Abstract
Inhaled nitric oxide (iNO) is used for acute vasoreactivity testing in pulmonary arterial hypertension (PAH) patients. Inhaled epoprostenol (iPGI2) has pulmonary selectivity and is less costly. We sought to compare acute hemodynamic effects of iNO (20 ppm) and iPGI2 (50 ng/kg/min) and determine whether their combination has additive effects. We conducted a prospective, single center, randomized, cross-over study in 12 patients with PAH and seven with heart failure with preserved ejection fraction (HFpEF). In PAH patients, iNO lowered mean pulmonary artery pressure (mPAP) by 9 ± 12% and pulmonary vascular resistance (PVR) by 14 ± 32% (mean ± SD). iPGI2 decreased mPAP by 10 ± 12% and PVR by 12 ± 36%. Responses to iNO and iPGI2 in mPAP and PVR were directly correlated (r2 = 0.68, 0.70, respectively, P < 0.001). In HFpEF patients, mPAP dropped by 4 ± 7% with each agent, and PVR dropped by 33 ± 23% with iNO, and by 25 ± 29% with iPGI2 (P = NS). Pulmonary artery wedge pressure (PAWP) increased significantly with iPGI2 versus baseline (20 ± 3 vs. 17 ± 2 mmHg, P = 0.02) and trended toward an increase with iNO and the combination (20 ± 2, 19 ± 4 mmHg, respectively). There were no additive effects in either group. In PAH patients, the vasodilator effects of iNO and iPGI2 correlated at the doses used, making iPGI2 a possible alternative for testing acute vasoreactivity, but their combination lacks additive effect. Exposure of HFpEF patients to inhaled vasodilators worsens the PAWP without hemodynamic benefit.
Keywords: pulmonary arterial hypertension, inhaled nitric oxide, inhaled epoprostenol, heart failure with preserved ejection fraction
Acute vasoreactivity testing during right heart catheterization of patients with pulmonary arterial hypertension (PAH) is used to screen for possible responsiveness to long-term calcium channel blocker therapy[1] and to obtain prognostic information.[2] Patients with idiopathic PAH (IPAH) who are acute vasoresponders by current criteria[1] have a significantly better survival than nonresponders.[2–4] In addition, even among non-responders, the degree of improvement in pulmonary hemodynamics during an acute vasodilator trial correlates with survival.[5]
Currently, inhaled nitric oxide (iNO) is commonly used to assess vasoreactivity because of its rapid onset, pulmonary specificity, minimal side effects, and short duration of action, but it is expensive and not available at many institutions. Intravenous epoprostenol and adenosine are recommended as alternative agents to test acute vasoreactivity.[1] In a previous study, patients with IPAH had similar acute vasodilator response to iNO and to intravenous epoprostenol.[6] The response to iNO was not dose-dependent, with maximal effect being achieved at 10 ppm, and its combination with intravenous epoprostenol did not produce additive vasodilatory effects.
The major disadvantages of intravenous epoprostenol for acute testing are systemic side effects at maximally tolerated doses, including hypotension, headache, nausea and vomiting, and an increase in catheterization time as the dose is uptitrated gradually, which is a potential problem in busy laboratories. Inhaled epoprostenol (iPGI2), on the other hand, mitigates systemic prostacyclin side effects by virtue of local delivery to the pulmonary circulation, and reduces administration time. In addition, iPGI2 is less expensive and more readily available than iNO.
While the correlation between responses to various vasodilators has been previously evaluated in IPAH patients, less is known about the magnitude of acute response to selective pulmonary vasodilators in patients with associated PAH, and in those with heart failure with preserved ejection fraction (HFpEF), in whom pulmonary hypertension is secondary to elevated left ventricular end diastolic pressure.
The aim of this study was to compare the acute hemodynamic effects of iNO and iPGI2 and their combination during right heart catheterization in patients with PAH and HFpEF.
MATERIALS AND METHODS
Patients
All patients referred for new evaluation of pulmonary hypertension and undergoing diagnostic right heart catheterization between November 2008 and October 2010 were considered for study inclusion. Adult patients (18 years of age and older) were included in the study if they had a resting mean pulmonary artery pressure (mPAP) ≥ 25 mmHg and a pulmonary artery wedge pressure (PAWP) ≤ 25 mmHg. Patients were excluded if they had already started Food and Drug Administration (FDA) approved PAH medications, had mitral or aortic valvular heart disease, left ventricular ejection fraction (LVEF) < 50%, ischemic heart disease, systemic hypotension (systolic systemic blood pressure (SBP) < 90 mmHg, or mean BP < 60 mmHg), known or suspected pulmonary veno-occlusive disease, or were pregnant. The study protocol was approved by the Tufts Medical Center Investigational Review Board and all subjects provided written informed consent.
The diagnosis of PAH or HFpEF was made according to the current World Health Organization (WHO) definition and European Cardiology Society/European Respiratory Society Guidelines:[1] (1) Patients with a PAWP ≤ 15 mmHg are categorized as having PAH; and (2) patients with a PAWP between 16-25 mmHg are classified as having HFpEF. We used a PAWP cut off of 25 mmHg to avoid the potential induction of acute pulmonary edema by the acute vasodilator agents.[7] Patients in WHO Groups III-V were excluded.
Right heart catheterization protocol
Hemodynamic Measurements. A Swan-Ganz catheter (Edwards Lifesciences, Irvine, Calif., USA) was inserted through an internal jugular vein under ultrasound and fluoroscopic guidance as previously described.[8] Following the measurement of baseline hemodynamic parameters, acute vasodilators (iNO, iPGI2, iNO + iPGI2) were administered serially by a respiratory therapist. Hemodynamic parameters were measured at baseline after 15 minutes of administration of each vasodilator, and after 20 minutes of a washout phase prior to administration of the next agent to ensure return of hemodynamics to baseline. Supplemental oxygen was administered as needed to maintain oxygen saturation > 90%.
Administration of iNO and iPGI2. Vasodilators were administered using the INOMAX delivery system (Ikaria, Hampton, N.J., USA). An inline system between the INOMAX and the patient was assembled (Fig. 1). The inline system had one port equipped with a continuous nebulizer for iPGI2 (or saline) delivery, another port connected directly to the INOMAX system for iNO delivery, and a third port connected to the patient via a tight fitting mask. The drugs could be delivered sequentially and in combination without disruption of the system. iNO (Ikaria, Hampton, N.J., USA) was administered at 20 ppm. Due to a risk of acute pulmonary edema associated with iNO at concentrations > 10 ppm,[9] patients with connective tissue disease associated PAH received iNO at 10 ppm, alone, or in combination with iPGI2. iPGI2 (Flolan; GlaxoSMithKline, Research Triangle Park, N.C.) was aerosolized using the Aeroneb nebulizer (Aerogen, Galway, Ireland) at 50 ng/kg/min, the highest nebulized dose reported in the literature.[10] iPGI2 was prepared using 48 ml of a 30,000 ng/ml concentration of epoprostenolin a 60 ml syringe, while another syringe had 60 ml of normal saline and both syringes were connected to the nebulizer cup. The dose for each patient was determined by adjusting the flow of the two syringes. If patients were on supplemental oxygen during baseline hemodynamics, they also received oxygen in the same concentration, alone during the iPGI2 phase, or blended with iNO during the iNO or combination phases.
Figure 1.
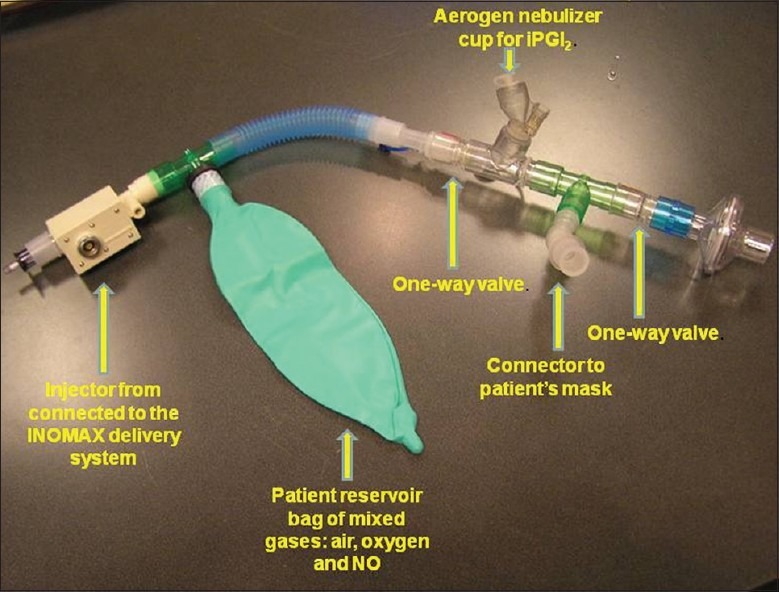
The inline system for delivery of inhaled vasodilators. The system has one port equipped with a continuous nebulizer for delivery of inhaled epoprostenol (iPGI2) or saline, another port connected directly to the INOMAX system for inhaled nitric oxide delivery and a third port connected to the patient via a tight fitting mask.
Administration of agents in randomized fashion. A respiratory therapist (RT), who was not a part of the investigative team, administered iNO, iPGI2, or iNO + PGI2 according to a predetermined computer-generated random schedule via the administration system. Only the RT was aware of the sequence; other clinicians were blinded to the order of administration during vasodilator testing and analysis of data.
Statistical analysis
Demographic characteristics and hemodynamic values are reported descriptively and as mean ± SD and percent change from baseline as appropriate. Student t-test was used to compare variables between PAH and HFpEF patients. One-way ANOVA for repeated measures was used to determine the statistical significance of differences between serial measurements within each group. Correlation was assessed using linear regression analysis. To ensure reversal of hemodynamics to baseline between each phase, ANOVA for repeated measures of various baselines was performed for mPAP at baseline and prior to each subsequent drug administration.
RESULTS
Patients and their characteristics
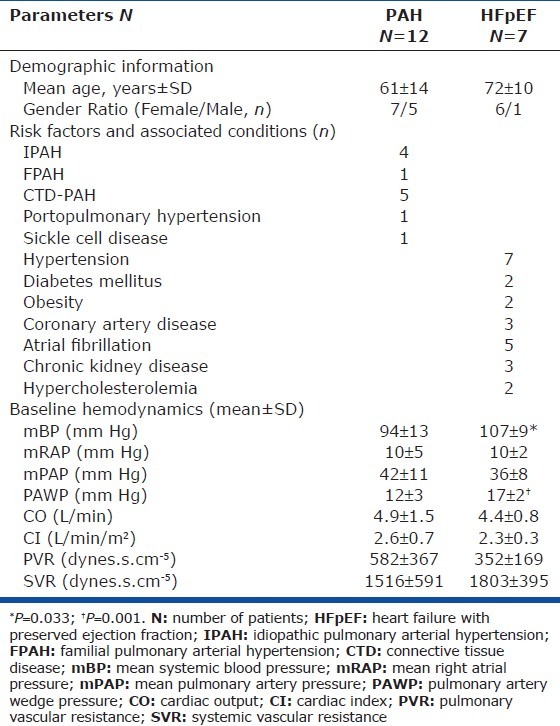
During the study period, 40 patients underwent evaluation for pulmonary hypertension at our Center (Fig. 2). All patients had dyspnea on exertion and a baseline echocardiogram suggestive of PH prior to the right heart catheterization. Out of patients eligible and enrolled to undergo acute testing, 12 patients had PAH and seven had HFpEF. Their baseline demographic and hemodynamic characteristics are presented in Table 1. Consistent with the demographics of PAH,[11] most patients were women. The majority of PAH patients had idiopathic, heritable, or connective tissue disease-associated PAH. All HFpEF patients had a history of systemic hypertension, and the majority had atrial fibrillation. Baseline mean SBP and PAWP were significantly higher in the HFpEF group.
Figure 2.
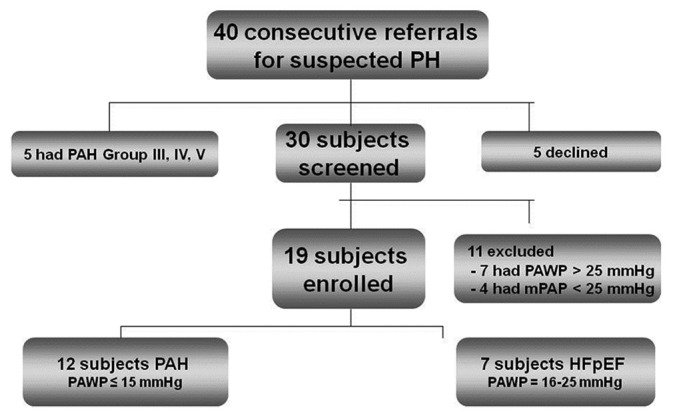
Screening and enrollment algorithm.
Table 1.
Patient baseline clinical characteristics and hemodynamic findings
Effects of inhaled vasodilators on PAH patients
Both iNO and iPGI2 significantly reduced mPAP and pulmonary vascular resistance (PVR) in PAH patients, but combining the two vasodilators did not produce additive effects (Table 2). iNO lowered mPAP by 9 ± 12% and PVR by 14 ± 32%. iPGI2 decreased mPAP by 10 ± 12% and PVR by 12 ± 36%. Responses to iNO and iPGI2 for mPAP and PVR were significantly and directly correlated (Figures 3 and 4), respectively). As expected, PVR/systemic vascular resistance (SVR) ratio decreased significantly with iNO, and the combination compared with baseline. In addition, iNO decreased PVR/SVR ratio significantly more than iPGI2, suggesting that iNO has a more potent selective pulmonary vasodilator effect. Oxygen saturation increased significantly and similarly with both inhalational agents, and with their combination. No significant changes were noted in mean BP, cardiac output (CO), cardiac index (CI), PAWP, or SVR during any of the three phases of testing.
Table 2.
Hemodynamic responses to each vasodilator alone and in combination in PAH patients, N=12
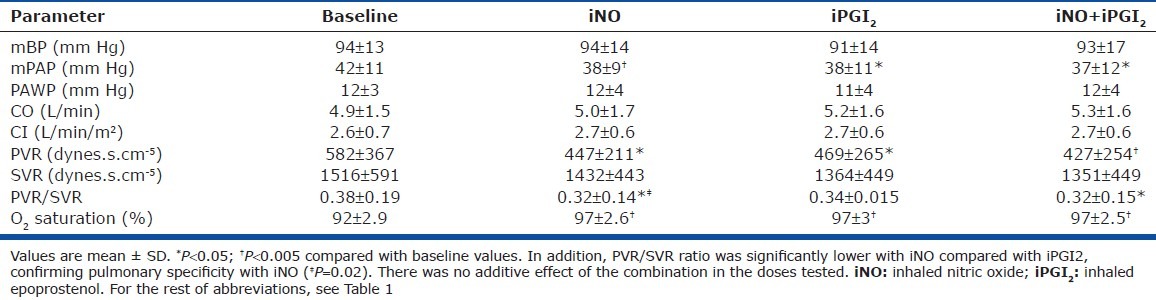
Figure 3.
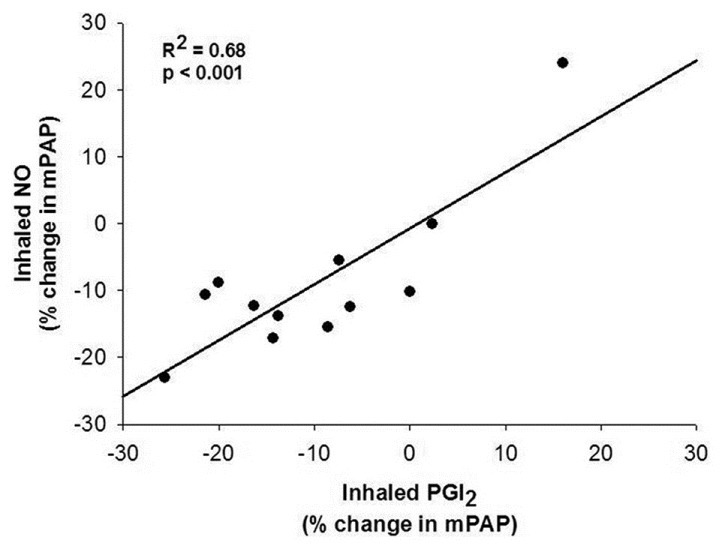
Comparison of percent change in mean pulmonary artery pressure between inhaled nitric oxide (20 ppm) and inhaled epoprostenol (iPGI2) (50 ng/kg/min) in 12 patients with pulmonary arterial hypertension.
Figure 4.
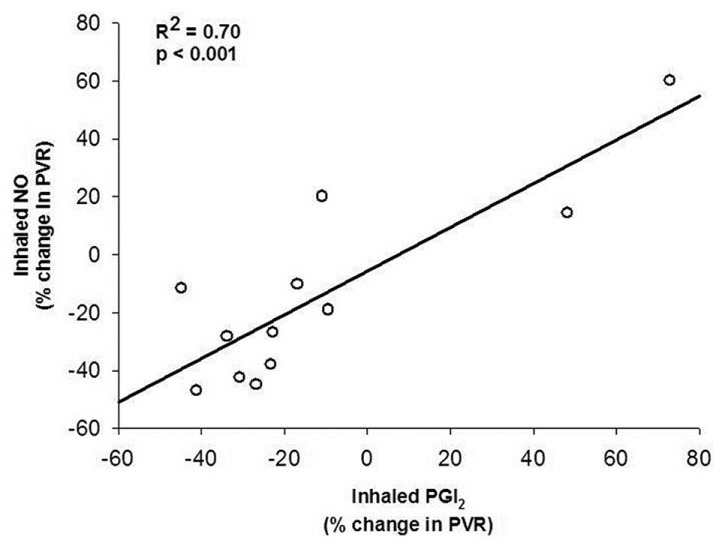
Comparison of percent change in pulmonary vascular resistance (PVR) between inhaled nitric oxide (20 ppm) and inhaled epoprostenol (PGI2) (50 ng/kg/min) in 12 patients with pulmonary arterial hypertension.
Effects of inhaled vasodilators on HFpEF patients
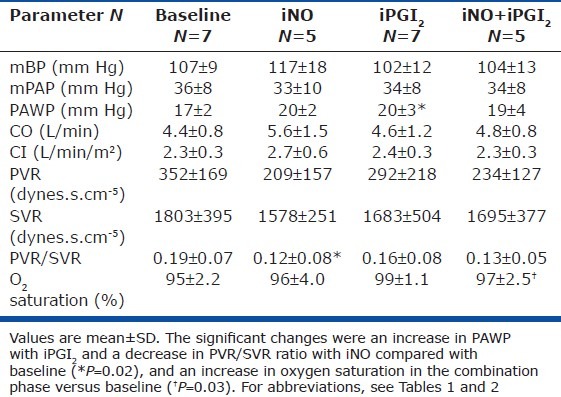
Among patients with HFpEF, neither vasodilator nor their combination had a significant effect on mPAP, PVR, SVR, CO, or CI (Table 3). During each of the three phases, mPAP dropped by an average of 4 ± 7%, while PVR dropped by 33 ± 23% with iNO, by 25 ± 29% with iPGI2, and by 18 ± 29% with their combination. CO increased by 17.5 ± 24% with iNO, 3.6 ± 12.6% with iPGI2, and 0.7 ± 7.9% with their combination. Of note, PAWP increased significantly with iPGI2 (P = 0.02) and trended toward an increase with both iNO (P = 0.066) and the combination (P = 0.067). The limited number of patients in this group who were exposed to all three phases (n = 5) rendered the analysis underpowered for detection of significant differences. Similar to the results in PAH patients, the PVR/SVR ratio decreased with iNO compared with baseline, suggesting a specific pulmonary vasodilatory effect in this population.
Table 3.
Hemodynamic responses to each vasodilator alone and in combination in HFpEF patients
Safety concerns
All PAH patients tolerated both agents alone and in combination. Two HFpEF patients completed only the baseline and the iPGI2 phase. Their baseline PAWP was 16 mmHg. One patient had an increase in PAWP to 19 mmHg at the end of the iPGI2 exposure, developed asymptomatic hypoxemia (oxygen saturation < 88%) despite increasing oxygen supplementation, and the procedure was aborted. The second patient tolerated iPGI2 with maintenance of PAWP to 16 mmHg, but developed dyspnea without oxygen desaturation upon exposure to iNO and the combination phase was not performed. PAWP was not recorded during the occurrence of dyspnea. Following discontinuation of the vasodilators in each instance, symptoms and oxygen saturations rapidly returned to baseline. Because of the occurrence of adverse effects in two of the seven patients in HFpEF, we elected to suspend further enrollment in this group.
DISCUSSION
We have shown that iNO and iPGI2 have similar pulmonary vasodilator effects in PAH patients of various etiologies. iPGI2 at 50 ng/kg/min exerted pulmonary vasodilator effects similar to iNO at 20 ppm, with comparable decreases in mPAP and PVR, while mBP and SVR remained unaltered. iNO was more selective on the pulmonary vasculature than iPGI2, producing a greater decrease in the PVR/SVR ratio. The combination of inhaled agents did not have additive vasodilatory effects.
In a comparison of the acute vasodilator effects of iNO and intravenous PGI2, Sitbon et al.,[6] showed a significant correlation between the two agents (r2 = 0.9, P < 0.001 for changes in mPAP) among IPAH patients who were acute vasoresponders, as defined by a decrease in total pulmonary resistance of ≥ 30%. In a subsequent report, the same investigators established that vasodilatory responses to iNO as defined by a fall in both mPAP and PVR by > 20% predict long-term responses to calcium channel blockers.[2] However, they did not address whether there was a correlation in the IPAH patients labeled as nonresponders, nor did they challenge patients with PH associated with other conditions.
Current consensus guidelines define a “positive” vasodilator response as a reduction in mPAP of at least 10 mmHg to a mPAP of < 40 mmHg.[4] IPAH patients meeting these criteria have a survival advantage over nonresponders and a higher likelihood of responding to long-term calcium channel blocker treatment.[4] More recent data suggest the presence of any vasodilatation has prognostic significance for PAH.[5] Therefore, our findings of a good correlation between the responses to the two inhaled vasodilators is of interest because either agent would be anticipated to be of equal value in prognostication.
Our results are different from those of Mikhail et al.,[10] who showed in a small group of PAH and secondary PH patients that iPGI2 (15-50 ng/kg/min) elicited significantly more vasodilatation than iNO (10-100 ppm) and the effect was not dose-dependent. Similar responses with more significant acute vasodilator effects on the pulmonary vasculature over iNO was demonstrated in six PAH patients who were given iPGI2 at 52-112 μg over 15 minutes and iNO at 10-28 ppm.[12] The discrepancies between these studies and ours may be attributable to unique delivery systems for iPGI2 used in each study. Unfortunately, we cannot ascertain the actual dose delivered to pulmonary tissues, which could vary between studies because of differences in condensation or impaction in the tubing, masks, and upper airways.
Considering that they work via different pathways that have shown additive effects in another clinical study,[13] we anticipated seeing additive effects of iNO and iPGI2 in the current one. The lack of additive effects may be due to maximizing the vasodilator capacity of the pulmonary vessels at the doses we administered.
In addition to PAH patients, we studied a small group of patients with HFpEF. Lam et al.,[14] investigated noninvasively a community based group of HFpEF patients and reported that the presence of PH was a strong predictor of mortality. Whether pulmonary reactivity of patients with PH-HFpEF predicts mortality remains to be determined.
In our HFpEF patients, iNO and iPGI2 tended to lower mPAP and PVR, the lack of statistical significance being related to the small number of patients and variable increases in cardiac output. The rise in PAWP that occurred in most patients and reached statistical significance with PGI2 testing contributed at least as much as the drop in mPAP to the narrowed transpulmonary gradient and hence decrease in PVR. We speculate that pulmonary vasodilation contributed to an accumulation of blood in the pulmonary veins in the presence of a stiff left ventricle and caused the increase in PAWP, which was responsible for adverse effects in some patients. These changes concur with those observed by Harralsson et al.,[7] who showed a significant increase in PAWP with both iNO and iPGI2 in patients with heart failure and a low ejection fraction, who were being evaluated for heart transplant. Semigran et al.,[15] compared the acute effects of iNO with intravenous nitroprusside in patients with HFpEF and also showed that while iNO decreased pulmonary vascular resistance to a greater degree than nitroprusside, it also increased PAWP.
While symptoms abated rapidly with cessation of drug inhalation in our patients who developed adverse effects, these observations suggest that selective pulmonary vasodilators should be administered to HFpEF patients with caution if at all.
Our study has a number of limitations. The small number of patients limits the statistical power, especially in our HFpEF patients. Nonetheless, we demonstrated highly significant correlations between the two agents among a variety of PH patients. We also lacked patients that would be considered positive acute vasodilator responders, and, thereby, were, unable to compare the ability of the two agents to elicit responses of that magnitude. In addition, practical limitations in the length of vasodilator studies in our catheterization lab prevented us from performing a dose-response study, although we selected doses that should have been in the maximal range based on prior literature,[2,10] and the lack of additivity between the two agents is consistent with that supposition.
In conclusion, we have shown that the vasodilator actions of iPGI2 correlate well with those of iNO in PAH patients and, at the doses used in this study, their combination lacks an additive effect. Our results support the use of iPGI2 as a possible alternative for testing acute vasoreactivity in PAH patients. Exposure of HFpEF patients to either iNO or iPGI2 increases PAWP with minimal if any pulmonary hemodynamic benefit and can induce adverse side effects.
ACKNOWLEDGMENTS
We acknowledge the support of the Respiratory Therapy department at Tufts Medical Center for the contribution to this study.
Footnotes
Source of Support: The Pulmonary, Critical Care and Sleep Division at Tufts Medical Center in Boston, Massachusetts, USA
Conflict of Interest: Ioana R. Preston has received grant support and/or honoraria for consultancies from Actelion, Aries, Bayer, GeNo, Gilead, Novartis, Pfizer, and United Therapeutics. Nicholas S. Hill has received grant support from Actelion, Bayer, GeNo, Gilead, Ikaria, Pfizer and United Therapeutics.
REFERENCES
- 1.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 2.Sitbon O, Humbert M, Jagot J, Taravella O, Fartoukh M, Parent F, et al. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium-channel blockers in primary pulmonary hypertension. Eur Respir J. 1998;12:265–70. doi: 10.1183/09031936.98.12020265. [DOI] [PubMed] [Google Scholar]
- 3.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1:250–8. doi: 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitbon O, Brenot F, Denjean A, Bergeron A, Parent F, Azarian R, et al. Inhaled nitric oxide as a screening vasodilator agent in primary pulmonary hypertension.A dose-response study and comparison with prostacyclin. Am J Respir Crit Care Med. 1995;151:384–9. doi: 10.1164/ajrccm.151.2.7842196. [DOI] [PubMed] [Google Scholar]
- 7.Haraldsson A, Kieler-Jensen N, Nathorst-Westfelt U, Bergh CH, Ricksten SE. Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest. 1998;114:780–6. doi: 10.1378/chest.114.3.780. [DOI] [PubMed] [Google Scholar]
- 8.Preston IR, Klinger JR, Houtches J, Nelson D, Farber HW, Hill NS. Acute and chronic effects of sildenafil in patients with pulmonary arterial hypertension. Respir Med. 2005;99:1501–10. doi: 10.1016/j.rmed.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Preston IR, Klinger JR, Houtchens J, Nelson D, Mehta S, Hill NS. Pulmonary edema caused by inhaled nitric oxide therapy in two patients with pulmonary hypertension associated with the CREST syndrome. Chest. 2002;121:656–9. doi: 10.1378/chest.121.2.656. [DOI] [PubMed] [Google Scholar]
- 10.Mikhail G, Gibbs J, Richardson M, Wright G, Khaghani A, Banner N, et al. An evaluation of nebulized prostacyclin in patients with primary and secondary pulmonary hypertension. Eur Heart J. 1997;18:1499–504. doi: 10.1093/oxfordjournals.eurheartj.a015478. [DOI] [PubMed] [Google Scholar]
- 11.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–87. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 12.Olschewski H, Walmrath D, Schermuly R, Ghofrani A, Grimminger F, Seeger W. Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med. 1996;124:820–4. doi: 10.7326/0003-4819-124-9-199605010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Saji K, Sakuma M, Suzuki J, Takahashi T, Demachi J, Nawata J, et al. Efficacy of acute inhalation of nitric oxide in patients with primary pulmonary hypertension using chronic use of continuous epoprostenol infusion. Circ J. 2005;69:335–8. doi: 10.1253/circj.69.335. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semigran MJ, Cockrill BA, Kacmarek R, Thompson BT, Zapol WM, Dec GW, et al. Hemodynamic effects of inhaled nitric oxide in heart failure. J Am Coll Cardiol. 1994;24:982–8. doi: 10.1016/0735-1097(94)90859-1. [DOI] [PubMed] [Google Scholar]


