Abstract
Purpose
We previously reported the eradication of human epidermal growth factor receptor 2 (HER2)– amplified human xenografts in mice by inhibition of the HER2 pathway with lapatinib and trastuzumab to block all homo- and heterodimer signaling as well as by blockade of estrogen receptor (ER) when expressed. In this clinical trial, we sought to translate these findings to patients using targeted therapy without chemotherapy.
Patients and Methods
Women with stages II to III HER2-positive breast cancers were eligible. They received trastuzumab once per week (4 mg/kg loading, then 2 mg/kg) and lapatinib 1000 mg once per day for 12 weeks. Women with ER-positive tumors also received letrozole (plus a luteinizing hormone–releasing hormone [LHRH] agonist if premenopausal). Pathologic response was assessed by ER status. Biopsies were obtained at baseline, weeks 2 and 8, and time of surgery.
Results
Sixty-six patients were enrolled, and 64 were eligible and evaluable for response. Median tumor size was 6 cm (range, 1.5 to 30 cm). Adverse events were mainly grades 1 to 2 (GI, 63%; skin, 46%). Grade 3 metabolic, GI, and liver (18%; 12 patients) and grade 4 liver toxicities (one patient) were also observed. Overall, in-breast pathologic complete response (pCR; ypT0-is) was 27% (ER positive, 21%; ER negative, 36%). The rate of low-volume residual disease (ypT1a-b) was 22% (ER positive, 33%; ER negative, 4%).
Conclusion
In patients with locally advanced HER2-positive breast cancer, our approach of targeted therapy only resulted in a high pCR rate without chemotherapy. Our data support the hypothesis that selected patients with HER2-positive tumors may not need chemotherapy, and more-complete blockade of HER receptors and ER is an effective strategy worthy of further study.
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2) is a member of a membrane tyrosine kinase receptor family (ie, HER1-4). HER2 has no known ligands, but other family members are activated by 11 different ligands.1–7 Ligand binding induces receptor homo- and heterodimerizations, which activate a phosphorylation signaling cascade. Downstream, the pathway regulates transcription of genes crucial for malignant progression, including cell proliferation, survival, angiogenesis, invasion, and metastasis.1–4,6,8,9
HER2 is amplified or overexpressed in 20% to 25% of breast cancers and is an ideal treatment target because it is the major driver of tumor progression in these tumors. Half of these tumors are estrogen receptor (ER) positive, and half are ER negative. HER2-overexpressing or -amplified (ie, HER2 positive) tumors have an aggressive behavior, with rapid growth and frequent metastasis.
Targeted therapy with the humanized monoclonal antibody trastuzumab, which interacts with the extracellular domain of HER2 to inhibit its function, is effective in the treatment of HER2-positive tumors. Studies have suggested that trastuzumab increases the effectiveness of chemotherapy, and regimens combining trastuzumab with cytotoxic agents have become standard.10,11 Adjuvant therapy combining chemotherapy with trastuzumab has markedly improved outcomes for patients with this subtype of breast cancer.12,13
The mechanism of action of trastuzumab is not completely clear, but it is known to effectively inhibit signaling from HER2 homodimers. However, it is only a weak inhibitor of signaling from HER2 heterodimers with HER1 and HER3.14,15 The result is downregulation of signaling through the phosphoinositide kinase-3 (PI3K)/AKT pathway and the induction of apoptosis in human tumors.6,8,16–19 Trastuzumab does not inhibit signaling by HER1 (epidermal growth factor receptor [EGFR]) homodimers or HER1 heterodimers with HER3.20–22
Trastuzumab may also work in part by inducing antibody-dependent cellular cytotoxicity. Although trastuzumab combined with chemotherapy is effective in reducing the risk of recurrence in patients, many HER2-positive tumors exhibit de novo or acquired resistance.12,13
Many mechanisms for resistance have been suggested, including downstream activation of the pathway by phosphatase and tensin homolog (PTEN) loss, activating mutations in PI3K, or amplification of cyclin E.23–27 In samples from two prior neoadjuvant trials, we reported that PTEN loss and PI3K mutations were associated with resistance to trastuzumab but not lapatinib.28
A short form of HER2 missing the extracellular domain, so-called p95, is constitutively active and unresponsive to trastuzumab because it lacks the trastuzumab-binding domain.29–31 Upregulation of receptor ligands or the receptors themselves has also been proposed as a mechanism of acquired resistance.32,33 Escape pathways such as ER or insulin-like growth factor receptor signaling have also been implicated in resistance.33,34
We previously hypothesized that one mechanism for resistance to trastuzumab is incomplete blockade of the HER receptor layer of the network because the drug does not block signaling from all HER family dimer pairs that can also activate downstream signaling. We also hypothesized that more-complete blockade of this major driver pathway along with ER (an escape pathway) when expressed might be effectively accomplished in the absence of chemotherapy.
Several drugs are available to inhibit the HER receptor layer more completely than trastuzumab alone. Gefitinib and erlotinib are potent kinase inhibitors of HER1.35 Pertuzumab binds to the heterodimerization domain of HER2 and blocks its interaction with HER1 and HER3, but it does not block HER1 homodimers or HER1:HER3 heterodimers.36 Lapatinib is a dual-kinase inhibitor (HER1 and HER2).37 We first showed in animal models that the three-drug combination of gefitinib, pertuzumab, and trastuzumab, which blocks signaling from all HER receptor homo- and heterodimer pairs, is much more effective than any of the single agents or two-drug combinations like trastuzumab and pertuzumab, and we reported that the combination was capable of eradicating HER2-overexpressing xenografts in mice.38 Later, we showed that the two-drug combination of lapatinib and trastuzumab was also effective in eradicating HER2-overexpressing xenografts.39 Inhibition of HER1 activity was required, even though this receptor is expressed at low levels in these models. In ER-positive tumors, endocrine therapy with estrogen deprivation was also required for optimal antitumor effects.38,39 Even-lower drug doses and intermittent therapy with lapatinib and trastuzumab were effective in eradicating most tumors.39 After these preclinical results, we proposed a neoadjuvant clinical trial with lapatinib plus trastuzumab combined with endocrine therapy for ER-positive tumors. Studies from other groups have also suggested that dual targeting with lapatinib and trastuzumab is more effective than either one alone.40–42
PATIENTS AND METHODS
This study was conducted in collaboration with the Translational Breast Cancer Research Consortium. Institutional review board and scientific committee approval were obtained at the lead site (Baylor College of Medicine) as well as all other participating sites. Written informed consent was obtained from all patients.
Eligible patients were women age ≥ 18 years with histologically confirmed invasive HER2-positive breast cancer. HER2 positivity was defined as overexpression by immunohistochemistry (3+) or amplification by fluorescent in situ hybridization. Breast tumors were required to be > 3 cm by clinical measurement or > 2 cm with a palpable ipsilateral axillary lymph node confirmed by two physicians.
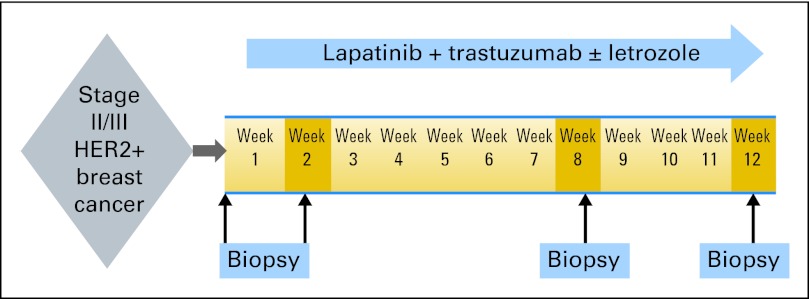
Figure 1 shows the study schema. This was a multicenter single-arm phase II study. All eligible patients received study treatment, which consisted of lapatinib (Tykerb, supplied by GlaxoSmithKline [London, United Kingdom]) 1,000 mg orally every day and trastuzumab 4 mg/kg loading dose followed by 2 mg/kg once per week. Patients whose tumors were ER and/or progesterone receptor (PR) positive, as defined by the testing laboratory, were also treated with letrozole 2.5 mg orally once per day (combined with LHRH agonist of choice in premenopausal women). Study treatment was administered for 12 weeks. Tumor biopsies were collected at baseline, weeks 2 and 8, and end of study treatment (surgical sample collection) for molecular studies.
Fig 1.
Study schema. HER2, human epidermal growth factor receptor 2.
If more therapy was deemed necessary after study treatment was completed, patients were allowed to receive trastuzumab-based chemotherapy. Adjuvant therapy after surgery occurred at the discretion of the treating physician.
Study goals were to measure the rate of pathologic complete response (pCR; disappearance of all invasive tumor in the breast; ypT0-is) as well as pathologic response rate, defined as pCR plus residual invasive disease of ≤ 1 cm (ypT1a-b) at the time of surgery. Pathologic assessment of response was assessed according to local institutional guidelines and practice. If patients did not proceed to surgery or received additional neoadjuvant therapy before surgery, they were considered as not achieving the primary end point of pathologic response. We also assessed the safety and toxicity of the combination regimen.
The study was stratified by ER status (ER positive, ER negative) and was designed to detect an increase in pathologic response rate (ypT0-is plus ypT1a-b) from 10% expected with trastuzumab to 35% in each stratum, using a Simon optimal two-stage design with one-sided alpha of 5% and power of 85%. Both strata met criteria to continue to full accrual (at least 21 per stratum).
RESULTS
Between June 11, 2008, and November 2, 2010, 66 women were enrolled at the five sites (Baylor College of Medicine, Vanderbilt University, University of Alabama in Birmingham, University of Chicago, and Mayo Clinic). One patient withdrew consent before starting treatment and was excluded from further analysis. One patient turned out to have an HER2-negative tumor (on further review by the local laboratory) and was therefore included in the safety analysis but excluded from the efficacy analysis. One patient was found, after treatment was initiated, to have been enrolled with a smaller tumor than was allowed in the study (1.5 cm). She was included in the analysis because she received treatment. Therefore, there were 65 participants evaluable for safety and 64 evaluable for efficacy.
Patient characteristics are listed in Table 1. The median age was 49 years (range, 31 to 74), and more than half of the patients were premenopausal. Enrollment reflected a diverse study population, with 22% being African American and 32% Hispanic. The study enrolled patients with large tumors, with a median tumor size of 6 cm (range, 1.5 to 30 cm), and 62% of patients had tumors that were > 5 cm (25th percentile, 4 cm;75th percentile, 8 cm)
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. | % |
| Age, years | ||
| ≤ 50 | 34 | 52 |
| > 50 | 31 | 48 |
| Median | 49 | |
| Range | 31-74 | |
| Race | ||
| White | 48 | 74 |
| Black | 14 | 21.5 |
| Asian | 1 | 1.5 |
| American Indian | 1 | 1.5 |
| Unknown | 1 | 1.5 |
| Ethnicity | ||
| Hispanic | 21 | 32 |
| Non-Hispanic | 43 | 66 |
| Unknown | 1 | 1.5 |
| Menstrual status | ||
| Premenopausal | 35 | 54 |
| Postmenopausal | 30 | 46 |
| ECOG status | ||
| 0 | 61 | 94 |
| 1 | 4 | 6 |
| Tumor size, cm | ||
| ≤ 5 | 25 | 38 |
| > 5 | 40 | 62 |
| Median | 6 | |
| Range | 1.5-30 | |
| ER | ||
| Positive | 40 | 62 |
| Negative | 25 | 38 |
| PR | ||
| Positive | 29 | 45 |
| Negative | 36 | 55 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PR, progesterone receptor.
Table 2 summarizes the efficacy of the study treatment. The overall rate of pCR in this nonchemotherapy-containing regimen was 27%.In the ER-positive group, pCR was 21%, whereas in the ER-negative group, it was 36%.
Table 2.
Pathologic Response Rates
| ER Status | pCR |
ypT1a-b |
NR |
Total No. of Patients | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Positive | 8 | 21 | 13 | 33 | 18 | 46 | 39 |
| Negative | 9 | 36 | 1 | 4 | 15 | 60 | 25 |
| Total | 17 | 27 | 14 | 22 | 33 | 52 | 64 |
Abbreviations: ER, estrogen receptor; NR, nonpathologic response; pCR, pathologic complete response.
The protocol-specified pathologic response rate (ypT0-is plus ypT1a-b) was 49% overall. In the ER-positive group, it was 54%, and in the ER-negative group, it was 40%. This shows that a subset of patients derived substantial clinical benefit from the 12-week treatment despite large initial tumor size, but some of them, especially those with ER-positive tumors, still had minimal residual disease at the time of surgery.
Overall, 89% of patients proceeded to surgery after completion of study treatment. The protocol allowed patients to receive additional therapy before surgery if deemed necessary by the treating physician. Five patients (8%) received additional neoadjuvant therapy before proceeding to surgery. Only two patients (3%) experienced progression during study treatment. Both patients had ER- and PR-negative tumors.
pCR in this study was defined as disappearance of invasive cancer in the breast, which is the most common definition used in other neoadjuvant trials. Table 3 also shows the rate of pCR defined as no residual invasive cancer in the breast or axilla (ypT0-is ypN0). Even with this stricter definition of pCR, our targeted therapy approach showed 22% of patients (ER negative, 28%. ER positive, 18%) with no residual invasive cancer in the breast or axilla
Table 3.
Pathologic Complete Response Rate in Breast and Lymph Nodes
| ER Status |
ypT0-isN0-3 |
ypT0-is N0 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Positive | 8 | 21 | 7 | 18 |
| Negative | 9 | 36 | 7 | 28 |
| Total | 17 | 27 | 14 | 22 |
Abbreviation: ER, estrogen receptor.
Sixty-five patients were evaluable for safety (Table 4). The study treatment was generally well tolerated. Treatment was discontinued because of toxicity in only 6% of patients. The most common toxicities were diarrhea (grades 1 to 2, 63%; grades 3 to 4, 3%) followed by rash (grades 1 to 2, 55%; grades 3 to 4, 1%), fatigue (32%), nausea (31%), and elevated liver function tests (grades 1 to 2, 18%; grade 3, 5%; grade 4, 2%). Only one grade 4 toxicity was observed in the study (elevated liver function tests), which completely normalized after 8 weeks.
Table 4.
Adverse Events
| Adverse Event | Grades 1 and 2 |
Grades 3 and 4 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| GI | ||||
| Diarrhea | 41 | 63 | 2 | 3 |
| Nausea | 20 | 31 | 0 | 0 |
| Heartburn/dyspepsia | 12 | 18 | 0 | 0 |
| Mucositis/stomatitis | 13 | 20 | 0 | 0 |
| Hepatic | ||||
| ALT | 13 | 20 | 3 | 5 |
| AST | 9 | 14 | 4 | 6 |
| Alkaline phosphatase | 8 | 12 | 0 | 0 |
| Elevated bilirubin | 4 | 6 | 1 | 2 |
| Skin | ||||
| Rash | 36 | 55 | 1 | 2 |
| Dry skin/other | 12 | 18 | 0 | 0 |
| Constitutional | ||||
| Fatigue | 21 | 32 | 0 | 0 |
| Hot flashes | 10 | 15 | 0 | 0 |
| Anorexia | 4 | 6 | 0 | 0 |
| Laboratory | ||||
| Anemia | 14 | 22 | 0 | 0 |
| Hypokalemia | 11 | 17 | 0 | 0 |
| Hyperglycemia | 10 | 15 | 1 | 1.5 |
| Hypocalcemia | 5 | 8 | 0 | 0 |
| Hyponatremia | 6 | 9 | 0 | 0 |
| Other | ||||
| Mood alteration | 8 | 12 | 0 | 0 |
| Hypertension | 6 | 9 | 1 | 2 |
| Insomnia | 7 | 11 | 0 | 0 |
| Taste alteration | 5 | 8 | 0 | 0 |
Serial tumor biopsies were an integral part of this trial. Table 5 summarizes the rate of collection. A majority of biopsies were performed successfully at the specified time points, despite the intense schedule of three scheduled biopsies over the course of treatment (fourth time point was surgical specimen collection).
Table 5.
Biopsy Sample Collection
| Time Point | Biopsies Performed |
|
|---|---|---|
| No. | % | |
| Baseline | 61 | 95 |
| Week 2 | 57 | 89 |
| Week 8 | 50 | 78 |
| Total | 168 | 88 |
DISCUSSION
Normal cells contain several redundant networks of signaling proteins that function to keep the cell alive when threatened by a noxious environment. Cancers arise because of hyperactivation of one or more of these normal proliferative and survival pathways through mutations such as amplification of normal genes that then become oncogenic.43,44 The oncogene-addiction hypothesis proposes that a cell hijacks and hyperactivates a normal pathway to become malignant, and then other potential survival pathways become unnecessary for cell survival. These redundant pathways may be reactivated when the driver pathway is inhibited. The hypothesis also suggests that if the driver pathway is completely blocked, cells addicted to this oncogenic pathway will die before reactivation of redundant escape pathways.43,44 Activation of HER2 in the mammary gland of transgenic mice causes breast cancer, but shutting down the pathway by downregulating HER2 causes complete tumor eradication, with few tumors re-growing by reactivation of another survival network.38,45,45
Here, we show in a neoadjuvant clinical trial in patients with large locally advanced HER2-positive tumors that a potent cocktail of drugs that more completely blocks the HER network causes pCR in the breast in a substantial percentage of patients. Achieving this rate of pCR with targeted therapy only and without using chemotherapy is noteworthy and clinically meaningful. The rate of residual invasive disease of ≤ 1 cm (ypT1a-b) in the ER-positive subset of 33% is also substantial because ER-positive tumors regress more slowly than ER- negative tumors, and it is possible that treatment beyond the 12 weeks studied here would result in a higher pCR rate even in these tumors. We are currently investigating this hypothesis in an ongoing follow-up study. Although pCR is associated with long-term favorable outcome of patients with ER-negative/HER2-positive disease, this end point may not accurately predict outcomes in ER-positive/HER2-positive tumors. The data suggest that there may be a subset of patients with HER2-positive breast cancer who do not need chemotherapy, a hypothesis that requires further study and the identification of biomarkers to help select the appropriate patients.
The results of our clinical study using lapatinib, trastuzumab, and endocrine therapy in the ER-positive subset were predicted by our preclinical observations.39 We had previously shown that a regimen that more completely blocked the pathway was effective in xenograft models and that the combination of trastuzumab and lapatinib was more effective than either of the single agents alone.38,39 Results of our earlier clinical trials indicating that the mechanism of action of trastuzumab and lapatinib is likely to involve different signaling pathways support these findings.28 We also demonstrated the efficacy of potent HER-pathway inhibition with the combination of trastuzumab and pertuzumab in preclinical models, which was more effective than either agent alone. However, its efficacy was further enhanced significantly by adding gefitinib to inhibit EGFR (HER1), even though EGFR is barely detectable by Western blot in these tumors xenografts.38 We also showed that blocking ER simultaneously with HER2 was required for eradication of tumors in mice, demonstrating that ER can function as an escape pathway in some tumors.38,39
The results of NeoSphere (A Randomized, Open Label Study to Compare the Complete Pathological Response Rate Achieved With 4 Combinations of Herceptin, Docetaxel and Pertuzumab in Patients With Locally Advanced, Inflammatory or Early Stage HER2 Positive Breast Cancer), a neoadjuvant clinical trial using trastuzumab and pertuzumab alone or in combination with chemotherapy in patients, were recently reported.46 The study showed the in-breast pCR rate in the biologic therapy–only arm (trastuzumab and pertuzumab without chemotherapy) to be 6% in ER-positive/HER2-positive tumors and 29% in ER-negative/HER2-positive tumors using the same pCR definition.46 This regimen does not inhibit EGFR activity, and the regimen did not contain hormonal therapy. On the basis of data from preclinical models, it is plausible to speculate that the efficacy of the regimen might have been enhanced if it had targeted EGFR and ER as well. In essence, these cumulative laboratory and clinical findings support the hypothesis that complete blockade of the HER receptor family in HER2-positive breast cancer, along with targeting of ER simultaneously when coexpressed, may be necessary for optimal therapy.38,39
The first results of the neoALTTO (Neo-Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial, a large randomized neoadjuvant trial of lapatinib, trastuzumab, or their combination with paclitaxel, have also been reported. This trial showed a much higher pCR rate with lapatinib plus trastuzumab combined with paclitaxel (ER positive, 42%; ER negative, 61%) than with lapatinib (ER positive, 16%; ER negative, 34%) or trastuzumab alone (ER positive, 23%; ER negative, 36%).47
Data from both these two large trials lend further support to the idea that targeting HER2-positive tumors with multiple agents to more effectively block the network will produce better results. They also demonstrate that chemotherapy adds to the benefit of targeted therapy in a substantial number of patients.
However, if pCR in neoadjuvant trials predicts long-term outcome, as supported by several studies, then our data do suggest that a sizeable subset of patients with HER2-positive tumors, if they can be identified a priori or selected by response to neoadjuvant targeted therapy, may not require chemotherapy. Biomarkers that may distinguish patients who do not require chemotherapy need to be determined and are now being studied in samples from this neoadjuvant trial. These tissue correlative studies are additionally focusing on alternative survival pathways known to be associated with resistance to this combination regimen and may yield new potential targets to reverse this resistance in patients.
Our endeavor to identify these biomarkers will be facilitated by the fact that we were successful in acquiring most serial tumor biopsies from study participants at various time points. Biomarker analysis is being currently performed on these samples. Our study demonstrates that neoadjuvant clinical trials with intense biopsy schedules are feasible and can play an important role in the development of new treatments and biomarker discovery.
This trial is unique in many other aspects. It enrolled patients with large breast tumors and yet showed meaningful efficacy of our approach. It also had a strong representation of minority patients who are more likely to have locally advanced breast cancer.
Our study may be limited by its single-arm phase II design; its results need to be validated in a larger setting. However, to our knowledge, this multicenter trial resulted in the highest rate of pCR to date using targeted therapy only and is the first one using this combination in the absence of chemotherapy. Its results raise the prospect of sparing a subset of patients in the future the toxicity and cost of chemotherapy, a worthy and meaningful goal.
Footnotes
Written on behalf of the Translational Breast Cancer Research Consortium (TBCRC).
Supported in part by Grant No. P30 CA125123 and Specialized Programs of Research Excellence (SPORE) Grant No. P50 CA58183 from the National Cancer Institute as well as grants from Komen Foundation for the Cure, Avon Foundation, Breast Cancer Research Foundation, Stand Up to Cancer, and Lee Jeans and a research grant from GlaxoSmithKline.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00548184.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Carolina Gutierrez, Novartis (C), Genentech (C); C. Kent Osborne, Genentech (C), Novartis (C), Pfizer (C), NanoString Technologies (C) Stock Ownership: None Honoraria: Angel A. Rodriguez, Genentech Research Funding: Mothaffar F. Rimawi, GlaxoSmithKline, Genentech; Andres Forero, GlaxoSmithKline, Genentech; Rachel Schiff, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mothaffar F. Rimawi, Rachel Schiff, C. Kent Osborne, Jenny C. Chang
Administrative support: Anne C. Pavlick
Provision of study materials or patients: Anne C. Pavlick
Collection and assembly of data: Mothaffar F. Rimawi, Ingrid A. Mayer, Andres Forero, Rita Nanda, Matthew P. Goetz, Angel A. Rodriguez, Anne C. Pavlick, Tao Wang, Susan G. Hilsenbeck, Carolina Gutierrez, C. Kent Osborne, Jenny C. Chang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Maurer CA, Friess H, Kretschmann B, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 2.Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 3.Wallasch C, Weiss FU, Niederfellner G, et al. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 5.Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: From orphanhood to multiple stromal ligands. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 7.Burgess AW, Cho HS, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 8.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 9.Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 12.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Wehrman TS, Raab WJ, Casipit CL, et al. A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc Natl Acad Sci U S A. 2006;103:19063–19068. doi: 10.1073/pnas.0605218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(suppl 2):1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- 17.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–2468. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 18.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Diermeier S, Horváth G, Knuechel-Clarke R, et al. Epidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activation. Exp Cell Res. 2005;304:604–619. doi: 10.1016/j.yexcr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62:3151–3158. [PubMed] [Google Scholar]
- 22.Ghosh R, Narasanna A, Wang SE, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871–1882. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 24.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 25.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 26.Scaltriti M, Eichhorn PJ, Cortés J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A. 2011;108:3761–3766. doi: 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittendorf EA, Liu Y, Tucker SL, et al. A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene. 2010;29:3896–3907. doi: 10.1038/onc.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2–overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 30.Arribas J, Baselga J, Pedersen K, et al. P95HER2 and breast cancer. Cancer Res. 2011;71:1515–1519. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 31.Liu CY, Yang W, Li JF, et al. Effect of trastuzumab on tumor cell lines shedding high or low level of HER-2 ECD [in Chinese] Zhonghua Zhong Liu Za Zhi. 2007;29:101–105. [PubMed] [Google Scholar]
- 32.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 33.Wang YC, Morrison G, Gillihan R, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers: Role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13:R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 35.Brand TM, Iida M, Li C, et al. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med. 2011;12:419–432. [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 37.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 38.Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 39.Rimawi MF, Wiechmann LS, Wang YC, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res. 2011;17:1351–1361. doi: 10.1158/1078-0432.CCR-10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 41.Nahta R, Yuan LX, Du Y, et al. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 42.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 45.Moody SE, Perez D, Pan TC, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]