cGMP (Fig. 3). We have demonstrated that a decrease in LZ+ MYPT1 expression produces a reduction in cGMP mediated smooth muscle relaxation [10, 11], and a decrease in both LZ+ MYPT1 expression and cGMP mediated smooth muscle relaxation has been documented in nitrate tolerance [33, 34], heart failure [12, 35], as well as other vascular diseases [36]. Similarly, our data demonstrate that myocardial ischemia produces a decrease in the expression of both MYPT1 and the LZ+ MYPT1 isoform in 3rd mesenteric vessels (Fig. 4). Although myocardial ischemia could produce endothelial dysfunction and a decrease in NO, these data demonstrate a defect at the level of the smooth muscle, specifically; the decrease in MYPT1 expression contributes to the molecular mechanism responsible for the reduction in the relaxation of the mesenteric vessels by NO-based vasodilators.
The ischemia induced decrease in MYPT1 expression (Fig. 4) could suggest that acute ischemia triggers calpain induced proteolysis or ubiquitin-proteasome based degradation. During depolarization with 80 mM KCl, intracellular Ca2+ concentration increases to a sustained steady state [37], which could suggest that the decrease in MYPT1 expression is due to a Ca2+ dependent process, such as calpain. But, calpain degradation produces large fragments of the intact protein [38], which we did not observe (Fig. 5). Ubiquitin covalently attaches to proteins that are subsequently delivered to the proteasome for rapid degradation. The 26S proteasome is responsible for the degradation of poly-ubiquitinated proteins [39] and is expressed in vascular smooth muscle [40, 41]. The 26S proteasome is composed of a 20S catalytic core and two 19S regulatory particles [39]. The 20S core is composed of two α rings (α1-7) and 2 β rings (β1-7); the α rings bind proteasome regulators and identifies ubiquitinated proteins targeted for degradation [39, 42], and the β rings contain catalytically active sites [39, 42]. Thus, an increase in poly-ubiquitinated-protein complexes (see Supplemental Figure 2) could suggest that the decrease in protein expression is due to protein degradation by the ubiquitin-proteasome pathway.
In addition to MYPT1, the expression of SM-22α was lower in the ischemic preparations (Fig. 5). SM-22α [43] is abundant in smooth muscle; SM-22α is a cytoskeletal protein that binds to actin filaments at a ratio of 1:6 actin monomers, and has been suggested to regulate the organization of thin filaments within the cytoskeleton of the smooth muscle cell [44]. SM-22α deficient mice have no phenotype; although smooth muscle tissue mechanics have not been reported [43]. Seow’s group has suggested that both actin and myosin filaments reorganize (shorten) in response to changes in smooth muscle length [45]. Thus, one possible explanation for the decrease in SM-22α in the ischemic preparations is that depolarization of the ischemic mesenteric vessel results in an actin filament depolymerization/repolymerization, which shortens the overall thin filament length resulting in free, unbound SM-22α that is subsequently targeted for degradation or proteolysis.
The mechanism and/or signaling responsible for triggering the changes in remote, non-ischemic vascular beds are unclear. Although we did not measure hemodynamics during the coronary ligation, studies establishing these parameters during acute coronary ischemia have been published for animals [46, 47] and humans [48]. These investigators have demonstrated that there is no significant change in either blood pressure or heart rate during 30 min of coronary artery ligation in dogs [46] or rats [47]. It is unlikely that production of free radicals in the coronary beds would result in a significant elevation in 3rd mesenteric arteries. The decrease in cardiac output during 30 min of ischemia could decrease pO2 and increase pCO2 in the mesenteric beds, which could increase the production of free radicals. However, we would expect free radical modification to be more widespread rather than limited to only MYPT1 and SM-22α. Further, changes in MYPT1 isoform expression have also been documented during development [49, 50], as well as animal models of portal hypertension [51] and pregnancy induced hypertension [52]; these investigators [51, 52] suggested that changes in pressure and flow regulate MYPT1 isoform expression. Nonetheless, the trigger for the changes in protein expression during ischemia will require further investigation.
CONCLUSION
Our data demonstrate that acute myocardial ischemia decreases force maintenance in 3rd mesenteric vessel stimulated to contract with either KCl, which leads to an increase in intracellular Ca2+, or PE, which increases Ca2+ and also activates signaling pathways for Ca2+ sensitization. Further, acute myocardial ischemia 1) changes the regulation of force from requiring the activation of NM myosin to activation of SM myosin, which results in an impairment in force maintenance and 2) decreases the expression of both MYPT1 and the LZ+ MYPT1 isoform, which produces a reduction in the relaxation of 3rd mesenteric vessels to NO-based vasodilators. These findings demonstrate that even acute myocardial dysfunction results in vascular dysfunction and in changes of the reactivity of the peripheral vasculature. Further, our results demonstrate that the activation of NM myosin participates in the regulation of vascular tone, and identifying the mechanism for the changes in the activation of NM/SM myosin and in the expression of MYPT1 will provide insight into the development of therapeutic strategies of myocardial ischemia.
Supplementary Material
Supplemental Figure 1. Desmin Expression is Similar in Perfused and Ischemic Mesenteric Vessels. Representative immunoblot of mesenteric arterial tissue lysates during KCl depolarization (0, 2, 10 min). The expression of desmin was similar (p>0.05) in perfused and ischemic vessels.
Supplemental Figure 2. Ischemia Increases Protein Ubiquitination. Western blots demonstrate that ischemia increases protein poly-ubiquitination. Bar graph demonstrates that the pool of ubiquitinated proteins is increased in ischemic 3rd mesenteric arterial smooth muscle (120±4% of perfused vessels, *P<0.05 vs perfused, n=4).
Acknowledgements
We would like to thank Dr. Ozgur Ogut for his comments and suggestions. This work was supported by the Mayo Clinic and the NIH T32 HL007111 (to Y.S.H.).
References
- 1.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez N, Martinez MA, Climent B, Garcia-Villalon AL, Monge L, Sanz E. Coronary reactivity to endothelin-1 during partial ischemia and reperfusion in anesthetized goats. Role of nitric oxide and prostanoids. Eur J Pharmacol. 2002;457(2–3):161–168. doi: 10.1016/s0014-2999(02)02684-5. [DOI] [PubMed] [Google Scholar]
- 3.Francis GS, Cohn JN. Heart failure: Mechanisms of cardiac and vascular dysfunction and the rationale for pharmacolgic intervention. FASEB J. 1990;4:3068. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]
- 4.Negrao CE, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD, Middlekauff HR. Impaired endothelium-mediated vasodilation is not the principal cause of vasoconstriction in heart failure. Am J Physiol. 2000;278:H168–H174. doi: 10.1152/ajpheart.2000.278.1.H168. [DOI] [PubMed] [Google Scholar]
- 5.Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992;267:14662–14668. [PubMed] [Google Scholar]
- 6.Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol. 2000;279:C1722–C1732. doi: 10.1152/ajpcell.2000.279.6.C1722. [DOI] [PubMed] [Google Scholar]
- 7.Ogut O, Yuen SL, Brozovich FV. Regulation of the smooth muscle contractile phenotype by nonmuscle myosin. J Muscle Res Cell Motil. 2007;28:409–414. doi: 10.1007/s10974-008-9132-2. [DOI] [PubMed] [Google Scholar]
- 8.Ekman M, Fagher K, Wede M, Stakeberg K, Arner A. Decreased phosphatase activity, increased ca2+ sensitivity, and myosin light chain phosphorylation in urinary bladder smooth muscle of newborn mice. J Gen Physiol. 2005;125:187–196. doi: 10.1085/jgp.200409212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furchgott RF. Endothelium-derived relaxing factor: Discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 10.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure. Role of myosin light chain phosphatase. Circ Res. 2004;95:612–619. doi: 10.1161/01.RES.0000142736.39359.58. [DOI] [PubMed] [Google Scholar]
- 11.Chen FC, Ogut O, Rhee AY, Hoit BD, Brozovich FV. Captopril prevents myosin light chain phosphatase isoform switching to preserve normal cgmp-mediated vasodilatation. J Mol Cell Cardiol. 2006;41:488–495. doi: 10.1016/j.yjmcc.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Ararat E, Brozovich FV. Losartan decreases p42/44 mapk signaling and preserves LZ+ mypt1 expression. PLoS ONE. 2009;4:e5144. doi: 10.1371/journal.pone.0005144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem. 2004;279:597–603. doi: 10.1074/jbc.M308496200. [DOI] [PubMed] [Google Scholar]
- 14.Chen FC, Ogut O. Decline of contractility during ischemia-reperfusion injury: Actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- 15.Han YS, Ogut O. Regulation of fibre contraction in a rat model of myocardial ischemia. PLoS One. 2010;5:e9528. doi: 10.1371/journal.pone.0009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopher B, Pizarro GO, Nicholson B, Yuen S, Hoit BD, Ogut O. Reduced force production during low blood flow to the heart correlates with altered troponin i phosphorylation. J Muscle Res Cell Motil. 2009;30:111–123. doi: 10.1007/s10974-009-9180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Mulvany MJ, Nilsson H, Flatman JA. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulvany MJ, Nilsson H, Flatman JA, Korsgaard N. Potentiating and depressive effects of ouabain and potassium-free solutions on rat mesenteric resistance vessels. Circ Res. 1982;51:514–524. doi: 10.1161/01.res.51.4.514. [DOI] [PubMed] [Google Scholar]
- 20.Yuen SL, Ogut O, Brozovich FV. Nonmuscle myosin is regulated during smooth muscle contraction. American journal of physiology. 2009;297:H191–H199. doi: 10.1152/ajpheart.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards CT, Ogut O, Brozovich FV. Agonist-induced force enhancement: The role of isoforms and phosphorylation of the myosin-targeting subunit of myosin light chain phosphatase. J Biol Chem. 2002;277:4422–4427. doi: 10.1074/jbc.M111047200. [DOI] [PubMed] [Google Scholar]
- 22.Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG. Identification and characterization of nonmuscle myosin ii-c, a new member of the myosin ii family. J Biol Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- 23.Phillips CL, Yamakawa K, Adelstein RS. Cloning of the cdna encoding human nonmuscle myosin heavy chain-b and analysis of human tissues with isoformspecific antibodies. J Muscle Res Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin iib: Functional adaptations for tension generation and maintenance. J Biol Chem. 2003;278:27439–27448. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin ii: Kinetic characterization of the non-muscle iia isoform. J Biol Chem. 2003;278:38132–38140. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- 26.Rhee AY, Ogut O, Brozovich FV. Nonmuscle myosin, force maintenance, and the tonic contractile phenotype in smooth muscle. Pflugers Arch. 2006;452:766–774. doi: 10.1007/s00424-006-0091-4. [DOI] [PubMed] [Google Scholar]
- 27.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin ii. J Muscle Res Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 28.El-Yazbi AF, Johnson RP, Walsh EJ, Takeya K, Walsh MP, Cole WC. Pressuredependent contribution of rho kinase-mediated calcium sensitization in serotoninevoked vasoconstriction of rat cerebral arteries. J Physiol. 2010;588:1747–1762. doi: 10.1113/jphysiol.2010.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh MP, Thornbury K, Cole WC, Sergeant G, Hollywood M, McHale N. Rhoassociated kinase plays a role in rabbit urethral smooth muscle contraction, but not via enhanced myosin light chain phosphorylation. Am J Physiol Renal Physiol. 2011;300:F73–F85. doi: 10.1152/ajprenal.00011.2010. [DOI] [PubMed] [Google Scholar]
- 30.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P. The large conductance, voltage-dependent, and calcium-sensitive k+ channel, hslo, is a target of cgmp-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273:32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cgmp signal transduction system: Regulation and mechanism of action. Biochem Biophys Acta. 1993;1173:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 32.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic gmp-dependent protein kinase activates cloned bkca channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 33.Dou D, Ma H, Zheng X, Ying L, Guo Y, Yu X, Gao Y. Degradation of leucine zipper-positive isoform of mypt1 may contribute to development of nitrate tolerance. Cardiovascular research. 2010;86:151–159. doi: 10.1093/cvr/cvp376. [DOI] [PubMed] [Google Scholar]
- 34.Ma H, He Q, Dou D, Zheng X, Ying L, Wu Y, Raj JU, Gao Y. Increased degradation of mypt1 contributes to the development of tolerance to nitric oxide in porcine pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2010;299:L117–L123. doi: 10.1152/ajplung.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogut O, Brozovich FV. The potential role of mlc phosphatase and mapk signalling in the pathogenesis of vascular dysfunction in heart failure. J Cell Mol Med. 2008;12:2158–2164. doi: 10.1111/j.1582-4934.2008.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aburto TK, Lajoie C, Morgan KG. Mechanisms of signal transduction during alpha 2-adrenergic receptor-mediated contraction of vascular smooth muscle. Circ Res. 1993;72:778–785. doi: 10.1161/01.res.72.4.778. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–S18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 39.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20s and 26s proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 40.Zong C, Young GW, Wang Y, Lu H, Deng N, Drews O, Ping P. Twodimensional electrophoresis-based characterization of post-translational modifications of mammalian 20s proteasome complexes. Proteomics. 2008;8:5025–5037. doi: 10.1002/pmic.200800387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res. 2010;107:1094–1101. doi: 10.1161/CIRCRESAHA.110.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, Kibbe MR. Nitric oxide regulates the 26s proteasome in vascular smooth muscle cells. Nitric Oxide. 2009;20:279–288. doi: 10.1016/j.niox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of sm22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol. 2001;21:1336–1344. doi: 10.1128/MCB.2001.21.4.1336-1344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapland C, Hsuan JJ, Totty NF, Lawson D. Purification and properties of transgelin: A transformation and shape change sensitive actin-gelling protein. J Cell Biol. 1993;121:1065–1073. doi: 10.1083/jcb.121.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali F, Chin L, Pare PD, Seow CY. Mechanism of partial adaptation in airway smooth muscle after a step change in length. J Appl Physiol. 2007;103:569–577. doi: 10.1152/japplphysiol.00216.2007. [DOI] [PubMed] [Google Scholar]
- 46.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 47.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 48.Freis ED, Schnaper HW, Johnson RL, Schreiner GE. Hemodynamic alterations in acute myocardial infarction. I. Cardiac output, mean arterial pressure, total peripheral resistance, central and total blood volumes, venous pressure and average circulation time. J Clin Invest. 1952;31:131–140. doi: 10.1172/JCI102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cgmp-mediated smooth muscle relaxation. J Biol Chem. 2001;276:37250–37257. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- 50.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. Journal of molecular and cellular cardiology. 2006;40:274–282. doi: 10.1016/j.yjmcc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. American journal of physiology. 2004;286:H1801–H1810. doi: 10.1152/ajpheart.00696.2003. [DOI] [PubMed] [Google Scholar]
- 52.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to snp in a rat l-name model of hypertension of pregnancy. Am J Physiol Cell Physiol. 2008;294:C564–C571. doi: 10.1152/ajpcell.00285.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Desmin Expression is Similar in Perfused and Ischemic Mesenteric Vessels. Representative immunoblot of mesenteric arterial tissue lysates during KCl depolarization (0, 2, 10 min). The expression of desmin was similar (p>0.05) in perfused and ischemic vessels.
Supplemental Figure 2. Ischemia Increases Protein Ubiquitination. Western blots demonstrate that ischemia increases protein poly-ubiquitination. Bar graph demonstrates that the pool of ubiquitinated proteins is increased in ischemic 3rd mesenteric arterial smooth muscle (120±4% of perfused vessels, *P<0.05 vs perfused, n=4).