Abstract
Abnormal within and across-joint synergistic behaviors have been reported in the lower limb post stroke. It is unknown, however, whether these impairments limit adaptive movement strategies in response to imposed kinematic constraints. In this context, the goal of this pilot study was to examine changes to three-dimensional swing phase kinematics of the paretic hip, knee, and ankle joints and pelvis induced by AFO use in subjects with chronic stroke. Overground gait analysis was performed on 9 ambulating hemiplegic subjects with and without their AFOs. Both the toeoff and peak ankle dorsiflexion angles were significantly decreased in the no AFO condition. Likewise, the peak and toeoff swing phase pelvic obliquity angles significantly increased when the AFO was removed (6.47° (2.0 SD) vs. 8.16° (2.8 SD), paired t-tests, p = 0.03 and 0.8° (3.1 SD) vs. 2.9° (1.1 SD), paired t-test, p = 0.02, respectively). These behaviors were consistent across subjects (7 of 9 subjects). The hip frontal plane, and hip and knee sagittal plane kinematics were unaffected by removal of the AFO. Finally, the minimum toe clearance was not affected by the removal of the AFO (1.39 cm ± 0.62 SD vs. 1.27 cm ± 0.47 SD, p > 0.05). Taken together, these findings suggest that pelvic obliquity is the primary compensatory degree of freedom utilized to achieve toe clearance in response to impaired dorsiflexion in the stroke population. We propose that this degree of freedom is exploited as it is not constrained by synergistic torque coupling of the lower limb.
Keywords: Rehabilitation, Kinematics, Pelvis, Motor control
1. Introduction
An estimated 20% of stroke survivors suffer from spastic drop foot, the inability to dorsiflex the foot which can lead to insufficient toe clearance during the swing phase of gait [1,2]. Consequently, ankle-foot orthoses (AFOs) are commonly prescribed to address the resulting body segment alignment and ankle joint motion [3]. The biomechanical implications of AFOs to moderately impaired subjects, however, are somewhat ambiguous. For example, benefits such as reduced metabolic demands [4,5] and improved self-reported confidence [6,7] while walking with AFOs have been observed in the chronic stroke population. However, inconsistent or insignificant alterations to spatial, temporal, and sagittal plane kinematic gait variables have hindered understanding of the biomechanical foundation underlying these benefits. Specifically, conflicting effects of AFO use on gait symmetry [8–10] and speed [7,11–13] have been reported, with the significant increases in gait speed failing to reach a level of functional improvement (increase of 20 cm/s) [14,15]. Moreover, only minimal adaptive changes to sagittal plane kinetics and kinematics at the hip and knee joints have been observed [11,13]. To achieve toe clearance despite impaired ankle dorsiflexion, individuals with stroke often employ hip hiking (defined as excessive up pelvic obliquity [16,17]) and circumduction (defined as excessive hip abduction angle, although this definition is currently debated [16,17]) strategies. However, the relative contribution of these two components to compensatory behavior is unclear.
In an earlier study, we reported that subjects with chronic stroke displayed within and across-joint torque synergies characterized by abnormal hip adduction and knee extension torque coupling and direction specific hip torque weakness [18]. Furthermore, these synergies appear to be strongly associated with kinematic deviations observed during overground walking [19], such that the more pronounced the torque synergies, the greater the observed kinematic deviations. It is unknown, however, if adaptive movement strategies in response to imposed kinematic constraints include motions associated with the observed torque synergies. If changes are observed, one could argue that kinematics at these joints are not constrained by underlying torque synergies. In this context, this pilot study will quantify adaptive changes in the three-dimensional kinematics of the paretic lower limb and pelvis of daily AFO users walking with and without their orthoses. While investigating adaptations under a more demanding task, such as obstacle negotiation or ground removal, would be in accordance with previous perturbation studies, we argue that this experimental paradigm constitutes a functionally relevant perturbation.
Consistent with previous investigations [20], we hypothesize that sagittal plane hip and knee flexion angles during swing will be unaffected by the use of AFOs. We also hypothesize that pelvic obliquity will be the primary compensatory action to achieve toe clearance in the absence of sufficient dorsiflexion provided by the AFO. Moreover, this compensatory action will be fine-tuned to maintain the minimal toe clearance level consistent with the AFO condition [21]. Given that the adaptive kinematics are proposed to be restricted to the frontal plane, we expect AFO usage to have minimal effect on spatial temporal properties of gait.
2. Methods
2.1. Subjects
Nine chronic ambulating hemiplegic cortical stroke subjects (7 males, 6 right hemiplegics) who use their AFOs daily were tested (age 51 years (SD 10.2), time post stroke 50.2 months (SD 39.9), height 177.7 cm (SD 13.3), and mass 83.6 kg (SD 21.3)). Subjects used their personal AFOs made from plastic copolymers (3 solid, 6 hinged). Lesion location was confirmed by imaging, with no evidence of brainstem, or cerebellar damage. Subjects were excluded if they could not walk at least 10 m overground without physical assistance or if their gait speed was >1.0 m/s, indicating a near full recovery of velocity. Exclusion criteria consisted of significant cardiorespiratory or metabolic disease including untreated cardiac failure, diabetes, or hypertension, and a history of previous orthopedic or neurological conditions which may limit walking ability.
2.2. Data collection
Overground gait analysis was performed using an eight camera motion capture system (Motion Analysis Corp, Santa Rosa, CA, 120 Hz) by tracking the three-dimensional (3D) motion of one inch, retro reflective markers affixed to the pelvis, thighs, shanks, and feet. Specifically, markers were placed on the posterior sacrum, the bilateral ASIS, medial and lateral femoral condyles, medial and lateral malleoli, posterior heel counter of the shoe, and dorsally over the second metatarsal head to identify segment ends. The motion of the thighs and shanks were tracked by three markers rigidly affixed to thermoplastic shells, which were in turn wrapped securely to the thighs and shanks. Subjects were asked to walk at their comfortable, self-selected pace across a ten meter walkway, first with their AFOs, then without their AFOs. No additional walking aids (canes or walkers) were used. A minimum of five trials were collected per condition, and subjects were given a five minute seated break between the AFO and non-AFO conditions.
2.3. Data processing
The marker trajectories were identified and low-pass filtered (6 Hz) to track the 3D motion of the pelvis and lower limb segments using EvaRT software (Motion Analysis Corp). The calibration error of the motion capture system was <1.0 mm. The relative positions and inter-segmental joint angles were calculated using a rigid body analysis [22]. Pelvic, hip, and knee 3D joint angles were normalized to stride cycle and averaged across strides using OrthoTrak 6.2.4.
2.4. Outcome measures and statistics
The kinematic variables (hip and knee flexion, ankle dorsiflexion, pelvic obliquity, and hip abduction angle) were assessed at discrete, subject-specific time points for the paretic limb. The peak swing phase angles were selected to reveal the greatest potential change with AFO use. These angles were also assessed at the initiation of swing (toeoff), as modeling studies have shown the importance of initial conditions for determining swing phase outcomes [23]. Spatial and temporal variables were calculated, including gait speed, step width, step length, and swing time, and normalized to subject height. Finally the minimum amount of toe clearance achieved during swing, as determined by the vertical displacement of the second metatarsal marker, was measured to assess potential changes in effective leg length [21]. Statistical analysis was performed using NCSS (2004). Paired t-tests were used to identify differences between conditions (p < 0.05, α = 0.05). Non-parametric analysis (Wilcoxon signed-rank test) was used for non-normal distributions.
3. Results
Significant differences in pelvic obliquity and ankle dorsiflexion angles were observed between the AFO and no AFO conditions (Fig. 1 and Table 1). Specifically, the peak swing phase pelvic obliquity angle significantly increased from 6.5° (2.0 SD) to 8.2° (2.8 SD) (Wilcoxon ranked sign test, p = 0.03) when the AFO was removed, while the peak swing phase dorsiflexion angle significantly decreased (−1.9° (SD 6.3) vs. 4.6° (SD 4.5), paired t-test, p = 0.01). At toeoff, the mean and standard deviation of the pelvic obliquity in the AFO condition was 2.9° and 1.1, respectively. A paired t-test revealed that this amplitude is significantly different (p = 0.02) than the corresponding values 0.8° (SD 3.1) observed during the no AFO condition. Likewise, at toeoff the ankle dorsiflexion angle was significantly greater in the AFO condition compared to the no AFO condition (7.9° (SD 5.3) vs. 10.1° (SD 6.0)). These behaviors were consistent across subjects with 7 of the 9 participants displaying increased pelvic obliquity and decreased ankle dorsiflexion when the AFO was removed.
Fig. 1.
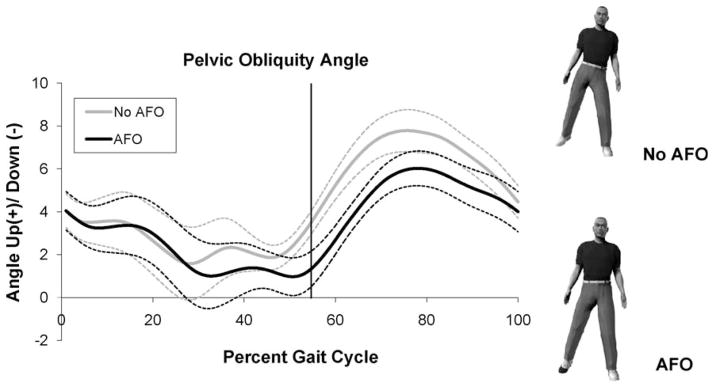
Pelvic obliquity of the paretic limb. Up (+)/Down (−). Vertical line indicates toeoff.
Table 1.
Frontal and sagittal plane gait kinematics.
| No AFO mean (SD) | AFO mean (SD) | P-value | |
|---|---|---|---|
| Frontal plane | |||
| Pelvic obliquity angle† | 2.9 (1.1) | .8 (3.1) | .02* |
| Peak pelvic obliquity angle | 8.2 (2.8) | 6.5 (2.0) | .03‡ |
| Hip abduction angle† | −4.0 (5.1) | −3.4 (4.0) | .63 |
| Peak hip abduction angle | 2.0 (2.3) | 2.6 (2.5) | .95 |
| Sagittal plane | |||
| Hip flexion angle† | 3.3 (11.1) | 1.7 (10.4) | .22 |
| Peak hip flexion angle | 27.9 (11.1) | 29.6 (11.6) | .20 |
| Knee flexion angle† | 19.3 (12.4) | 14.4 (11.8) | .25 |
| Peak knee flexion angle | 31.9 (7.7) | 27.7 (13.2) | .32 |
| Ankle dorsiflexion angle† | 7.9 (5.3) | 10.1 (6.0) | .02* |
| Peak ankle dorsiflexion angle | −1.9 (6.3) | 4.6 (4.5) | .01* |
Up pelvic obliquity, hip adduction, hip flexion, knee flexion, and ankle dorsiflexion are defined as positive (+).
Statistically significant differences between AFO and non AFO conditions by paired t-test.
Calculated at toeoff.
Statistically significant differences between AFO and non AFO conditions by Wilcoxon signed-rank test.
On the contrary, the paretic limb hip frontal plane behavior was not impacted by the use of AFOs (Fig. 2 and Table 1). There was neither a change in peak swing phase paretic hip abduction/adduction angle (2.0° (SD 2.3) vs. 2.6° (SD 2.5), p = 0.95), nor a change to paretic hip joint angle at toeoff (−4.0 (SD 5.1) vs. −3.4 (SD 4.0), p = 0.63) when the AFO was removed.
Fig. 2.
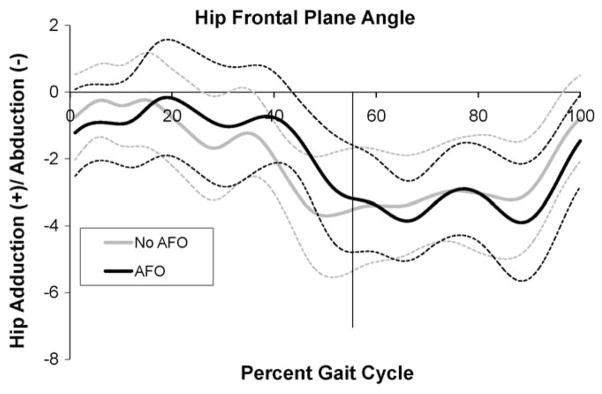
Hip frontal plane angle of the paretic limb. Adduction (+)/Abduction (−). Vertical line indicates toeoff.
The swing phase sagittal plane kinematics at the knee and hip joints were also unaffected by removal of the AFO (Table 1). Specifically, the hip flexion angles were not significantly different between the AFO and no AFO conditions neither at peak swing phase (29.6° (SD 11.6) vs. 27.9° (SD 11.1), p = 0.20), nor at toeoff (1.7° (SD 10.4) vs. (3.3° (SD 11.1), p = 0.22). Similarly, the knee flexion angle at peak swing phase (19.3° (SD 12.4) vs. 14.4° (SD 11.8), p = 0.25) and toeoff (31.9° (SD 7.72) vs. (27.7° (SD 13.23), p = 0.32) were not significantly different across conditions.
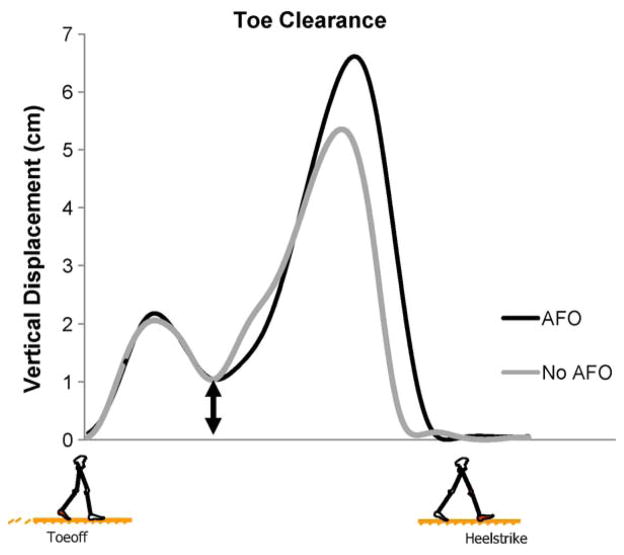
The minimal amount of toe clearance was not affected by the removal of the AFO (1.39 cm ± 0.62 SD vs. 1.27 cm ± 0.47 SD, p > 0.05, Fig. 3 for example data). More specifically, the upstream kinematics of the paretic limb were adjusted to maintain the same level of minimal toe clearance when the AFO was removed.
Fig. 3.
Example of toe clearance from one stroke subject. Double arrow indicates the minimum vertical displacement of the paretic limb. Toeoff to Heelstrike.
Minimal changes to spatial variables were observed when the AFO was removed (Table 2). The normalized overground gait speed was not significantly different between AFO and no AFO conditions (0.42 (SD 0.07) vs. 0.39 (SD 0.08); p = 0.19). There were also no differences in normalized step width (0.12 (SD 0.03) vs. 0.13 (SD 0.03) p = 0.22). Differences were, however, observed in the step length of the non-paretic limb, which was significantly shorter when the AFO was removed (0.24 (SD 0.06) vs. 0.27 (SD 0.06), p = 0.03). This influence did not extend to the paretic limb step length, which was not affected by the no AFO condition (0.29 (SD 0.05) vs. 0.31 (SD 0.05), p = 0.30.) The removal of the AFO did not affect the temporal gait variable (% swing time) of either the paretic (47.65% (SD 4.6) vs. 46.15% (SD 3.5)) or non-paretic limb (31.02% (SD 2.9) vs. 30.95% (SD 3.7)).
Table 2.
Gait speed, step width and step length variables are normalized to height (mean and SD).
| No AFO | AFO | P-value | |
|---|---|---|---|
| Gait speed (s−1) | .39 (.08) | .42 (.07) | .19 |
| Step width | .13 (.03) | .12 (.03) | .22 |
| Paretic step length | .29 (.05) | .31 (.05) | .30 |
| Non-paretic step length | .24 (.06) | .27 (.06) | .03* |
| Paretic swing time (% gait cycle) | 47.65 (4.6) | 46.15 (3.5) | .16 |
| Non-paretic swing time (% gait cycle) | 31.02 (2.9) | 30.95 (3.7) | .95 |
Statistically significant differences between AFO and non AFO conditions by paired t-test.
4. Discussion
This work explored the effects of AFO use on frontal and sagittal plane kinematics of the paretic limb in the chronic stroke population. Our findings indicate that increased peak and toeoff pelvic obliquity of the paretic limb were used to maintain constant minimum toe clearance during swing in response to decreased ankle dorsiflexion in the absence of AFOs. At the same points in the gait cycle, the corresponding knee and hip sagittal plane and frontal plane hip angles were unaffected. Furthermore, the compensatory pelvic obliquity resulted in the consistent minimal toe clearance when the AFO was removed.
The adaptation of pelvic obliquity, combined with the absence of kinematic changes at the hip and knee joints, may be reflective of the available motor control options post-stroke. Specifically, a failure to compensate for impaired ankle dorsiflexion through increased knee or hip flexion angle may be due to abnormal synergistic coupling of the hip and knee joints, clinically defined as extension (hip adduction, extension and internal rotation coupled with knee extension) and flexion (hip flexion, abduction, and external rotation coupled with knee flexion) synergies [18,24]. These synergies reduce the motor system’s ability to manipulate individual degrees of freedom in response to kinematic constraints. Indeed, our previous work has found statistical associations between torque synergies and abnormal pelvic obliquity behavior [19]. The exploitation of an additional degree of freedom beyond those constrained by synergies found here is similar to reports from upper limb reaching movements in which trunk lean is recruited to compensate for impaired shoulder and elbow coordination in stroke subjects [25]. In the presence of an AFO, however, the motor system may limit pelvic obliquity to reduce the metabolic [4,5] and energetic [13] demands of walking.
Proprioceptive impairments have been suggested as one of the factors contributing to abnormal motor control behaviors post-stroke [26,27]. To the contrary, our data revealed that compensatory adaptations maintained similar toe clearance with and without AFO. Furthermore, the amplitude of the minimum toe clearance observed in the stroke group was consistent with those reported in healthy control subjects (1.39 cm ± 0.62 vs. 1.29 cm ± 0.45 [21]). These findings may be interpreted as a demonstration of spatial awareness of the lower limb during gait by stroke subjects.
Plantarflexion power at the initiation of swing, or pushoff, has been shown to significantly contribute to the forward progression of the body during healthy gait [28]. As such, it has been suggested that the reduction in pushoff is one of the limiting factors to maximal walking speed post stroke [29]. In the context of this study, we propose that AFO use may be considered an experimental model that largely eliminates the contribution of plantarflexors to pushoff. The insignificant changes to preferred walking speed with and without AFO found in this investigation suggest that plantarflexors have little influence on pushoff and gait speed after stroke. This finding, however, is in contrast to existing literature [15]. This disparity may be due to differences in normalization strategies. We repeated our analysis with non-normalized gait speed (m/s) and no statistical difference was found between conditions. While statistical significance of gait speed as a function of AFO use has been reported, the magnitude of these changes do not constitute a functional improvement [14]. Therefore plantar-flexion power may restrict maximal gait speed, but it appears to have a limited role in determining preferred gait speed post stroke [29,30].
Despite the potential influence of AFO use on gait stability, little change was observed in the base of support (as defined by the step length and width) between conditions. The observed increase in non-paretic step length is consistent with reports that stride length may be longer with AFOs than without [15], although the breakdown of paretic and nonparetic step length has not previously shown significant differences [7]. The consistent paretic step length and step width across conditions suggests that the AFO use does not affect paretic foot placement. This further supports the assertion that only swing phase changes to the limb were affected at the pelvis and ankle.
The major limitation of this study is the relatively small sample size (n = 9), which may have contributed to the insignificant difference in gait speeds observed across conditions. A second limitation was the non-randomization of gait conditions, as the AFO condition was tested first for all subjects. Fatigue, however, is not thought to influence the outcomes, as experiment time was brief and subjects were given rest between trials and conditions. Finally, the type of AFO was not consistent across subjects. Given the swing phase focus of this investigation, differences in AFO type are not anticipated to influence results because both hinged and solid AFOs prevent excessive plantar-flexion during swing.
Clinically, the adaptation of the pelvic movement to maintain constant toe clearance suggests that hip hiking may not be an intrinsic motor change resulting from stroke, but rather the primary compensatory motion. This compensatory adaptation may be largely constrained by the abnormal torque synergies observed in the lower limb post stroke [18]. Therefore, a rehabilitation strategy designed specifically to reduce synergistic within and across joint coupling may lead to significant changes in locomotor behaviors after stroke.
Acknowledgments
We would like to thank Preeti Nair, PT PhD for her contribution in editing this manuscript. We would also like to thank Jennifer Moore, MPT, and Heidi Roth, MSPT for assistance with data collection and analysis. Sources of funding: This research was made possible by funding from the Searle Fund, the American Heart Association (Predoctoral Fellowship #0610062Z) and by the U.S. Department of Education, National Institute on Disability and Rehabilitation Research (Field Initiated Grant # H133A990008).
Footnotes
Conflicts of interest
The authors and collaborators have no conflicts of interest.
References
- 1.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11(3):201–10. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 2.Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104(3):431–49. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- 3.Shurr D, Michael J. Prosthetics and Orthotics. Upper Saddle River. NJ: Prentice Hall; 2002. [Google Scholar]
- 4.Danielsson A, Sunnerhagen KS. Energy expenditure in stroke subjects walking with a carbon composite ankle foot orthosis. J Rehabil Med. 2004;36(4):165–8. doi: 10.1080/16501970410025126. [DOI] [PubMed] [Google Scholar]
- 5.Danielsson A, Willen C, Sunnerhagen KS. Measurement of energy cost by the physiological cost index in walking after stroke. Arch Phys Med Rehabil. 2007;88(10):1298–303. doi: 10.1016/j.apmr.2007.06.760. [DOI] [PubMed] [Google Scholar]
- 6.de Wit DC, Buurke JH, Nijlant JM, Ijzerman MJ, Hermens HJ. The effect of an ankle-foot orthosis on walking ability in chronic stroke patients: a randomized controlled trial. Clin Rehabil. 2004;18(5):550–7. doi: 10.1191/0269215504cr770oa. [DOI] [PubMed] [Google Scholar]
- 7.Tyson SF, Thornton HA. The effect of a hinged ankle foot orthosis on hemiplegic gait: objective measures and users’ opinions. Clin Rehabil. 2001;15(1):53–8. doi: 10.1191/026921501673858908. [DOI] [PubMed] [Google Scholar]
- 8.Hesse S, Werner C, Matthias K, Stephen K, Berteanu M. Non-velocity-related effects of a rigid double-stopped ankle-foot orthosis on gait and lower limb muscle activity of hemiparetic subjects with an equinovarus deformity. Stroke. 1999;30(9):1855–61. doi: 10.1161/01.str.30.9.1855. [DOI] [PubMed] [Google Scholar]
- 9.Hesse S, Luecke D, Jahnke MT, Mauritz KH. Gait function in spastic hemiparetic patients walking barefoot, with firm shoes, and with ankle-foot orthosis. Int J Rehabil Res. 1996;19(2):133–41. doi: 10.1097/00004356-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Pohl M, Mehrholz J. Immediate effects of an individually designed functional ankle-foot orthosis on stance and gait in hemiparetic patients. Clin Rehabil. 2006;20(4):324–30. doi: 10.1191/0269215506cr951oa. [DOI] [PubMed] [Google Scholar]
- 11.Gok H, Kucukdeveci A, Altinkaynak H, Yavuzer G, Ergin S. Effects of ankle-foot orthoses on hemiparetic gait. Clin Rehabil. 2003;17(2):137–9. doi: 10.1191/0269215503cr605oa. [DOI] [PubMed] [Google Scholar]
- 12.Fatone S, Hansen AH. Effect of ankle-foot orthosis on roll-over shape in adults with hemiplegia. J Rehabil Res Dev. 2007;44(1):11–20. doi: 10.1682/jrrd.2006.08.0090. [DOI] [PubMed] [Google Scholar]
- 13.Bleyenheuft C, Caty G, Lejeune T, Detrembleur C. Assessment of the Chignon(®) dynamic ankle-foot orthosis using instrumented gait analysis in hemiparetic adults. Ann Readapt Med Phys. 2008;51(3):154–60. doi: 10.1016/j.annrmp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 15.Leung J, Moseley A. Impact ofankle-footorthosis ongait and leg muscle activityin adults with hemiplegia: systematic literature review. Physiotherapy. 2003;89:39–55. [Google Scholar]
- 16.Kerrigan DC, Frates EP, Rogan S, Riley PO. Hip hiking and circumduction: quantitative definitions. Am J Phys Med Rehabil. 2000;79(3):247–52. doi: 10.1097/00002060-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: SLACK; 1992. [Google Scholar]
- 18.Cruz T, Dhaher Y. Evidence of abnormal lower limb torque synergies after stroke: an isometric study. Stroke. 2008;39(1):139–47. doi: 10.1161/STROKEAHA.107.492413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz TH, Dhaher YY. Compensatory gait movements post stroke: the influence of synergies. American Society of Biomechanics; Ann Arbor, MI: 2008. [Google Scholar]
- 20.Burdett RG, Borello-France D, Blatchly C, Potter C. Gait comparison of subjects with hemiplegia walking unbraced, with ankle-foot orthosis, and with Air-Stirrup brace. Phys Ther. 1988;68(8):1197–203. [PubMed] [Google Scholar]
- 21.Winter DA. Foot trajectory in human gait: a precise and multifactorial motor control task. Phys Ther. 1992;72(1):45–56. doi: 10.1093/ptj/72.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–44. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg SR, Ounpuu S, Delp SL. The importance of swing-phase initial conditions in stiff-knee gait. J Biomech. 2003;36(8):1111–6. doi: 10.1016/s0021-9290(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 24.Brunnstrom S. A neurophysiological approach. New York: Harper & Row; 1970. Movement therapy in hemiplegia. [Google Scholar]
- 25.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–53. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 26.Leibowitz N, Levy N, Weingarten S, Grinberg Y, Karniel A, Sacher Y, et al. Automated measurement of proprioception following stroke. Disabil Rehabil. 2008;30(24):1829–36. doi: 10.1080/09638280701640145. [DOI] [PubMed] [Google Scholar]
- 27.Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil. 2008;22(8):758–67. doi: 10.1177/0269215508090674. [DOI] [PubMed] [Google Scholar]
- 28.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- 29.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol Avon) 1999;14(2):125–35. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 30.Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2008;29(1):129–37. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]