Abstract
Purpose
Prostate cancer has been described as a component tumor of Lynch syndrome (LS), with tumors obtained from mutation carriers demonstrating the DNA mismatch repair deficiency phenotype. Previous studies quantifying prostate cancer risk in LS have provided conflicting results.
Methods
We examined cancer histories of probands and their first- through fourth-degree relatives for 198 independent mutation-positive LS families enrolled in two US familial cancer registries. Modified segregation analysis was used to calculate age-specific cumulative risk or penetrance estimates, with accompanying Wald-type CIs. Cumulative lifetime risks and hazard ratio (HR) estimates for prostate cancer were calculated and compared with those of the general population.
Results
Ninety-seven cases of prostate cancer were observed in 4,127 men. Median age at prostate cancer diagnosis was 65 years (range, 38 to 89 years), with 11.53% of affected individuals diagnosed before age 50 years. The cumulative risk of prostate cancer at ages 60 and 80 years was 6.30% (95% CI, 2.47 to 9.96) and 30.0% (95% CI, 16.54 to 41.30), as compared with the population risk of 2.59% and 17.84%, respectively. The overall prostate cancer HR among carriers was 1.99 (95% CI, 1.31 to 3.03).
Conclusion
The cumulative lifetime risk of prostate cancer in individuals with LS is two-fold higher than in the general population and is slightly higher in carriers diagnosed before age 60 years (HR, 2.48; 95% CI, 1.34 to 4.59). These estimates are clinically valuable to quantify risk for both patients and providers.
INTRODUCTION
Lynch syndrome (LS) is an inherited cancer predisposition syndrome with elevated lifetime risk of colorectal cancer (CRC) ranging from 35% to 80%.1–4 Germline mutations in the mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2 are the leading cause of LS. In addition to CRC, other cancers are known to be associated with LS. For women, the risk of endometrial cancer (EC) is high, ranging from 34% to 71%.1–4 Other cancers, including gastric, ovarian, urinary tract, pancreatic, brain, and sebaceous tumors, are also associated with LS, and there is an elevated risk in mutation carriers.5–8
Prostate cancer is the most common cancer in men, with a Surveillance, Epidemiology, and End Results (SEER)9 estimated lifetime risk of 17.84% and median age at diagnosis of 67 years. Prostate cancer has been described as a component tumor of LS10,11; however, the lifetime risk of prostate cancer in men with LS has been difficult to quantify. Prostate cancer has a high background population risk, which makes it difficult to identify significant modifications to the lifetime risk estimate. Most studies that have aimed to estimate prostate cancer risk in LS have been performed in families identified through high-risk cancer genetics clinics, with strong personal and/or family histories of cancer, subjecting them to ascertainment bias and leading to an overestimation of cancer risk. In addition, incomplete information on germline mutation status of both affected and unaffected family members leads to a smaller number of informative individuals available for analysis. Modified segregation analysis is a statistical tool used to limit ascertainment bias by conditioning on the genotype and phenotype of the proband. Additionally, the method uses the observed genotype information available on a limited number of family members to infer unobserved genotype status of other members within the pedigree who have not undergone germline genetic testing. We have previously used this method to estimate the risk of CRC and EC in LS and to demonstrate an increased risk of pancreatic cancer in LS.1,12 In this analysis, we used modified segregation analysis to quantify the lifetime cumulative risk and hazard ratio (HR) of prostate cancer for MMR mutation carriers recruited through high-risk cancer genetics clinics.
METHODS
Kindred with a pathogenic mutation in the MMR genes MLH1, MSH2, or MSH6 were identified through cancer genetics clinics at the Dana-Farber Cancer Institute (DFCI), Boston, Massachusetts, and the University of Michigan Comprehensive Cancer Center (UMCCC), Ann Arbor, Michigan. Participants presented to these clinics by either self-referral or physician referral on the basis of personal and/or family history of cancer. Probands, the first person in the family to undergo MMR gene testing, enrolled in a familial cancer registry through protocols approved by the institutional review board of each center. Family pedigrees in which one or more family members were confirmed carriers of a pathogenic MMR gene mutation by clinical genetic testing were included in this analysis. Kindred recruited to the respective registries from their inception (DFCI, 1994; UMCCC, 2002) through December 2010 were eligible for inclusion.
Cancer histories of independent probands were analyzed. Ages and cancer diagnoses of all first-, second-, third-, and fourth-degree relatives were compiled, and cancer diagnoses were confirmed with medical record reports or death certificates, when available. Each pedigree was reviewed to determine whether the genetic mutation originated from the proband's maternal or paternal lineage based on mutation status of relatives and/or cancer family history; individuals from the unaffected side of the family were excluded from the analysis.
Information on the age of occurrence of prostate cancer in relatives of MMR mutation–positive index individuals was used to estimate age-specific incidences of prostate cancer in MMR mutation carriers by modified segregation analysis based on a Cox proportional hazards model. Calculations were performed using MENDEL (version 8.0) as well as vintage MENDEL (version 3.3.5) software.13,14 Relatives were assumed to have been observed from 20 years of age and to have been censored at age at diagnosis of prostate cancer, age at death, age at last follow-up, or age 80 years, whichever occurred earliest. Penetrance analysis included information from both genotyped and ungenotyped relatives. Information on MMR mutation status in relatives was included whenever available. The likelihood makes an assumption of standard Mendelian genetics on the probability of unobserved genotype conditional on observed genotypes in a pedigree and marginalizes over all possible genotype configurations. For individuals with missing age information, age was imputed based on relationship to proband, age of proband, and deceased status at last follow-up (Table 1 lists information on the extent of missing age information). Sensitivity analysis was performed without imputing age information to ensure that age imputation did not artificially inflate estimates of penetrance and relative risk (Appendix Tables A1 to A4, online only).
Table 1.
Descriptive Statistics and Characteristics of the Study Population
| Characteristic | DFCI |
UMCCC |
Combined |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Individual families | 117 | 81 | 198 | |||
| MLH1 | 49 | 41.9 | 25 | 30.9 | 74 | 40.4 |
| MSH2 | 54 | 46.2 | 47 | 58.0 | 101 | 48.6 |
| MSH6 | 14 | 11.9 | 9 | 11.1 | 23 | 11.0 |
| Total individuals | 5,064 | 3,250 | 8,314 | |||
| Current age known | 2,691 | 1,713 | 4,404 | |||
| Male sex | 2,546 | 50.3 | 1,581 | 48.6 | 4,127 | 50.2 |
| Cases of prostate cancer | 45 | 52 | 97 | |||
| Age known at diagnosis | 34 | 44 | 78 | |||
| Total No. of individuals genotyped | 314 | 283 | 597 | |||
| Cases of prostate cancer | 6 | 10 | 16 | |||
| Mutation positive | 6 | 9 | 15 | |||
| Unaffected with prostate cancer | 308 | 273 | 581 | |||
| Mutation positive | 216 | 181 | 397 | |||
| Age, years | ||||||
| Median at diagnosis | 63 | 67 | 65 | |||
| Range | 44-89 | 38-82 | 38-89 | |||
| Interquartile range | 16.5 | 10.5 | 12.75 | |||
| Standard deviation | 11 | 9.5 | 10.1 | |||
| Diagnosed at age < 50 years | 11.7 | 11.3 | 11.5 | |||
Abbreviations: DFCI, Dana-Farber Cancer Institute; UMCCC, University of Michigan Comprehensive Cancer Center.
We assumed that probands in the registries were ascertained based on phenotype status of the two primary LS cancers (CRC and EC). To correct for this ascertainment bias, we maximized the conditional likelihood of observing prostate cancer status and genotypes (mutations in MLH1, MSH2, or MSH6) of the entire pedigree given the phenotypic and genotypic information of the proband. Because sampling was performed in terms of other cancer phenotypes, rather than prostate cancer, there were no significant differences between conditional and unconditional likelihood in this study. We present results from the conditional likelihood, which are slightly more conservative and reflect our sampling scheme for the pedigrees more accurately. Results without conditioning on the proband are available in Appendix Tables A5 and A6 (online only).
Cancer incidence in carriers was assumed to follow a piecewise proportional hazards model, with λ(t) = λ0(t)exp[g(t)], where λ0(t) is the background incidence, which was assumed to follow the population incidence from the SEER 13 database.9 The age-specific relative risks in carriers as compared with the general population rates are modeled through the function exp[g(t)]. Age-specific HR parameters βk were estimated for the two age intervals of 20 to 59 and 60 to 80 years. The function g(t) takes the form Σ2k=1 exp[βk], a piecewise constant HR in the kth age band k = 1,2. Cancer incidences in noncarriers were assumed to follow the population cohort–specific rates as obtained through SEER 13.
To construct CIs for the log(HR) estimates, we assumed that the maximum likelihood estimates of the parameters were asymptotically normally distributed with covariance matrix given by the inverse of the Fisher information matrix. Cumulative risk and 95% Wald-type CIs were calculated from the cumulative incidence Λ(t) given by Λ(t) = Σnk=1iktk exp[βk] where ik is the population incidence, tk is the length, and βk is the log(RR) in the kth age interval. The cumulative risk is given by F(t) = 1 − exp[−Λ(t)], and a 95% CI for F(t) is 1 − exp[−Λ(t) ± 1.96Var(Λ(t))] where Var(Λ(t)) = Σnk=1ik2tk2 exp[2βk] + 2 Σnj<k,k=1ikijtktj[Var(βk)Var(βj)]1/2 exp(βk + βj)corr(βk,βj).14
Permission for research was obtained from the institutional review boards at the UMCCC (Ann Arbor, MI) and DFCI (Boston, MA).
RESULTS
As of December 2010, 198 families had been enrolled in the research registries of DFCI (N = 117) and UMCCC (N = 81) with pathogenic MMR mutations and were included for analysis (MLH1 mutation, 74; MSH2, 101; MSH6, 23; Table 1). Total number of at-risk individuals was 8,314. Mutation status was known for 597 individuals; 412 were mutation positive, and 185 were true negative. Included in the analysis for prostate cancer were 4,127 men, 97 of whom (2.4%) had a personal history of prostate cancer and were considered patient cases. Median age at prostate cancer diagnosis was 65 years, with an age range of 38 to 89 years (interquartile range, 12.75; standard deviation, 10.1) and 11.53% of patient cases diagnosed with prostate cancer at age younger than age 50 years (Table 1).
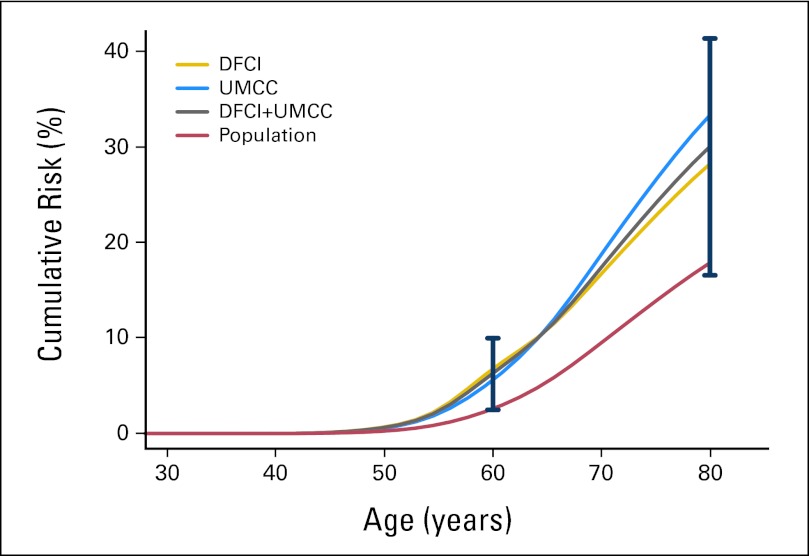
Cumulative lifetime risk of prostate cancer (to age 80 years) was 30.0% in MMR mutation carriers (95% CI, 16.54 to 41.30; P = .07), as compared with the 17.84% general population risk by SEER estimates. The risk of prostate cancer in mutation carriers is elevated as early as age 50 years, where the risk is 0.64% (95% CI, 0.24 to 1.01; P = .06), as compared with the general population risk of 0.26%, although this difference does not reach statistical significance. This elevated risk was observed in the independent and combined data sets (Table 2; Fig 1; Appendix Table A7, online only). Cumulative incidence findings were similar to cumulative risk findings (Appendix Table A8, online only).
Table 2.
Age-Specific CR Estimates Corresponding to MMR Mutations Compared With Population Rates Reported in SEER 13
| Age (years) | CR in Population (%) | DFCI |
UMCCC |
Combined |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR (%) | 95% CI | P | CR (%) | 95% CI | P | CR (%) | 95% CI | P | ||
| 40 | 0 | 0.002 | 0.00 to 0.004 | .10 | 0.002 | 0.00 to 0.004 | .30 | 0.002 | 0.00 to 0.004 | .06 |
| 50 | 0.26 | 0.69 | 0.16 to 1.18 | .10 | 0.57 | 0.00 to 1.13 | .29 | 0.64 | 0.24 to 1.01 | .06 |
| 60 | 2.59 | 6.77 | 1.69 to 11.57 | .10 | 5.60 | 0.00 to 11.08 | .30 | 6.30 | 2.47 to 9.96 | .06 |
| 70 | 9.49 | 16.77 | 7.71 to 24.80 | .11 | 18.74 | 6.22 to 29.56 | .14 | 17.39 | 10.10 to 24.07 | .03 |
| 80 | 17.84 | 28.18 | 11.50 to 41.72 | .20 | 33.28 | 9.55 to 50.79 | .18 | 30.00 | 16.54 to 41.30 | .07 |
NOTE. Penetrance estimates and 95% Wald-type CIs obtained by modified segregation analysis; P value corresponds to Wald test that age-specific cumulative risk in MMR carriers is different from age-specific cumulative risk observed in population.
Abbreviations: CR, cumulative risk; DFCI, Dana-Farber Cancer Institute; MMR, mismatch repair; SEER, Surveillance, Epidemiology, and End Results; UMCCC, University of Michigan Comprehensive Cancer Center.
Fig 1.
Cumulative risk of prostate cancer in mismatch repair gene mutation carriers compared with general population rates reported in SEER 13; 95% Wald-type CIs are included at ages 60 and 80 years. DFCI, Dana-Farber Cancer Institute; UMCC, University of Michigan Comprehensive Cancer Center.
Overall HR (to age 80 years) for prostate cancer in MMR mutation carriers in the combined data set was 1.99 (95% CI, 1.31 to 3.03; P = .0013). Among younger men, ages 20 to 59 years, this HR is slightly higher at 2.48 (95% CI, 1.34 to 4.59; P = .0038; Table 3). Because of limitations in sample size, we did not have power to carry out mutation-specific penetrance analysis; however, more case occurrences were noted in the MSH2 mutation group. Table 4 summarizes descriptive statistics of patient cases of prostate cancer stratified by mutation type.
Table 3.
HR Estimates and 95% Wald-Type CIs for Penetrance Models
| Age (years) | DFCI |
UMCCC |
Combined |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| 20 to 59 | 2.67 | 1.25 to 5.69 | .01 | 2.20 | 0.78 to 6.21 | .14 | 2.48 | 1.34 to 4.59 | .0038 |
| 60 to 80 | 1.53 | 0.71 to 3.31 | .28 | 2.04 | 0.87 to 4.78 | .10 | 1.71 | 0.95 to 3.07 | .07 |
| Overall HR | 1.93 | 1.12 to 3.31 | .017 | 2.10 | 1.08 to 4.09 | .029 | 1.99 | 1.31 to 3.03 | .0013 |
NOTE. Penetrance models reported in Table 2; two-parameter piecewise proportional hazards model is used, with separate parameters for ages 20 to 59 and 60 to 80 years.
Abbreviations: DFCI, Dana-Farber Cancer Institute; HR, hazard ratio; UMCCC, University of Michigan Comprehensive Cancer Center.
Table 4.
Descriptive Statistics of Patient Cases and Age of Onset Stratified by Study Site and Mutation Subtype
| Statistic | DFCI |
UMCCC |
Combined |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MLH1 | MSH2 | MSH6 | MLH1 | MSH2 | MSH6 | MLH1 | MSH2 | MSH6 | |
| No. of cases of prostate cancer | 11 | 30 | 4 | 23 | 24 | 5 | 34 | 54 | 9 |
| Age known at onset | 10 | 22 | 2 | 21 | 21 | 2 | 31 | 43 | 4 |
| Age at diagnosis, years | |||||||||
| Median | 69 | 62 | 76 | 69 | 60 | 68 | 69 | 60 | 74 |
| Range | 52-80 | 44-89 | 75-76 | 55-79 | 38-82 | 63-72 | 50-80 | 38-89 | 63-76 |
| Diagnosed at age < 50 years, % | 0 | 18.8 | 0 | 0 | 4.7 | 0 | 0 | 11.6 | 0 |
Abbreviations: DFCI, Dana-Farber Cancer Institute; UMCCC, University of Michigan Comprehensive Cancer Center.
DISCUSSION
Prostate cancer is the most common male cancer and has been proposed as a component tumor of LS. LS-associated tumors often demonstrate microsatellite instability (MSI) and absent staining of the MMR gene proteins via immunohistochemistry. These hallmarks of DNA MMR deficiency have been described in some prostate cancer tumors and human prostate cancer cell lines.15,16 Ahman et al17 obtained 77 prostate cancer tumors from 73 men belonging to 33 families enrolled in a hereditary prostate cancer study and identified five unrelated patients (6.5%) with microsatellite-unstable prostate cancer. Three families showed high levels of MSI at two or more markers. Two families showed low levels of MSI. All of the families with MSI-high tumors had histories of familial cancer, including two with early-onset CRC and one with hereditary breast cancer. We previously analyzed 31 prostate tumors from independent families enrolled in our prostate cancer genetics program who reported a positive family history of CRC. Three MSI tumors (9.7%) were identified.18 Two tumors were MSI high and were from men with family histories that met the clinical diagnostic criteria for LS (Amsterdam I criteria). Additional tumor testing performed on one of these MSI-high tumors noted absent immunohistochemical staining of the MSH2 and MSH6 proteins, with intact expression of the MLH1 and PMS2 proteins. This individual tested positive for a germline MSH2 mutation, confirming the diagnosis of LS and the evidence of DNA MMR deficiency in prostate cancer.18 Although these studies demonstrate the presence of the DNA MMR deficiency phenotype in prostate cancer, studies of familial prostate cancer have found a low prevalence of MSI, suggesting that LS is unlikely to be implicated in the majority of cases of familial prostate cancer.17,18
Previous reports have suggested an elevated incidence of prostate cancer among men with MMR gene mutations, but the contribution of LS to the development of these prostate cancers is unclear. In 60 Brazilian families with mutations in MLH1 or MSH2, da Silva et al19 reported 16 (20.8%) of 77 male carriers developed prostate cancer. Prostate cancer was the most common extracolonic cancer, but most patients were diagnosed at age older than 70 years, leading the investigators to conclude that the elevated incidence of prostate cancer was unlikely to be related to LS. Goecke et al20 identified eight cases of prostate cancer among 423 German men who were positive or obligate carriers of MSH2 mutations and an additional two cases (2.4%) in men whose mutation status was assumed positive and belonged to an MSH2-positive family. Incidence of prostate cancer was not significantly higher than expected (adjusted P = .11), but median age at diagnosis was 59 years, younger than the average age at diagnosis, leading the investigators to suggest a marginal association between MSH2 mutation and risk of prostate cancer.
Given the findings in observational studies, investigators have used standardized incidence ratios (SIRs) to estimate prostate cancer risk in LS, but the findings have been limited by small sample size and failure to control for ascertainment bias. In 1999, Aarnio et al3 identified four cases of prostate cancer among 360 individuals who were mutation positive or obligate carriers of MLH1 or MSH2 mutations and estimated an SIR of 2.9 (95% CI, 0.8 to 7.4). In 2001, Scott et al21 identified one case of prostate cancer among 12 MSH2 mutation–positive families and estimated an SIR of 1.02 (95% CI, 0.1 to 13.6). Although suggesting an elevated trend, neither of these SIRs were statistically significant.3,21 In 2009, Grindedal et al22 reviewed 34 Norwegian MMR mutation–positive families and reported nine cases of prostate cancer among 106 men who were carriers or obligate carriers of MMR mutations (8.5%). SIR was estimated at 5.9 (95% CI, 4.1 to 17.1). No cases of prostate cancer were observed in the 68 brothers (29 true negative, 39 not tested) of these 106 mutation carriers (P = .01). Age at onset of prostate cancer in the MMR carriers was significantly younger than expected: 60.4 versus 66.6 years at diagnosis (P = .006). Estimated cumulative lifetime risk of prostate cancer (to age 70 years) was 29% in the MMR mutation carriers, as compared with the general population risk of 8%. A 2006 analysis of 18 Amsterdam I–positive LS families identified through the Utah Population Database identified a significantly elevated risk of prostate cancer among the 509 first-degree relatives of those with CRC (standardized morbidity ratio, 2.2; 95% CI, 1.31 to 3.39; P = .002).23 Win et al24 prospectively observed a cohort of 446 unaffected MLH1, MSH2, MSH6, and PMS2 carriers and estimated a prostate cancer SIR of 2.49 (95% CI, 0.51 to 7.27; P = .18). Median age at diagnosis in three patient cases of prostate cancer from this prospective cohort was 54 years (range, 50 to 62 years), younger than expected in the general population. In a more recent study, Win et al25 estimated prostate cancer risk in 382 men after primary CRC diagnosis and estimated an SIR of 2.05 (95% CI, 1.23 to 3.01). Median age at prostate cancer diagnosis in the 19 patient cases was 64 years (range, 55 to 77 years). When stratified by gene, this risk was more pronounced in MSH2 carriers (SIR, 3.62; 95% CI, 2.07 to 5.36).
Our study has several unique strengths, including a larger sample size than previous studies and use of more-rigorous statistical methods. Modified segregation analysis carried out in this study used conditioning on the genotype and phenotype of the proband to reduce ascertainment bias and gain a more accurate prostate cancer risk estimate. The analysis also allowed for inclusion of ungenotyped individuals, thus increasing the effective sample size. The power of the analysis was increased by combining two independent registries and carrying out careful age imputation and sensitivity analysis.1,12 Using this analytic approach in 198 families with LS resulting from mutations in MLH1, MSH2, or MSH6, cumulative lifetime risk of prostate cancer (to age 80 years) was 30.0%, as compared with the general population risk of 17.8%. Overall HR was approximately 2.0; it was greater, nearing 2.5, in younger men diagnosed at age younger than 60 years. The large sample size in this analysis provided adequate power to detect significance.
It is important to mention the limitations with this study design, which include the inability to confirm all prostate cancer diagnoses with medical records. Additionally, with the available data, we were unable to use clinical features such as prostate-specific antigen (PSA) or Gleason score to differentiate between low-risk (clinically insignificant) and intermediate- or high-risk prostate cancer.26 It is true that patients in families with a diagnosis of LS undergo enhanced cancer screening and surveillance. However, until this year, prostate cancer had not been known as an LS-associated cancer, and enhanced prostate cancer screening has not been included in screening recommendations for patients with LS. Therefore, the likelihood that the elevated risk of prostate cancer demonstrated in this study is the result of overdiagnosis of low-risk prostate cancer in asymptomatic individuals is small, but it cannot be ruled out. The fact that Win et al25 also demonstrated an increased risk of prostate cancer with a different study sample, study design, and statistical analysis further supports our finding.
The US Preventive Services Task Force recently issued a recommendation against screening with PSA in asymptomatic men younger than age 75 years.27 However, early-detection strategies may be of benefit for men at increased risk of the disease, including those with family histories of prostate cancer or inherited predisposition to prostate cancer. The National Comprehensive Cancer Network clinical guidelines advocate for annual clinical assessment beginning at age 40 years for men with a family history of prostate cancer.26 Some familial prostate cancer may be explained by mutations in one of several known cancer predisposition genes. Men from families with hereditary breast and ovarian cancer syndrome and mutations in BRCA1/2 have also been found to have increased risks of prostate cancer.28,29 A recent study identified a novel HOXB13 variant associated with an increased risk of prostate cancer, especially early-onset and hereditary forms of the disease.30 These study results indicate LS should be included in the group of inherited cancer syndromes with elevated risk of prostate cancer.
Prospective follow-up of men with MMR gene mutations will elucidate the natural history of prostate cancer in MMR carriers and determine useful techniques for reducing the morbidity and mortality from prostate cancer in this specific subset of patients. The increased risk for prostate cancer in men with LS is supported by recent estimates by Win et al,25 who also demonstrated a two-fold increased risk when using a different study design and analytic tools. Despite the study limitations, our finding that LS is associated with an increased risk of prostate cancer is clinically valuable for patients and clinicians when weighing risks and benefits of screening, and we suggest that prostate cancer screening be offered to this high-risk group. It would be reasonable to consider screening male MMR mutation carriers with digital rectal examination and PSA beginning at age 40 years. Additional studies to quantify the potential risks, benefits, and cost effectiveness of this screening will offer guidance about optimal strategies to manage prostate cancer risk in patients with LS. Analysis of the grade of prostate cancer, both symptomatic and asymptomatic, identified in men with LS compared with those diagnosed in the general population as well as comparisons of clinical outcomes will provide further insight into differences in the natural history of prostate cancer in these two groups. Additional studies will also help clarify whether the prognostic advantage observed in MSI CRCs is also observed in prostate cancer, which may lead to specific therapies targeting the MSI phenotype.31–33
Appendix
Table A1.
Sensitivity Analysis of CR: No Age Imputation, Conditioned on Genotype and Phenotype Status of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| 50 | 0.26 | 0.72 | 0.26 | 0.61 | 0.26 | 0.68 |
| 60 | 2.59 | 7.04 | 2.59 | 6.03 | 2.59 | 6.65 |
| 70 | 9.49 | 13.63 | 9.49 | 15.01 | 9.49 | 13.27 |
Abbreviations: CR, cumulative risk; MMR, mismatch repair; UM, University of Michigan.
Table A2.
Sensitivity Analysis of HRs: No Age Imputation, Conditioned on Genotype and Phenotype Status of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 20 to 59 | 2.781 | 1.313 to 5.891 | 2.370 | 0.842 to 6.668 | 2.621 | 1.425 to 4.823 |
| 60 to 80 | NA | NA | 1.366 | 0.445 to 4.190 | 1 | 1 to 1 |
| Overall HR | 1.352 | 0.702 to 2.603 | 1.738 | 0.834 to 3.620 | 1.495 | 0.916 to 2.441 |
Abbreviations: HR, hazard ratio; NA, not applicable; UM, University of Michigan.
Table A3.
Sensitivity Analysis of CR: No Age Imputation, No Conditioning on Genotype or Phenotype Status of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| 50 | 0.26 | 0.63 | 0.26 | 0.60 | 0.26 | 0.61 |
| 60 | 2.59 | 6.16 | 2.59 | 5.88 | 2.59 | 6.03 |
| 70 | 9.49 | 12.82 | 9.49 | 18.89 | 9.49 | 14.44 |
Abbreviations: CR, cumulative risk; MMR, mismatch repair; UM, University of Michigan.
Table A4.
Sensitivity Analysis of HRs: No Age Imputation, No Conditioning on Genotype or Phenotype Status of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 20 to 59 | 2.424 | 1.130 to 5.201 | 2.308 | 0.924 to 5.765 | 2.371 | 1.317 to 4.268 |
| 60 to 80 | NA | NA | 2.023 | 0.974 to 4.201 | 1.274 | 0.692 to 2.347 |
| Overall HR | 1.322 | 0.709 to 2.467 | 2.123 | 1.192 to 3.782 | 1.649 | 1.080 to 2.517 |
Abbreviations: HR, hazard ratio; NA, not applicable; UM, University of Michigan.
Table A5.
Sensitivity Analysis of CR: Age-Imputed Data, Without Conditioning on Genotype or Phenotype of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | CR in Population (%) | CR in MMR Carriers (%) | |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40 | 0 | 0.01 | 0 | 0.01 | 0 | 0.01 |
| 50 | 0.26 | 0.60 | 0.26 | 0.56 | 0.26 | 0.58 |
| 60 | 2.59 | 5.94 | 2.59 | 5.56 | 2.59 | 5.76 |
| 70 | 9.49 | 16.16 | 9.49 | 21.69 | 9.49 | 18.39 |
Abbreviations: CR, cumulative risk; MMR, mismatch repair; UM, University of Michigan.
Table A6.
Sensitivity Analysis of HRs: Age-Imputed Data, Without Conditioning on Genotype or Phenotype of Proband
| Age (years) | Harvard |
UM |
Combined |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| 20 to 59 | 2.334 | 1.082 to 5.036 | 2.178 | 0.870 to 5.454 | 2.261 | 1.251 to 4.085 |
| 60 to 80 | 1.563 | 0.766 to 3.189 | 2.548 | 1.322 to 4.908 | 1.957 | 1.205 to 3.180 |
| Overall HR | 1.831 | 1.084 to 3.093 | 2.411 | 1.397 to 4.159 | 2.067 | 1.417 to 3.015 |
Abbreviations: HR, hazard ratio; UM, University of Michigan.
Table A7.
Person-Years, No. of Patient Cases, and No. of At-Risk Individuals Based on Observed Age Data for Combined DFCI and UMCC Data Sets (N = 4,404)
| Age (years) | No. of Individuals | Person-Years | No. of Patient Cases | No. at Risk |
|---|---|---|---|---|
| 0 to 20 | 844 | 12,412 | 0 | 4,341 |
| 21 to 30 | 384 | 11,544 | 0 | 4,274 |
| 31 to 40 | 712 | 28,709 | 2 | 4,125 |
| 41 to 50 | 647 | 32,355 | 11 | 3,942 |
| 51 to 60 | 732 | 43,869 | 28 | 3,630 |
| 61 to 70 | 423 | 29,547 | 22 | 3,406 |
| 71 to ≥ 80 | 661 | 56,673 | 15 | 2,998 |
NOTE. Patient case numbers add up to 78 (those for whom we have observed age of onset) instead of 97.
Abbreviations: DFCI, Dana-Farber Cancer Institute; UMCCC, University of Michigan Comprehensive Cancer Center.
Table A8.
CR and CIN Among MMR Carriers
| Age (years) | CR in Population (%) | DFCI |
UMCCC |
Cumulative |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR (%) | 95% CI | P | CIN (%) | 95% CI | CR (%) | 95% CI | P | CIN (%) | 95% CI | CR (%) | 95% CI | P | CIN (%) | 95% CI | ||
| 40 | 0 | 0.002 | 0.00 to 0.004 | .10 | 0.002 | 0.00 to 0.004 | 0.002 | 0.00 to 0.004 | .30 | 0.002 | 0.00 to 0.004 | 0.002 | 0.00 to 0.004 | .06 | 0.002 | 0.00 to 0.004 |
| 50 | 0.26 | 0.69 | 0.16 to 1.18 | .10 | 0.68 | 0.17 to 1.12 | 0.57 | 0.00 to 1.13 | .29 | 0.56 | 0.00 to 1.14 | 0.64 | 0.24 to 1.01 | .06 | 0.63 | 0.24 to 1.02 |
| 60 | 2.59 | 6.77 | 1.69 to 11.57 | .10 | 7.00 | 1.71 to 12.30 | 5.60 | 0.00 to 11.08 | .30 | 5.75 | 0.00 to 11.72 | 6.30 | 2.47 to 9.96 | .06 | 6.50 | 2.50 to 10.53 |
| 70 | 9.49 | 16.77 | 7.71 to 24.80 | .11 | 18.33 | 8.03 to 28.51 | 18.74 | 6.22 to 29.56 | .14 | 20.76 | 6.42 to 35.05 | 17.39 | 10.10 to 24.07 | .03 | 19.05 | 10.70 to 27.54 |
| 80 | 17.84 | 28.18 | 11.50 to 41.72 | .20 | 33.10 | 12.22 to 54.03 | 33.28 | 9.55 to 50.79 | .18 | 40.45 | 10.07 to 70.93 | 30.00 | 16.54 to 41.30 | .07 | 35.63 | 18.01 to 53.28 |
NOTE. Penetrance models reported in Table 2.
Abbreviations: CIN, cumulative incidence; CR, cumulative risk; DFCI, Dana-Farber Cancer Institute; MMR, mismatch repair; UMCCC, University of Michigan Comprehensive Cancer Center.
Footnotes
Processed as a Rapid Communication manuscript; See accompanying article on page 1782
Supported by Grants No. K24 113433 from NIH/NCI (S.S.), R01 CA136621 and P50 CA69568 from NCI (K.A.C.), and P30 CA014089 and P30 CA046592 from NIH/NCI (S.B.G.).
Presented at the 15th Annual Meeting of the Collaborative Group of the Americas on Inherited Colorectal Cancer, Montreal, Quebec, Canada, October 10-11, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Victoria M. Raymond, Myriad Genetics (C); Sapna Syngal, Archimedes (C), Myriad Genetics (U), Quest Diagnostics (C); Stephen B. Gruber, Myriad Genetics (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Victoria M. Raymond, Bhramar Mukherjee, Elena M. Stoffel, Kathleen A. Cooney, Stephen B. Gruber
Financial support: Sapna Syngal, Stephen B. Gruber
Administrative support: Victoria M. Raymond, Stephen B. Gruber
Provision of study materials or patients: Fay Kastrinos, Sapna Syngal, Stephen B. Gruber
Collection and assembly of data: Victoria M. Raymond, Shu-Chen Huang, Elena M. Stoffel, Fay Kastrinos, Sapna Syngal, Stephen B. Gruber
Data analysis and interpretation: Victoria M. Raymond, Bhramar Mukherjee, Fei Wang, Fay Kastrinos, Sapna Syngal, Kathleen A. Cooney, Stephen B. Gruber
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarnio M, Mecklin JP, Aaltonen LA, et al. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- 3.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 6.van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47:464–470. doi: 10.1136/jmg.2010.076992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dores GM, Curtis RE, Toro JR, et al. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: Insight into Muir-Torre syndrome. Cancer. 2008;113:3372–3381. doi: 10.1002/cncr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: A report of 121 families with proven mutations. Clin Genet. 2009;75:141–149. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute: Surveillance, Epidemiology, and End Results. http://seer.cancer.gov.
- 10.Soravia C, van der Klift H, Bründler MA, et al. Prostate cancer is part of the hereditary non-polyposis colorectal cancer (HNPCC) tumor spectrum. Am J Med Genet A. 2003;121A:159–162. doi: 10.1002/ajmg.a.20106. [DOI] [PubMed] [Google Scholar]
- 11.Wagner DG, Gatalica Z, Lynch HT, et al. Neuroendocrine-type prostatic adenocarcinoma with microsatellite instability in a patient with lynch syndrome. Int J Surg Pathol. 2010;18:550–553. doi: 10.1177/1066896910379406. [DOI] [PubMed] [Google Scholar]
- 12.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange K, Weeks D, Boehnke M. Programs for pedigree analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5:471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger M, Denzinger S, Hammerschmied CG, et al. Elevated microsatellite alterations at selected tetranucleotides (EMAST) and mismatch repair gene expression in prostate cancer. J Mol Med (Berl) 2006;84:833–841. doi: 10.1007/s00109-006-0074-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Wang J, Fraig MM, et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 2001;61:4112–4121. [PubMed] [Google Scholar]
- 17.Ahman AK, Jonsson BA, Damber JE, et al. Low frequency of microsatellite instability in hereditary prostate cancer. BJU Int. 2001;87:334–338. doi: 10.1046/j.1464-410x.2001.00104.x. [DOI] [PubMed] [Google Scholar]
- 18.Bauer CM, Ray AM, Halstead-Nussloch BA, et al. Hereditary prostate cancer as a feature of Lynch syndrome. Fam Cancer. 2011;10:37–42. doi: 10.1007/s10689-010-9388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva FC, de Oliveira LP, Santos EM, et al. Frequency of extracolonic tumors in Brazilian families with Lynch syndrome: Analysis of a hereditary colorectal cancer institutional registry. Fam Cancer. 2011;9:563–570. doi: 10.1007/s10689-010-9373-2. [DOI] [PubMed] [Google Scholar]
- 20.Goecke T, Schulmann K, Engel C, et al. Genotype-phenotype comparison of German MLH1 and MSH2 mutation carriers clinically affected with Lynch syndrome: A report by the German HNPCC Consortium. J Clin Oncol. 2006;24:4285–4292. doi: 10.1200/JCO.2005.03.7333. [DOI] [PubMed] [Google Scholar]
- 21.Scott RJ, McPhillips M, Meldrum CJ, et al. Hereditary nonpolyposis colorectal cancer in 95 families: Differences and similarities between mutation-positive and mutation-negative kindreds. Am J Hum Genet. 2001;68:118–127. doi: 10.1086/316942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grindedal EM, Møller P, Eeles R, et al. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2460–2467. doi: 10.1158/1055-9965.EPI-09-0058. [DOI] [PubMed] [Google Scholar]
- 23.Maul JS, Warner NR, Kuwada SK, et al. Extracolonic cancers associated with hereditary nonpolyposis colorectal cancer in the Utah Population Database. Am J Gastroenterol. 2006;101:1591–1596. doi: 10.1111/j.1572-0241.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 24.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: A prospective cohort study. J Clin Oncol. 2012;30:958–964. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst. 2012;104:1363–1372. doi: 10.1093/jnci/djs351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology: Prostate Cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 27.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 28.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 29.Ostrander EA, Udler MS. The role of the BRCA2 gene in susceptibility to prostate cancer revisited. Cancer Epidemiol Biomarkers Prev. 2008;17:1843–1848. doi: 10.1158/1055-9965.EPI-08-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, An JY, Noh SH, et al. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol. 2011;26:585–592. doi: 10.1111/j.1440-1746.2010.06487.x. [DOI] [PubMed] [Google Scholar]
- 33.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]