Abstract
The following state-of-the-art seminar was delivered as part of the Aspen Lung Conference on Pulmonary Hypertension and Vascular Diseases held in Aspen, Colorado in June 2012. This paper will summarize the lecture and present results from a nonhuman primate model of infection with Simian (Human) Immunodeficiency Virus - nef chimeric virions as well as the idea that polymorphisms in the HIV-1 nef gene may be driving the immune response that results in exuberant inflammation and aberrant endothelial cell (EC) function. We will present data gathered from primary HIV nef isolates where we tested the biological consequences of these polymorphisms and how their presence in human populations may predict patients at risk for developing this disease. In this article, we also discuss how a dysregulated immune system, in conjunction with a viral infection, could contribute to pulmonary arterial hypertension (PAH). Both autoimmune diseases and some viruses are associated with defects in the immune system, primarily in the function of regulatory T cells. These T-cell defects may be a common pathway in the formation of plexiform lesions. Regardless of the route by which viruses may lead to PAH, it is important to recognize their role in this rare disease.
Keywords: human immunodeficiency virus, herpes virus, pulmonary vasculopathy, nef, SHIV-nef, inflammation, endogenous retroviruses
HUMAN IMMUNODEFICIENCY VIRUS-ASSOCIATED PULMONARY HYPERTENSION
Morbidity and mortality associated with human immunodeficiency virus (HIV) have decreased, whereas long-term, noninfectious complications have increased. One complication, PAH is a rapidly progressive disease, which is more prevalent in individuals infected with HIV[1] (2,500 times more frequent in untreated HIV-infected population) compared with the uninfected population. The diagnosis of PAH includes identification of specific clinical hemodynamic parameters measured by right heart catheterization. Symptoms and signs frequently present only after the pulmonary vascular disease becomes advanced. Patients with HIV-PAH have poorer survival rates compared with uninfected patients with PAH.[2] Severe arteriopathy showing a gradient of vessel involvement is a histological hallmark, but it is almost impossible to link the histological lesions to the severity of the hemodynamic alterations in humans, since human tissues are infrequently available.
Microscopically, one finds intimal fibrosis, increased medial thickness, and pulmonary arteriolar occlusion.[3,4,5,6] The concept that pulmonary hypertension is a cancer-like disease where the ECs lose their differentiated phenotype and acquire a highly proliferative phenotype in response to environmental or genetic triggers has been postulated and supported by empirical data.[5,7]
While the pathogenesis of HIV-PAH is unclear, the histological similarities to idiopathic pulmonary hypertension are striking: Presence of plexiform lesions composed of ECs and cells that express markers characteristic of an undifferentiated, highly proliferative cell.[8,9]
Systemic arteriopathy in SIV-infected macaques
The Simian Immunodeficiency Virus (SIV)-infected macaque model closely recapitulates HIV infection and immunodeficiency and, therefore, has been used to study pathogenesis of lentiviral infections. Early studies by Chalifoux et al.[10] reported pulmonary arteriopathy in rhesus macaques infected with SIV. On the other hand, Simian (Human) Immunodeficiency Virus (SHIV) models use chimeric virions specifically designed to dissect contributions to pathogenesis by specific HIV-1 genes. In the SHIV-nef model, rhesus macaques are infected with a recombinant virion where the SIV-nef gene has been replaced with the HIV-nef from a cloned virus isolated from an HIV-infected individual to create a recombinant chimeric SHIV-nef (Fig. 1). We infected a cohort of monkeys with one such chimeric virion and performed histological analyses of pulmonary vessels. We uncovered lesions histologically indistinguishable from those in end-stage human PAH.[11] We infected a second cohort of monkeys and again found vascular remodeling. Furthermore, organs such as the brain, kidney, liver, and spleen did not show evident vasculopathy. Interestingly, periarteriolar mononuclear cell infiltrates were also observed (Fig. 2), red arrow). Fluorescence immunophenotyping of SHIV-nef-infected macaque lungs at various times postinfection indicated CD34, smooth muscle actin, and even Nef accumulated in the lungs over time. Survival time postinfection ranged from 12 to 62 weeks with median 30.5 weeks and mean 33 weeks (95% CI: 11.7, 54.3). After testing individual blood parameters during the acute phase (Week 2) as predictors of survival, we found a strong positive linear relationship between overall survival time and mean corpuscular hemoglobin and mean corpuscular volume (Fig. 3). Multiple observations for each animal per time point (mixed-effects model) were used to estimate the effect of SIV viral load in plasma on each blood parameter, adjusted for time. Analyses showed that increased viral load in the periphery was positively correlated with viral load in the lymph nodes ( P = 0.0041) and decrease in platelets ( P = 0.0421). The role of platelets and thrombus formation in pulmonary hypertension is an area that has been grossly understudied, particularly in the context of the human disease. Studies have characterized the phenotype of platelets in idiopathic pulmonary arterial hypertension (IPAH), showed a defect in their ability to be activated in vitro, and reduced levels of endothelial nitric oxide synthase (eNOS) levels, impacting their ability to regulate their function appropriately.[12]
Figure 1.
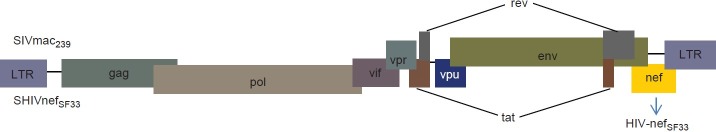
Genetic organization of the chimeric SHIV-nef virus. Both HIV and its counterpart in simians (SIV), have similar genetic organization. The SIV-nef from SIVmac239 was replaced by the HIV-nef isolated from an AIDS patient (designated as SF33), whereas the rest of the SIV genes remained intact.[26] Hence, the resulting chimeric virus was further designated as SHIV-nef-SF33, which was used to infect rhesus macaques (Macaca mulatta) in these studies.
Figure 2.
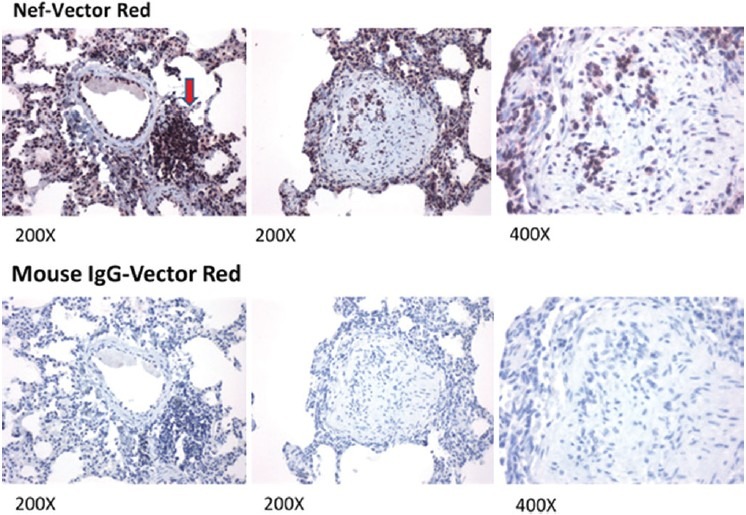
Detection of HIV-nef in the lung of an SHIV-nef-infected macaque. HIV-Nef was detected in paraffin-embedded lung sections by immunohistochemistry using mouse anti-HIV Nef antibody (Santa Cruz Biotech), NovaRed substrate (Vector Labs), and counterstained with hematoxylin. The red arrow points to mononuclear cell infiltrates. Tissue sections were also stained with mouse IgG (Vector Labs) as negative control (bottom row). Images were captured in a Nikon ECLIPSE E600 Microscope and the SPOT Advance v3.2 software; magnifications are indicated at the bottom of each image.
Figure 3.
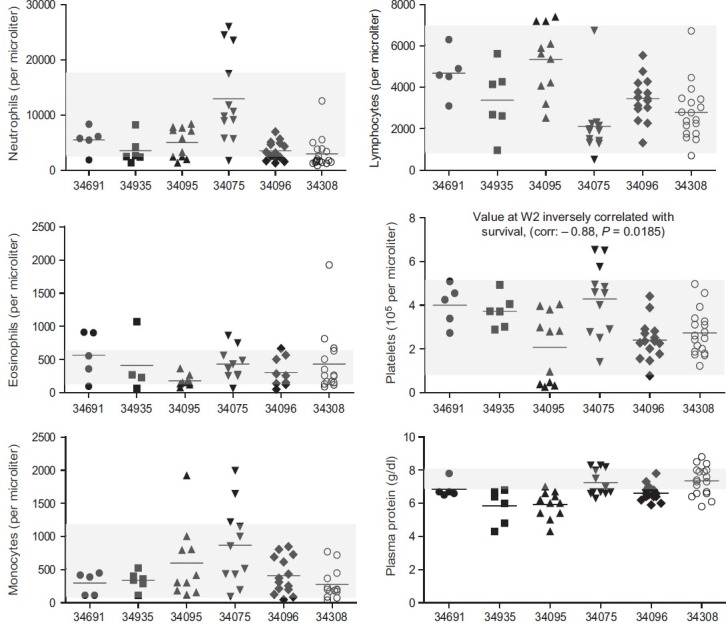
Complete blood counts (CBC) in each macaque infected with chimeric SHIV-nef. Specific tests are indicated on the Y-axis of each panel, whereas monkey identity is shown on the X-axis. Symbols represent data collected from the macaques at different time points; the line indicates the mean values. CBC values collected from healthy uninfected adult males ( n= 20) from the California National Primate Research Center colony, are indicated by shaded boxes. Data were plotted in GraphPad Prism v5 for PC.
Monocrotaline-induced PAH in rats is associated with increased platelet and leukocyte activation and platelet-leukocyte interactions in vivo, which may play important roles in pathogenesis.[13] In fact, one of the typical pathological changes observed histologically in PAH is thrombosis in situ. The importance of platelets is highlighted by the fact that aspirin attenuates PAH in monocrotaline-treated rats by reducing plasma 5-hydroxytryptamine levels and increasing survival. Aspirin treatment also reduced right ventricular hypertrophy and pulmonary arteriole proliferation.[14] Increased platelet, leukocyte, and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension.[15]
An interesting observation that may link platelet aggregation and HIV-1 nef is the concept of molecular mimicry to HIV peptides in HIV-1-related thrombocytopenia. Autoantibodies directed against a variety of HIV proteins, most significantly nef, have been identified as culprits in platelet aggregation and increased morbidity and mortality in humans.[16] In fact, several HIV-1 proteins are homologous to 22 known AIDS autoantigens; antibodies to T-cell receptors, CD4, CD95, complement components, immonoglobulin G, tumor necrosis factor (TNF), and other immune-related proteins have been described as relevant to AIDS pathogenesis.[17] Investigators have postulated that autoimmunity may actually define early stages of AIDS and provide a link to the autoimmune abnormalities observed in PAH rodent models. Perros et al.[18] reported that circulating autoantibodies against vascular wall components, generated in tertiary (ectopic) lymphoid tissues, may have a pathogenetic link in IPAH.[18] Additional studies from Nicoll's group have concluded that regulatory T cells normally function to limit vascular injury and may protect against the development of PAH.[19] Thus, it appears that immune dysregulations are common pathogenetic mechanisms in IPAH and PH mediated by hypoxia.
We examined cytokine levels in the plasma of SHIV-nef compared with SIV-infected macaques; 20 nonhuman primate cytokines and interleukins were measured. We compared cytokine/chemokine levels in plasma from six SHIV-nef with levels in four SIV-infected macaques using multiplex cytokine panels.[20] Comparisons of cytokines by group were not performed for six cytokines with completely missing data, or six of fewer observations across all animals in the control group, i.e., IL-1B, IL-6, Macrophage-derived chemokine, macrophage inflammatory protein (MIP)-1B, MIP-1a, and TNF-α. The concentrations of macrophage-produced cytokines Granulocyte-macrophage colony-stimulating factor, IL-12, IL-18, IL-8, and IL-10, as well as MCP-1 and MIP-1b, were significantly higher in SHIV-nef-infected macaques compared with SIV alone (data not shown). Conversely, soluble CD40 ligand, and growth-related oncogene alpha protein (GRO-α), were significantly lower in the SHIV-nef-infected animals. Estimates for the group-specific intercepts and slopes (from a mixed-effects model) showed that there was no relationship between changes in cytokine levels and weeks post infection (data not shown). One macaque in particular showed increased expression of chemokines related to production of granulocytes (GM-CSF), acute phase reaction (TNF-α), and acute neutrophilic inflammation (MIP-1a, MIP-1b) after 24 weeks of infection. Maximal levels of IL-6 were detected at 37 weeks. In summary, monkeys infected with SHIV-nef chimeric virus swarms developed lung inflammation, specifically perivascular inflammation. Later in the infection, pulmonary vascular remodeling was detected. Nef and markers of inflammation and remodeling were found in the lungs and peripheral circulation and were directly correlated with nef at different times post-infection.
Although a cause-and-effect relationship between HIV-1 nef and development of pulmonary lesions remains to be determined, the results in the SHIV-nef-infected monkeys strongly suggest that nef may play a role in the pathogenesis of HIV-PAH.
Human immunodeficiency virus-1 negative factor
HIV-1 nef (negative factor) gene codes for an accessory protein made early in infection and whose major function is as an adaptor that interacts with cellular proteins; its three-dimensional structure plays a critical role in these interactions. Nef downregulates CD4[21] and major histocompatibility antigen-I (MHC-I) trafficking to the membrane.[22] Nef expression increases Extracellular signal-regulated kinases (ERK), Mitogen-activated protein kinase kinase (MEK), and Elk mitogen-activated protein (Elk-MAP) kinase activity, thus affecting T-cell activity, viral replication, and viral infectivity.[23] Nef-mediated increases in MIP-1, IL-1b, IL-6, and TNF-α in monocyte-derived macrophages required the domains critical for the interaction with the endocytotic machinery because mutants (i.e., EE155-156QQ and DD174-175AA) were ineffective.[24] Acheampong et al.[25] demonstrated for the first time that either recombinant nef added to cultures or intracellular nef-induced apoptosis of human brain microvascular EC.
We hypothesized that nef signature sequences are associated with PH and predict disease progression in individuals at risk; these naturally occurring alleles may trigger a cascade of events leading to the selection of a rapidly growing, apoptosis-resistant EC population. We found four amino acid polymorphisms consistently in nef alleles recovered from SHIV-nef-infected macaques after passage in vivo: V to I; H to Y; A to P/D; and L to V. These were similar to those reported by Dr. Paul Luciw in his cohort of monkeys.[26] We recently reported seven polymorphic areas in nef functional domains that were over-represented in peripheral blood cells of PH-subjects compared with normotensives.[27] None of the polymorphisms were predicted to have major structural changes on the core, but rather the impact would be on residues located on the surface; these would be predicted to disrupt solvent and intermolecular interactions. Nevertheless, we sought to investigate whether nef alleles associated with HIV-PH induce angiogenic/proliferative/tumorigenic responses in human pulmonary artery ECs. We cloned primary nef isolates from individuals into a HaloTag mammalian expression vector (Promega), and measured whether the polymorphisms would interfere with nef-dependent CD4 downregulation. Nef-HaloTag fusion constructs were nucleofected into HeLa-CD4 cells and CD4 expression assessed by flow cytometry. Baseline CD4 expression was 97-99%; the presence of nef resulted in a 34-86% range of CD4 expression. Denaturing/nonreducing immunoblots of cell extracts showed that nef proteins from the various constructs were expressed at different levels. Given that nef interacts with numerous cellular proteins, we also analyzed the nef-HaloTag constructs under native conditions. We observed the presence of higher molecular weight forms of nef, suggesting that these cloned variants retained the ability to form putative intracellular oligomers/complexes.[27] We are in the process of measuring the proliferative properties of pulmonary ECs exposed to the various nef constructs.
What do all of these observations/results mean? Is HIV-nef having a direct impact on EC biology or are the effects just epiphenomena? The finding of significantly more polymorphisms in nef functional domains in the HIV-PH groups will hopefully stimulate discussions regarding selective pressures on nef. Genetic divergence in nef has been ascribed to the CD8+ T-cell-mediated viral control. Accumulation of mutations in the HIV genome is conceivable, especially in chronically HIV-infected subjects exposed to antiretroviral therapies, which was a feature of our validation cohort from San Francisco. We found no evidence that the nef polymorphisms found in subjects with HIV-PH were affected by the presence of antiretroviral therapies, length of HIV infection, or age, at least in the cohorts we analyzed. However, we can also invoke that nef immunogenicity and the ability to mimic autoantigens, which coupled with a failure of regulatory T cells to control endothelial injury, may lead to a “perfect storm” of events culminating in the vascular endothelial damage that results in vascular remodeling. The vascular pathology seen in severe PAH is remarkably similar despite the fact that it arises in diverse conditions including collagen vascular diseases, abnormal blood flow, and use of anorexigen drugs. The pathogenesis of severe PAH is clearly complex, and probably results from the interaction of multiple modulating genes with environmental factors.
THE ROLE OF HERPES VIRUSES IN PULMONARY HYPERTENSION
In 2003, Cool et al.[28] analyzed lung tissue from 10 of 16 patients with primary pulmonary hypertension for the presence of human herpesvirus-8 (HHV-8), latency-associated nuclear antigen-1 (LANA-1) via immunohistochemistry and the viral cyclin via polymerase chain reaction (PCR) analysis. No LANA-1 was detected in lung tissue from patients with secondary pulmonary hypertension, although one such patient had PCR evidence of viral cyclin. Plexiform lesions from patients with primary pulmonary hypertension had a histological and immunohistochemical resemblance to cutaneous Kaposi's sarcoma lesions. The authors concluded that infection with the vasculotropic virus HHV-8 may have a pathogenetic role in primary pulmonary hypertension.[28] However, more recent studies failed to reproduce these results. Hsue and colleagues[29] concluded that a role for HHV-8 infection in PAH remains less than definitive; subsequent studies concluded that human γ-herpes viruses, Epstein-Barr virus, and HHV-8 were not detected in the lungs of patients with severe PAH,[30] and HHV-8 was not detected in patients with PAH.[31] Thus, the potential association between HHV-8 and with IPAH is not supported by recent evidence. Using quantitative PCR with a variety of gene-specific primers for a spectrum of viruses of the Herpesviridae family, we queried peripheral blood DNA isolated from peripheral blood of HIV-infected hypertensive and normotensive individuals. In these studies, we did not detect any Herpes simplex virus type 2 (HSV-2) or Varicella zoster virus (VZV) in any of the samples; we only detected CMV in one PAH and HHV-8 in one non-PAH sample. Interestingly, HSV-1 was detected in all samples, but we found higher copy numbers in HIV-PAH. These results have to be interpreted with caution, since the sample sizes were quite small ( n = 6). More studies with larger sample sizes are imperative.
In closing, we have identified nef polymorphisms associated with HIV-PAH. Although these polymorphisms may cause Nef structural changes that alter interactions with important vascular pulmonary cell proteins and in turn affect EC function, an alternative and not necessarily mutually exclusive explanation is the possibility that there is molecular mimicry by nef and possibly other HIV proteins. Certainly, loss of tolerance associated with bronchus-associated lymphoid tissue expansion in experimental pulmonary hypertension has been proposed. Nef polymorphisms may uncover new epitopes that drive immune responses; the more immunogenic the HIV proteins, the more profound the response. Finally, we would like to propose an intriguing hypothesis. We have been examining the contribution of exogenous viral infections that impact vascular ECs and the immune responses these infections elicit. We would like to suggest the possibility that endogenous mobile genetic elements, which compose as much as two-thirds of the mammalian genome, may also play a role in immune dysregulation and EC dysfunction in pulmonary hypertension. Mobile genetic elements include retrotransposons and endogenous retroviruses. Human endogenous retroviruses (HERV) are ancestral proviruses that resulted from germ cell infections with exogenous retroviruses.[32] Although they have lost the ability to replicate and infect other cells, they can be transmitted vertically. In somatic cells, these elements may still be active or they may be reactivated through infections with HIV or herpesviruses[33,34] or via environmental stresses. Once activated, they can be either transcribed or mobilized; mobilization and transposition then contribute to the development of autoimmune diseases[35] and/or cancer[36] through genomic rearrangements such as insertions, deletions, inversions, and duplications.[37] Thus, a combination of environmental stresses and virus infections may activate some of these endogenous elements and result in some of the functional abnormalities and chromosomal instability[38] observed in vascular cells of remodeled arteries in HIV-PAH or IPAH.
Footnotes
Source of Support: NIH/NHLBI R01 HL083491 and R01-HLO90480 (S.C.F.)
Conflict of Interest: None declared.
REFERENCES
- 1.Opravil M, Sereni D. Natural history of hiv-associated pulmonary arterial hypertension: Trends in the HAART era. AIDS. 2008;22:S35–40. doi: 10.1097/01.aids.0000327514.60879.47. [DOI] [PubMed] [Google Scholar]
- 2.Pellicelli AM, Barbaro G, Palmieri F, Girardi E, D’Ambrosio C, Rianda A, et al. Primary pulmonary hypertension in hiv patients: A systematic review 2. Angiology. 2001;52:31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- 3.Aiello VD, Canzian M. Histopathology images of pulmonary vascular disease: Part i. PVRI Review. 2011;1:34–38. [Google Scholar]
- 4.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2006;29:462–8. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–74. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeager ME, Golpon HA, Voelkel NF, Tuder RM. Microsatellite mutational analysis of endothelial cells within plexiform lesions from patients with familial, pediatric, and sporadic pulmonary hypertension. Chest. 2002;121:61S. [PubMed] [Google Scholar]
- 8.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, et al. Peroxisome proliferator-activated receptor gamma (ppargamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–9. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 9.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers.Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–9. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992;67:338–49. [PubMed] [Google Scholar]
- 11.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, et al. Hiv-1 nef is associated with complex pulmonary vascular lesions in shiv-nef-infected macaques. Am J Respir Crit Care Med. 2006;174:437–45. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aytekin M, Aulak KS, Haserodt S, Chakravarti R, Cody J, Minai OA, et al. Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: Role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2012;302:L512–20. doi: 10.1152/ajplung.00289.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu XS, Du CQ, Zeng CL, Yao L, Zhang FR, Wang K, et al. Systemic evaluation of platelet and leukocyte activation and interaction in a rat model of pulmonary arterial hypertension. Cardiology. 2010;117:44–53. doi: 10.1159/000320107. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Shen J, Pu J, He B. Aspirin attenuates pulmonary arterial hypertension in rats by reducing plasma 5-hydroxytryptamine levels. Cell Biochem Biophys. 2011;61:23–31. doi: 10.1007/s12013-011-9156-x. [DOI] [PubMed] [Google Scholar]
- 15.Diehl P, Aleker M, Helbing T, Sossong V, Germann M, Sorichter S, et al. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis. 2011;31:173–9. doi: 10.1007/s11239-010-0507-z. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to hiv-1 peptides in hiv-1-related immunologic thrombocytopenia. Blood. 2005;106:572–6. doi: 10.1182/blood-2005-01-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter CJ. Extensive viral mimicry of 22 aids-related autoantigens by hiv-1 proteins and pathway analysis of 561 viral/human homologues suggest an initial treatable autoimmune component of aids. FEMS Immunol Med Microbiol. 2011;63:254–68. doi: 10.1111/j.1574-695X.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 18.Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–21. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 19.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, et al. Regulatory t cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109:867–79. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giavedoni LD. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J Immunol Methods. 2005;301:89–101. doi: 10.1016/j.jim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Piguet V, Schwartz O, Le GS, Trono D. The downregulation of cd4 and mhc-i by primate lentiviruses: A paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 22.Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. Hiv-1 nef blocks transport of mhc class i molecules to the cell surface via a pi 3-kinase-dependent pathway. Virology. 2001;282:267–77. doi: 10.1006/viro.2000.0816. [DOI] [PubMed] [Google Scholar]
- 23.Schrager JA, Der MV, Marsh JW. Hiv nef increases t cell erk map kinase activity. J Biol Chem. 2002;277:6137–42. doi: 10.1074/jbc.M107322200. [DOI] [PubMed] [Google Scholar]
- 24.Olivetta E, Percario Z, Fiorucci G, Mattia G, Schiavoni I, Dennis C, et al. Hiv-1 nef induces the release of inflammatory factors from human monocyte/macrophages: Involvement of nef endocytotic signals and nf-kappa b activation. J Immunol. 2003;170:1716–27. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 25.Acheampong EA, Parveen Z, Muthoga LW, Kalayeh M, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J Virol. 2005;79:4257–69. doi: 10.1128/JVI.79.7.4257-4269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandell CP, Reyes RA, Cho K, Sawai ET, Fang AL, Schmidt KA, et al. Siv/hiv nef recombinant virus (shivnef) produces simian aids in rhesus macaques. Virology. 1999;265:235–51. doi: 10.1006/viro.1999.0051. [DOI] [PubMed] [Google Scholar]
- 27.Almodovar S, Knight R, Allshouse AA, Roemer S, Lozupone C, McDonald D, et al. Human immunodeficiency virus nef signature sequences are associated with pulmonary hypertension. AIDS Res Hum Retroviruses. 2012;28:607–18. doi: 10.1089/aid.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. New Engl J Med. 2003;349:1113–22. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 29.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of hiv and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22:825–33. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valmary S, Dorfmuller P, Montani D, Humbert M, Brousset P, Degano B. Human gamma-herpesviruses epstein-barr virus and human herpesvirus-8 are not detected in the lungs of patients with severe pulmonary arterial hypertension. Chest. 2011;139:1310–6. doi: 10.1378/chest.10-1200. [DOI] [PubMed] [Google Scholar]
- 31.Henke-Gendo C, Mengel M, Hoeper MM, Alkharsah K, Schulz TF. Absence of kaposi's sarcoma-associated herpesvirus in patients with pulmonary arterial hypertension. Am J Respir Criti Care Med. 2005;172:1581–5. doi: 10.1164/rccm.200504-546OC. [DOI] [PubMed] [Google Scholar]
- 32.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 33.Contreras-Galindo R, Kaplan MH, Contreras-Galindo AC, Gonzalez-Hernandez MJ, Ferlenghi I, Giusti F, et al. Characterization of human endogenous retroviral elements in the blood of hiv-1-infected individuals. JVirol. 2012;86:262–76. doi: 10.1128/JVI.00602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nellaker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus w family by viral infection. Retrovirology. 2006;3:44. doi: 10.1186/1742-4690-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balada E, Vilardell-Tarres M, Ordi-Ros J. Implication of human endogenous retroviruses in the development of autoimmune diseases. Int Rev Immunol. 2010;29:351–70. doi: 10.3109/08830185.2010.485333. [DOI] [PubMed] [Google Scholar]
- 36.Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ, 3rd, et al. Landscape of somatic retrotransposition in human cancers. Science. 2012;337:967–71. doi: 10.1126/science.1222077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohne A, Brunet F, Galiana-Arnoux D, Schultheis C, Volff JN. Transposable elements as drivers of genomic and biological diversity in vertebrates. Chromosome Res. 2008;16:203–215. doi: 10.1007/s10577-007-1202-6. [DOI] [PubMed] [Google Scholar]
- 38.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1153–60. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


