Abstract
Our aim is to assess the safety and potential clinical benefit of intravenous iron (Ferinject) infusion in iron deficient patients with idiopathic pulmonary arterial hypertension (IPAH). Iron deficiency in the absence of anemia (1) is common in patients with IPAH; (2) is associated with inappropriately raised levels of hepcidin, the key regulator of iron homeostasis; and (3) correlates with disease severity and worse clinical outcomes. Oral iron absorption may be impeded by reduced absorption due to elevated hepcidin levels. The safety and benefits of parenteral iron replacement in IPAH are unknown. Supplementation of Iron in Pulmonary Hypertension (SIPHON) is a Phase II, multicenter, double-blind, randomized, placebo-controlled, crossover clinical trial of iron in IPAH. At least 60 patients will be randomized to intravenous ferric carboxymaltose (Ferinject) or saline placebo with a crossover point after 12 weeks of treatment. The primary outcome will be the change in resting pulmonary vascular resistance from baseline at 12 weeks, measured by cardiac catheterization. Secondary measures include resting and exercise hemodynamics and exercise performance from serial bicycle incremental and endurance cardiopulmonary exercise tests. Other secondary measurements include serum iron indices, 6-Minute Walk Distance, WHO functional class, quality of life score, N-terminal pro-brain natriuretic peptide (NT-proBNP), and cardiac anatomy and function from cardiac magnetic resonance. We propose that intravenous iron replacement will improve hemodynamics and clinical outcomes in IPAH. If the data supports a potentially useful therapeutic effect and suggest this drug is safe, the study will be used to power a Phase III study to address efficacy.
Keywords: exercise capacity, ferric carboxymaltose, iron, pulmonary arterial hypertension, pulmonary vascular resistance
Intracellular iron and body iron stores are tightly regulated, and iron deficiency, with or without anemia, plays an important role in chronic disease modulation.[1] There is increasing evidence that iron homeostasis is important in pulmonary hypertension with data from three centers showing iron deficiency is (1) common in idiopathic pulmonary arterial hypertension (IPAH), and (2) associated with reduced exercise capacity and increased mortality.[2–4]
True iron status can be difficult to ascertain in chronic diseases such as IPAH using standard laboratory techniques due to the presence of inflammation. Inflammation induces ferritin while repressing serum iron and transferrin saturations.[5] Circulating soluble transferrin receptor (sTfR) levels are largely unaffected by inflammation.[6] Using a sTfR value greater than 28.1 nmol/l as a cut-off for iron deficiency, we have previously shown a high prevalence (63%) of iron deficiency without overt anaemia in 98 patients with IPAH.[2] sTfR levels above this cut-off for iron deficiency with lower 6-Minute-Walk Distance (6MWD), worse World Health Organization (WHO) functional class and predicted survival independent of WHO functional class, and age.[2] Circulating hepcidin, the key inhibitor of iron absorption from the gut, was inappropriately raised in this cohort, suggesting that poor iron uptake contributes to iron deficiency. In support of this, oral iron replacement was ineffective in normalizing serum iron levels in another cohort of IPAH patients.[3]
It is unclear whether iron deficiency reduces exercise capacity and influences the natural history of IPAH directly or is simply an epiphenomenon. Interestingly, iron deficiency is also a common finding in heart failure and is associated with reduced exercise performance and poor survival.[7–9] Studies have shown that intravenous iron supplementation improves the functional status and well-being of patients with iron deficiency and chronic heart failure.[10,11] This has led us to propose that intravenous iron supplementation will have a favorable effect on pulmonary hemodynamics and exercise in IPAH patients who are iron deficient. We present our protocol and discuss the rationale behind its design and potential mechanisms of action for intravenous iron.
We have designed a proof-of-concept study to examine the safety and potential therapeutic benefit of intravenous ferric carboxymaltose (Ferinject) in IPAH. A 36-week, double-blind, randomized, placebo-controlled, crossover study will investigate whether a single dose of 1 g of Ferinject will improve cardiopulmonary hemodynamics, exercise capacity, and quality of life, and is well-tolerated. The primary outcome measure is the effect of intravenous iron replacement on resting pulmonary vascular resistance (PVR) from baseline to 12 weeks, measured by cardiac catheterization.
Patients with IPAH and iron-deficiency who have been stable on their current therapy for the preceding 1 month will be treated with Ferinject® 1000 mg or 15 mg/kg if weight < 66.7Kg. Iron deficiency will be defined by sTfR levels > 28.1 nmol/l. Where not available at screening, patients must have one of the following: ferritin < 37 μg/l or iron < 10.3 μmol/l or transferrin saturations < 16.4%.
The study will take place in three centers—Hammersmith Hospital, London; Royal Hallamshire Hospital, Sheffield; and Papworth Hospital, Cambridge. Each center will recruit 20 patients following the same protocol. All patients will be followed for up to 36 weeks after dosing with Ferinject to measure the kinetics of iron repletion.
The study is funded by the British Heart Foundation. Ferinject is provided as a gift from Vifor (International) Inc., Rechenstrasse, Switzerland, who will remain independent of the study design and have no role with forthcoming data collection or analysis.
MATERIALS AND METHODS
Study subjects
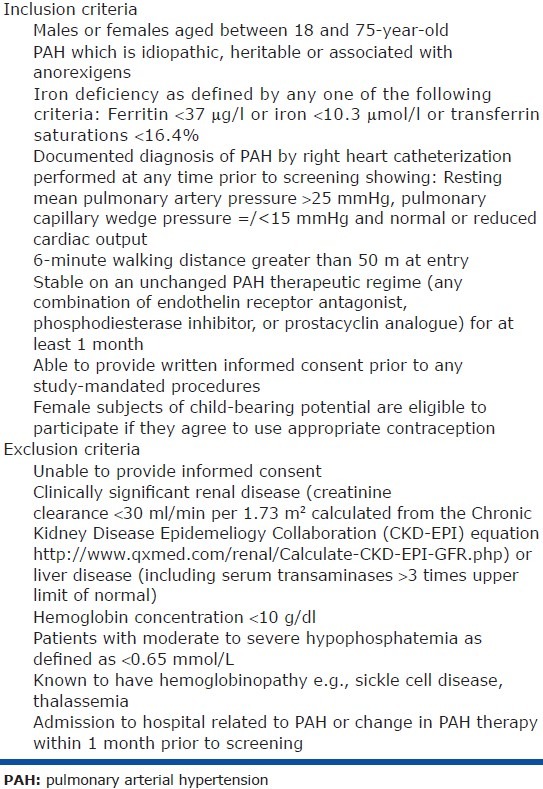
We aim to recruit 60 patients with symptomatic pulmonary arterial hypertension (idiopathic, heritable, or associated with anorexigens). A documented diagnosis of PAH by right heart catheterization is required prior to screening with evidence of a resting mean pulmonary artery pressure > 25 mmHg, a pulmonary capillary wedge pressure equal or less than 15 mmHg, and a normal or reduced cardiac output.[12] The inclusion and exclusion criteria are given in Table 1.
Table 1.
Entry criteria
Study design rationale
The three main considerations in study design were (a) the study definition of iron deficiency, (b) the dose and frequency of administration of intravenous iron, and (c) the timing of endpoints to capture onset and duration of effect.
-
(a)
Soluble transferrin receptor (sTfR > 28.1 nmol/l), the best least-invasive measure of iron deficiency, is not routinely available across all three sites. Profiling data from Rhodes et al.[2] suggest that we can correctly identify 83% of patients with sTfR > 28.1 nmol/l if any one of the following criteria are met: Ferritin < 37 μg/l, iron < 10.3 μmol/l, or transferrin saturations < 16.4%. We estimate that 55% of our patient cohort will be eligible for the study on these criteria.
-
(b)
In the FERRIC-HF trial of iron supplementation in congestive heart failure, patients received 200 mg iron sucrose (Venofer) weekly until iron repletion according to the Ganzoni formula, unless ferritin exceeded 500 μg/l, and then 200 mg every four weeks until the end of the study.[10] By four and 16 weeks, 781 ± 94 and 1269 ± 297 mg iron sucrose had been given to the nonanemic group (Hb > 12.5 g/dl) and 1051 ± 219 and 1583 ± 366 mg had been given to the anemic group. Ferinject, a slow release preparation, can be given in doses up to 1,000 mg. As IPAH patients live throughout the UK, to minimize the number of hospital visits, Ferinject will be given as a single 1,000 mg dose or 15 mg/kg if weight < 66.7 kg. Follow-up iron profiling will be used to determine how long this dose maintains iron repletion.
-
(c)
It is unclear whether iron replacement would have an early effect, perhaps related to vasorelaxation or improved muscle bioenergetics, or a late effect related to vascular remodeling. To capture an early effect, exercise capacity will be measured by cardiopulmonary exercise testing at two weeks and 12 weeks. To capture a meaningful effect on pulmonary hemodynamic, pulmonary artery pressure and cardiac output will be measured by cardiac catheterization at 12 weeks. A crossover design shown in Figure 1, was selected to improve the power to detect an effect on exercise. A repeat third cardiac catheter after crossover was considered unacceptable to patient comfort.
Figure 1.
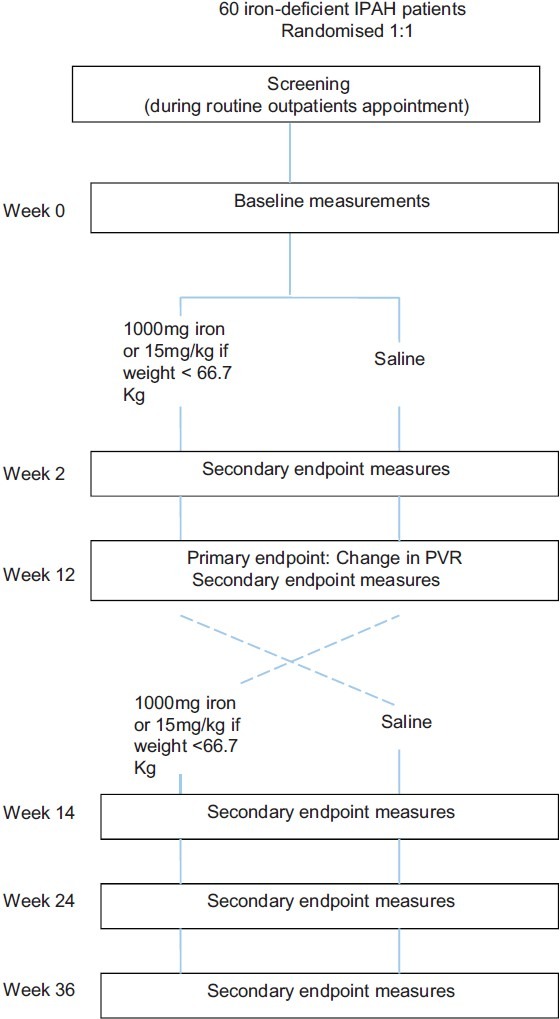
Schematic overview of study to determine the effect of intravenous iron supplementation on cardiopulmonary hemodynamics and exercise capacity in iron-deficient patients with idiopathic pulmonary arterial hypertension.
Subjects will be considered complete for the purpose of this study once they have completed all procedures at the end of Week 24. The end of study is defined as the last visit of the last subject undergoing the trial.
Recruitment and randomization
Potential participants will be identified and screened using data collected during their routine outpatient appointment at each of the Pulmonary Hypertension centers.
Patients will be randomized on 1:1 ratio to each treatment arm. Randomization will be performed at the Baseline (Week 0) visit using InForm. Randomization will be stratified by gender, with an appropriate fixed block size, in order to ensure equal allocation to Ferinject and placebo. The computer-generated randomization list will be prepared by a statistician independent of the study team and will be provided to Imperial College staff (who are not otherwise involved in the study) responsible for building the InForm system.
Investigational product(s)
Patients who are eligible to participate in the study will receive Ferinject or normal saline (placebo). Ferinject is currently licensed up to doses of 1,000 mg as an intravenous infusion in the UK for the treatment of iron deficiency when oral preparations cannot be used or are ineffective.
Ferinject has recently been used in a large Phase III trial in heart failure.[11] Adverse events were few in 304 patients assigned to receive Ferinject and were not significantly different from the placebo group (n = 105). Only two patients reported injection site pain and no allergic reactions were described.
Outcome measures and data collection
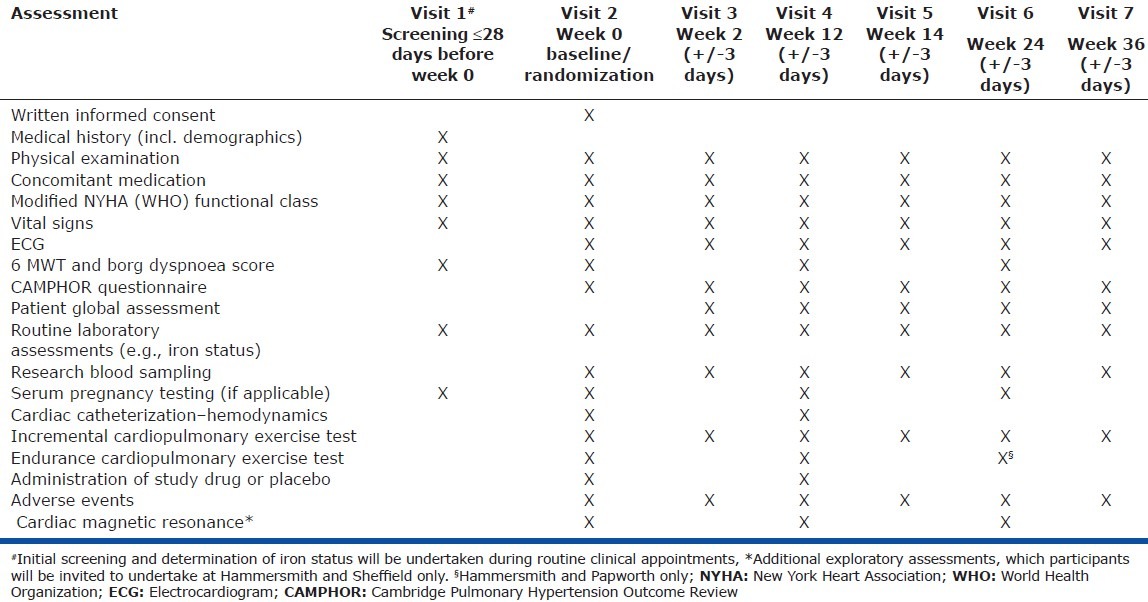
For a tabulated summary of all visits and assessment described in the following section, see Table 2.
Table 2.
Visit and assessment schedule
Primary outcome measures. The primary efficacy measure will be the change in resting pulmonary vascular resistance from baseline at 12 weeks, measured by cardiac catheterization.
Secondary measures. It is possible that iron may be beneficial in PAH through mechanisms not directly related to pulmonary hemodynamic. To address this, exercise capacity, N-terminal pro-brain natriuretic peptide (NT-pro BNP) data, and cardiac magnetic resonance data will be recorded.
A summary of the measurements that will be made is as follows:
Incremental bicycle cardiopulmonary exercise testing according to ATS guidelines[13]–measurements will include peak rate of oxygen consumption (VO2 ml/min/kg), VO2 at metabolic threshold, slope of ventilation / CO2 production (VE/VCO2 slope), ventilatory equivalent for CO2 at metabolic threshold, VO2/WR slope, and oxygen (O2) pulse
Endurance time on bicycle cardiopulmonary exercise test at 80% peak work rate (from the incremental test), with measurement of steady-state gas exchange
Resting cardiopulmonary hemodynamic—right atrial pressure, pulmonary arterial pressure, pulmonary wedge pressure, cardiac output, stroke volume, and pulmonary vascular resistance; measurements indexed where appropriate
Exercise cardiopulmonary hemodynamics at work rate equivalent to 40% peak VO2 (derived from the incremental exercise test) at Hammersmith and Sheffield sites and at a fixed resistance and cadence estimated to correspond to 40% peak work rate at Papworth site—including pulmonary arterial pressure, pulmonary capillary wedge pressure, cardiac output, stroke volume, and pulmonary artery saturation, measurements indexed where appropriate
Iron indices—serum iron, transferrin saturations, ferritin, soluble transferrin receptor, red cell distribution width (RDW), erythropoietin (EPO), and unsaturated iron binding capacity (UIBC)
6 minute walk distance (6 MWD) and Borg dyspnea scale conducted according to American Thoracic Society (ATS) guidelines[14]
WHO functional class
NT-proBNP
Quality of life using the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) questionnaire[15] and the self-reported Patient Global Assessment
Safety—the occurrence of adverse events
Cardiac magnetic resonance (CMR)—right ventricular volumes, mass, ejection fraction, stroke volume, and diastolic function
RESULTS
This is a proof-of-concept study, thus the sample size has been chosen with respect to safety (in terms of exposure to the drug and investigations) and feasibility (patient population) and with the aim of measuring effect size. We expect that a sample size of 60 will be sufficient to detect clinically meaningful changes in pulmonary hemodynamic. We also expect to see trends in exercise end-points, NT-proBNP and quality of life in the direction consistent with benefit.
The null hypothesis is that iron replacement has no effect on PVR in patients with iron deficiency and IPAH, with a two-sided alternative that iron replacement changes PVR in patients with iron deficiency and IPAH. Assuming a standard deviation of 250 dynes.s/cm5 and a drop-out rate of 10%, 60 patients randomized 1:1 would give this study an 80% power to detect a 194 dynes.s/cm5 reduction in PVR with iron treatment at a significance of P = 0.05 using a two-sample t-test. In previous trials in IPAH the mean treatment effect versus placebo using bosentan[16] was 415 dynes.s/cm5 (223 decrease vs. 191 increase) with iloprost[17] 335 dynes.s/cm5 after inhalation and sildenafil[18] 310 dynes.s/cm5.
The placebo-to-treatment crossover group provides an extra data set (60 each arm) for cardiopulmonary exercise testing two and 12 weeks post iron dosing. Assuming a 12-weekly drop-out rate of 10% and standard deviation of 5 ml/min/kg for peak VO2, we would have 80% power to detect a mean change of 1.94 ml/min/kg at a significance level of alpha = 0.05 using a paired analysis (cf 2.2 ml/min/kg difference in FERRIC-HF with 35 patients[10]). Other prespecified, secondary endpoints will be change in cardio-pulmonary exercise test (CPET) measurements at two weeks, change in cardiac output at 12 weeks, and change in 6 MWD and circulating biochemical markers at 12 weeks. A full statistical analysis plan will be developed for each endpoint, using repeated measures regression models to allow for intra- and inter-subject variation. Primary analysis will be intention-to-treat, with sensitivity analyses to explore the effect of dropout. Due to the exploratory nature of the study, no multiplicity adjustment will be made to P values for secondary analyses, though results will be interpreted with caution if the primary endpoint does not achieve significance.
Although soluble transferrin receptor (sTfR > 28.1 nmol/l) has been shown to be the best least-invasive measure of iron deficiency, the measurement is not routinely available. It will only be possible to measure sTfR in all patients after enrolment
In order to examine the effect of Ferinject on iron deficiency defined by the sTfR criterion, plasma samples will be analyzed to establish the number of patients enrolled with sTfR > 28.1 nmol/l on recruitment of the 20th, 40th, and 60th patient, and an additional number of patients will be recruited to achieve at total of 60 subjects with iron deficiency based on the sTfR criterion.
Interaction analysis will be performed to analyze if the effect of Ferinject differs in patients with sTfR > 28.1 nmol/l compared to patients with sTfR < 28.1 nmol/L. While sTfR > 28.1 nmol/l identifies patients with iron deficiency, data from our cohort show that an increased mortality signal appears above a cutoff of 25 nmol/l. An exploratory analysis will also be performed to examine if there is any difference in treatment effects with this lower cutoff.
Data from the three centers will be pooled and analyzed at Hammersmith. Due to the small sample sizes in each center, the assessment of consistency of results among the three centers will be made on an informal basis.
We will endeavor to minimize loss of patient follow-up and resulting missing outcome data. All causes of missing data will be collected and reviewed to determine the type of missing outcome data. If it is missing at random, this should have little bias on results with the proposed analysis. If informative missing outcome data is suspected, we will perform sensitivity analysis to investigate robustness of the results from the proposed analysis base on plausible assumption about the missing outcome data.
DISCUSSION
This study aims to establish the safety and efficacy of intravenous Ferinject in patients with idiopathic pulmonary arterial hypertension. Using several modalities to assess the response to Ferinject, the study also aims to provide insight into the mechanism of action. We, and others, previously showed that iron deficiency is associated with worse outcomes in IPAH, including exercise capacity, functional class, and mortality. The null hypothesis in our study is that intravenous Ferinject is not associated with an improvement in outcomes, which, if true, would suggest that iron deficiency may simply be a an epiphenomenon in IPAH, perhaps as a result of inflammation.
We previously reviewed the mechanisms through which iron may act as a potential therapeutic target in pulmonary arterial hypertension,[19] but here we discuss these mechanisms in relation to trial design. One of the principal conduits through which iron replacement may act is the regulation of pulmonary vascular tone. One of us (Peter A. Robbins) showed a critical role for iron status in the modulation of pulmonary artery pressure in response to hypoxia. Iron chelation in man using desferrioxamine mimics the rise in pulmonary arterial pressure on acute hypoxic exposure.[20] Furthermore, the administration of intravenous iron sucrose is sufficient to attenuate the rise in pulmonary arterial pressure on exposure to acute hypoxia and during acclimatization to altitude.[21] On the other hand, a recent publication showed that desferrioxamine inhibits pulmonary vascular remodeling in response to chronic hypoxia in rats, with a commensurate reduction in right ventricular systolic pressure and mass.[22] This may be due to a decrease in iron-dependent reactive oxygen species production and signaling.
The mechanism by which iron interacts with hypoxia is not fully understood, although its critical role in facilitating hypoxia-inducible factor (HIF) breakdown through oxygen-sensitive prolyl hydroxylation is a likely candidate; thus an iron-deficient state is akin to a pseudo-hypoxic environment. For this reason, pulmonary vascular resistance was chosen as the primary outcome measure to gain an insight into mechanism as well as efficacy. The apparently paradoxical results of studies examining the role of iron in acute hypoxic pulmonary vasoconstriction[21] and experimental hypoxia-induced pulmonary hypertension in rats[22] further highlights the importance of studying central hemodynamics as well as exercise and quality of life end-points.
Iron may affect physiological function outside the pulmonary circulation, and indeed a lack of direct effect on the pulmonary vascular resistance would not negate the further use or study of iron replacement in pulmonary hypertension, if other endpoints showed promise. Iron is critical to the synthesis of hemoglobin and myoglobin, both of which impact on oxygen delivery and intracellular oxygen storage. While patients with overt anemia (hemoglobin < 10 g/dl) have been excluded from the study, some patients with IPAH run high hemoglobin levels, often due to hypoxia, and thus may be relatively anemic. Nonetheless, two studies in heart failure have shown that both anemic and nonanemic patients benefit from intravenous iron replacement therapy, with improved exercise capacity, indicating a role for iron replacement beyond hemoglobin synthesis.[11,12] Three exercise end-points have been selected: 6-Minute-Walk Test was included as a standard comparator in pulmonary hypertension trials; incremental cardiopulmonary exercise testing was also included to provide data on peak capacity and prognostic markers; and endurance exercise testing at 80% of the peak work rate from the incremental test was included as a novel end-point. In other diseases, in particular chronic obstructive pulmonary disease, it has shown promise as a sensitive marker for change since small changes in peak oxygen consumption will result in large changes in endurance time; however, the measurement suffers from high variability. In order to maximize exercise data, the study was designed as a crossover.
Iron replacement may also affect cardiac function, either indirectly by reducing right ventricular afterload, or directly through effects on myocardial oxygen metabolism or regulation of the right ventricular hypertrophic response.[23] In addition to cardiac catheterization at rest and on exercise (at 40% peak VO2 estimated to coincide with peak stroke volume), cardiac function will be assessed using cardiac magnetic resonance and plasma biomarkers, including NT-proBNP.
The landmark study of intravenous Ferinject in congestive cardiac failure, FAIR-HF, used the self-reported patient global self-assessment as a primary end-point with exercise capacity as measured by 6-Minute-Walk Distance as secondary end-point.[11] Both measures were considered to be too variable to provide a robust measure as a primary end-point with the sample size planned for this study; nonetheless, we included both these measures as well as a pulmonary hypertension-specific quality of life tool, CAMPHOR.[15]
The investigators in FAIR-HF chose to titrate the dose of Ferinject to a calculated iron deficit according to the Ganzoni formula. Iron was administered to achieve this dose in 200 mg doses every week, unless iron saturation was achieved (ferritin 800 ng/mL or ferritin 500 ng/mL when transferrin saturations > 50% or hemoglobin > 16 g/dL). Following the “correction phase,” 200 mg Ferinject was administered every four weeks in the maintenance phase, starting at Week 8 or Week 12, depending on the duration of the correction phase. The doses of Ferinject ultimately prescribed in FAIR-HF were not reported, but following a very similar protocol in FERRIC-HF,[10] 1269 ± 297 and 1583 ± 366 mg was administered to patients with hemoglobin > 12.5 g/dl and < 12.5 g/dl, respectively. Since Ferinject is licensed at doses up to 1,000 mg or 15 mg/kg (whichever is the smaller) in a single infusion, we chose to simplify the protocol to a single dose of 1,000 mg, or 15 mg/kg (if < 66.7 kg). We included a 12/24-week washout period to examine the kinetics of iron redepletion to gain some understanding of timing for repeat infusion. Patients often travel long distances to their nearest pulmonary hypertension expert center and thus there is need to reduce visits.
We have chosen to limit the study to patients with IPAH. This is based, first, on the fact that data on the prevalence of iron deficiency is only available in this subgroup. In addition, we have excluded other forms of PAH since other factors may be at play. Specifically, in connective tissue disease, gastrointestinal bleeding may be a significant factor in iron deficiency and patients may need to be treated according to a different clinical algorithm. Patients with congenital heart disease are often extremely hypoxaemic and tend to run high hemoglobin levels.[24] While oral iron replacement has been shown to be associated with improvements in exercise capacity,[25] there is the safety issue of a single dose of 1000mg Ferinject in this group, since hemoglobin levels may rise dramatically, resulting in marked erythrocytosis and possible hyperviscosity. Further studies in these patient groups will need to be undertaken.
Reduced iron absorption and decreased iron bioavailability is a physiological response to infection and inflammation. It is conceivable that this is a protective mechanism in IPAH as well as in the context of acute infection. Furthermore, iron overload is known to impair cardiac function. Although safety data will be collected, should this study show efficacy, there will be a need for a larger study to monitor adverse outcomes in addition to other efficacy end-points suited to larger studies, such as morbidity and mortality.
This is a biomarker-driven study. The biomarker of interest is circulating sTfR. As the assay is not available at all centres, patients must meet one of the following criteria: sTfR levels > 28.1 nmol/l or ferritin < 37 μg/l or iron < 10.3 μmol/l or transferrin saturations < 16.4%. A planned analysis of sTfR levels after recruitment of the 20th, 40th and 60th patients permits inclusion of more patients to achieve 60 patients with sTfR > 28.1 nmol/l. The design also permits a planned exploratory analysis on the effect of iron replacement in only patients with sTfR > 28.1 nmol/l and > 25 nmol/l, as two thresholds associated with poor clinical outcome.[2]
There is a need for further therapy in PAH. There is an additional, desirable requirement that it is cost effective and convenient. By comparison with other new treatments in design, Ferinject would represent good value, with the added convenience that it can be administered quickly by intravenous injection as soon as iron studies become available. Although sTfR remains the gold standard for the diagnosis, this study also uses commonly available assays which in most centers can be turned around in a few hours. Our study will also address the likely need for reinfusion at 12 weeks, the standard follow-up interval for patients with IPAH.[12]
In conclusion, this study of intravenous Ferinject is ambitious in its design. We are attempting to address mechanism of action as well as efficacy and safety. In drawing up a crossover design, we intend to increase the power to detect changes in important secondary end-points, in particular relating to exercise function and cardiac structural changes with magnetic resonance scanning. In the event that the primary end-point is not met, but there are important changes in these other end-points, we anticipate that these will provide proof-of-concept for further study. We also anticipate that the results of the study will power therapeutic strategies designed to improve iron homeostasis in idiopathic and potentially other subsets of pulmonary hypertension.
Footnotes
Source of Support: This work was sponsored by the Imperial College Academic Health Science Centre and funded by the British Heart Foundation. EudraCT Number: 2010-024585-22
Conflict of Interest: None declared.
REFERENCES
- 1.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–93. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: Clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58:300–9. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegaman S, Westerhof N, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37:1386–91. doi: 10.1183/09031936.00100510. [DOI] [PubMed] [Google Scholar]
- 4.Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66:326–32. doi: 10.1136/thx.2010.147272. [DOI] [PubMed] [Google Scholar]
- 5.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 6.Cook JD, Dassenko S, Skikne BS. Serum transferrin receptor as an index of iron absorption. Br J Haematol. 1990;75:603–9. doi: 10.1111/j.1365-2141.1990.tb07806.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YI, Haas JD. Altered metabolic response of iron-depleted nonanemic women during a 15-km time trial. J Appl Physiol. 1998;84:1768–75. doi: 10.1152/jappl.1998.84.5.1768. [DOI] [PubMed] [Google Scholar]
- 8.Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–43. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 9.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–80. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 10.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: A randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–12. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Comin CJ, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 12.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. ESC Committee for Practice Guidelines (CPG).Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.McKenna SP, Doughty N, Meads DM, Doward LC, Pepke-Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): A measure of health-related quality of life and quality of life for patients with pulmonary hypertension. Qual Life Res. 2006;15:103–15. doi: 10.1007/s11136-005-3513-4. [DOI] [PubMed] [Google Scholar]
- 16.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 17.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 18.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes CJ, Wharton J, Howard L, Gibbs JS, Vonk-Noordegraaf A, Wilkins MR. Iron deficiency in pulmonary arterial hypertension: A potential therapeutic target. Eur Respir J. 2011;38:1453–60. doi: 10.1183/09031936.00037711. [DOI] [PubMed] [Google Scholar]
- 20.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, et al. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol. 2008;586:5999–6005. doi: 10.1113/jphysiol.2008.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: Two randomized controlled trials. JAMA. 2009;302:1444–50. doi: 10.1001/jama.2009.1404. [DOI] [PubMed] [Google Scholar]
- 22.Wong CM, Preston IR, Hill NS, Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodelling. Free Radic Biol Med. 2012;53:1738–47. doi: 10.1016/j.freeradbiomed.2012.08.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park AM, Wong CM, Jelinkova L, Liu L, Nagase H, Suzuki YJ. Pulmonary hypertension-induced GATA4 activation in the right ventricle. Hypertension. 2010;56:1145–51. doi: 10.1161/HYPERTENSIONAHA.110.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaemmerer H, Fratz S, Braun SL, Koelling K, Eicken A, Brodherr-Heverlein S, et al. Erythrocyte indexes, iron metabolism, and hyperhomocysteinemia in adults with cyanotic congenital cardiac disease. Am J Cardiol. 2004;94:825–8. doi: 10.1016/j.amjcard.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Tay EL, Peset A, Papaphylactou M, Inzuka R, Alonso-Gonzalea R, Giannakoulas G, et al. Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the Eisenmenger syndrome. Int J Cardiol. 2011;151:307–12. doi: 10.1016/j.ijcard.2010.05.066. [DOI] [PubMed] [Google Scholar]


