Abstract
Gender differences in neuromuscular control of the lower extremity may contribute to increased injury risk in females, but the neurophysiological mechanisms underlying these differences remain unclear. In this study, we sought to explore the effect of gender on volitional and reflex neuromuscular responses to a rapid valgus perturbation at the knee applied under “intervene” and “do not intervene” conditions. Multiple 7° ramp-and-hold valgus perturbations were applied at the neutrally extended knee of 12 male and 12 female healthy subjects, while surface electromyography over the quadriceps and hamstrings recorded the neuromuscular response. Volitional responses did not vary between groups, perhaps reflecting the relative novelty of the loading direction. However, reflex responses observed under the “do not intervene” paradigm did vary by gender. Males demonstrated much more frequent and consistent reflex muscle activation than females. Moreover, muscle activation patterns were gender-specific. Diminished responses in female subjects may indicate that the position-based valgus perturbation did not produce the necessary mechanical stimulus to elicit reflexes. These gender differences in reflex control of the knee provide new insight into the control of frontal-plane knee joint movement and loading and may elucidate the neuromechanical underpinnings associated with neuromuscular control.
Keywords: frontal plane, gender, knee, motor control, reflex
It has been suggested that a deficit in proper neuromuscular control of the lower extremity in female athletes contributes to an increased knee injury rate compared with male athletes in the same sports.1 Indeed, numerous investigators have documented neuromuscular differences during sporting activities, including gender-specific muscle activation strategies as well as lower limb kinematics and kinetics.2–5 These differences may in part be due to inherent biomechanical differences between the genders, such as musculoskeletal alignment,6 muscle anatomy/physiology,7,8 and passive joint compliance.9,10 Conversely, Field et al.11 proposed that the differences in movement organization between genders are primarily due to variations in descending input from cortical and subcortical regions, which may be mediated by hormonal concentration differences. However, it is likely that gender differences observed under functional tasks are due to a combination of both biomechanical and neural factors.
The execution of a task-specific motor template requires the integration of very complex sensory signals and descending drive.12–14 Potentially, gender differences at any level along the neural axis (cortical, subcortical, and spinal) could influence these motor templates. Investigations utilizing functional magnetic resonance imaging (fMRI) have demonstrated disparities in neural activation between males and females when they perform similar motor tasks in several motor and premotor areas of the brain,15,16 which may suggest variations in both descending drive and the integration of sensory signals. In an attempt to isolate the neural pathways associated with somatosensory feedback, investigators have examined gender differences in muscle reflex activity under controlled loading conditions at the knee, primarily in response to tibial internal/external rotation17,18 and anterior translation19 loading. These investigations have shown gender-specific temporal activation of knee muscles. However, the organization and scaling of the muscle activation patterns across knee muscles were not addressed.
Accordingly, the central goal of this study was to further examine gender differences in neuromuscular strategies to resist isolated loading at the knee. We applied a controlled valgus positional perturbation at the knee under non–weight-bearing conditions and recorded the resulting neuromuscular response in the quadriceps and hamstrings muscles. Our rationale for choosing the valgus perturbation was twofold: first, there is a body of evidence to suggest that frontal-plane loading may increase knee injury risk, particularly in female athletes.3,20 Second, familiarity with a given loading condition or movement may lead to modifications in neuromuscular control.21,22 Thus, our use of a novel valgus positional perturbation, with which subjects have limited prior experience, provides an experimental platform to evaluate gender differences while minimizing potential biases associated with motor learning. Joint perturbations were applied under both “intervene” and “do not intervene” testing conditions to facilitate an exploration of the differences in muscular response due to anticipation of the perturbation and differentiate between reflex and volitional control.19,23,24 Neuromuscular responses were quantified in terms of muscle onset latency, activation intensity, activation pattern, and, for the “do not intervene” trials, the probability of muscle firing. To this end, consistent with previous reports,23,24 we expect that there will be within-group differences between reflex and volitional control of the joint, in terms of both response latency and activation patterns. We hypothesize that there will be differences in the reflex behavior across gender, which may be attributable to variations in structural and/or neurophysiological properties. However, we hypothesize that volitional control, observed during the “intervene” trials, will be similar between genders. We believe that the choice of perturbation direction (i.e., novel loading in a relatively constrained degree of freedom) will predominantly affect the volitional response. The results of this study will further elucidate our understanding of the interaction between gender and neurophysiology as they relate to motor control.
METHODS
All experimental procedures were approved by the institutional review board of Northwestern University and complied with the principles of the Declaration of Helsinki. Twelve male subjects and 12 female subjects, with no history of neurological or musculoskeletal disorders, participated in the study after providing informed consent. All subjects reported a moderate level of activity, which mainly included running/jogging or recreational sports. Subjects were excluded if they had a history of injuries to the knee or lower extremity, or tested positive to manual joint laxity tests administered by a physical therapist. Prior to testing, subjects were evaluated by a physical therapist to measure joint alignment and balance. Frontal-plane joint alignment was assessed by determining the quadriceps angle (Q angle), defined as the acute angle between the line connecting the tibial tubercle and the middle of the patella, and the line extending from the middle of the patella to the anterosuperior iliac spine (ASIS).25 Balance was tested using a modified “foam-and-dome” test, which gauges subjects’ reliance on visual, proprioceptive, and vestibular cues to maintain balance, to insure they were within normal limits for their age group and to screen for potential proprioceptive deficits.26,27 Subjects were asked to maintain a single-limb stance for up to 30 s under four categories: eyes open; eyes closed; eyes open while standing on foam; and eyes closed while standing on foam. The amount of time each subject could maintain the single-limb stance under each condition was recorded. A summary of subjects’ demographics as well as the results from these screening tests are reported in Table 1.
Table 1.
Subjects’ demographics.
| Male | Female | |
|---|---|---|
| Age | 25.9 (3.8) | 24.9 (4.2) |
| Height (m) | 1.80 (0.07) | 1.65 (0.08) |
| Weight (kg) | 79.9 (8.4) | 58.8 (5.1) |
| Q angle (°) | 8.3 (4.7) | 12.4 (3.0) |
| Balance tests26* | ||
| Eyes open | 30.0 (0.0) | 29.3 (2.3) |
| Eyes closed | 22.7 (10.7) | 26.3 (8.0) |
| Eyes open on foam | 27.3 (7.2) | 30.0 (0.0) |
| Eyes closed on foam | 13.7 (11.6) | 16.0 (10.0) |
Values presented as mean (SD).
All subjects were within normative values for this age group.27
The fluctuation of female sex hormones may potentially affect neuromuscular control and passive joint properties.28 Hence, to minimize any possible confounding effects, all female subjects were tested at approximately the same time-point in the menstrual cycle. Subjects who were not using hormonal contraceptives (6 of 12) were tested within 2 days of their self-reported start of menstrual bleeding, whereas the remaining female subjects were tested during the off-week of hormonal contraceptive use, which is similar to the early follicular phase in non-users.29
Experimental Protocol
The detailed experimental procedures have been described in previous work from our laboratory30,31 and are only briefly presented here. Muscle activations were recorded from the quadriceps and hamstrings muscles using surface electromyography (EMG) electrodes. Although surface EMG may be susceptible to cross-talk from neighboring muscles,32 previous studies have demonstrated that cross-talk is negligible in the large thigh muscles.33,34 After an alcohol swab was used to abrade and clean the skin at the electrode placement site, pre-amplified surface EMG electrodes (Bagnoli 3.1; Delsys, Boston, Massachusetts) were used to record from the rectus femoris (RF), vastus medialis oblique (VMO), vastus medialis longus (VML), vastus lateralis (VL), semitendinosus (ST), and biceps femoris (BF).
Maximum voluntary contraction (MVC) levels in knee flexion and extension were recorded with the subject seated and the knee at 60° of flexion. The EMG response to tendon tap was recorded to establish monosynaptic stretch reflex latencies in the quadriceps for all subjects. A minimum of 15 repeated taps were delivered to the patellar tendon over a period of 30 s, using a hammer mounted with a load cell (Kistler Instrument Corp., Amherst, New York).
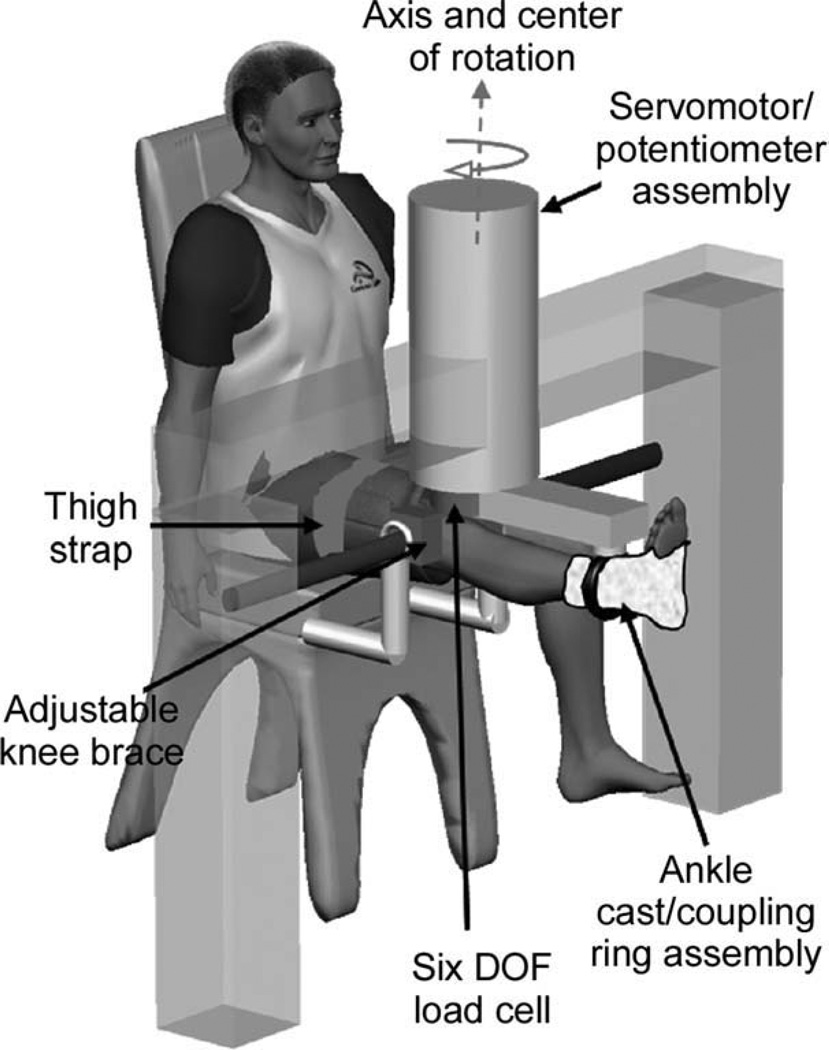
For the mechanical perturbation protocol, subjects were seated in an experimental chair with the right knee neutrally extended (0° of knee flexion) and allowed to assume their natural frontal-plane knee joint alignment (Fig. 1). The subject’s right ankle was placed in a cast and then secured within a coupling ring, which fixed the limb to a servomotor actuator via a rigid cantilever beam. The center of motor rotation was aligned with the apex of the patella, roughly the joint’s frontal plane center of rotation. A 6 degree-of-freedom load cell (JR3, Inc., Woodland, California), attached to the servomotor, was used to record the force and torque signals, and a precision potentiometer and tachometer mounted on the actuator were used to record position/velocity signals. Brackets were securely fastened around the knee joint to prevent medial/lateral translation of the knee during the mechanical perturbation, and a strap was placed over the right thigh to prevent movement of the proximal limb.30,31
FIGURE 1.
Experimental set-up. Subjects were seated in an experimental chair with the right leg extended. The apex of the patella was aligned with the center of rotation of the servomotor. The right leg was fixed within a coupling ring with a cast placed around the ankle joint. The coupling ring was fixed to a servomotor actuator with a precision potentiometer and tachometer. The knee was fitted within a bracket mounted firmly at the medial and lateral femoral epicondyles. Together with a thigh strap, the brackets isolated the knee varus–valgus movement from the frontal plane movement of the hip joint.
During subject evaluation, manual laxity tests were performed by a physical therapist as a qualitative screening tool for potential injury. In addition to these tests, a quantitative assessment of frontal-plane stiffness in the experimental posture was performed by applying a quasi-static frontal-plane stretch to the knee joint at a constant velocity (3°/s). Starting from the neutral position, the servomotor rotated each subject’s knee to 7° of valgus, then to 7° of varus, and then back to the neutral position. The resulting frontal-plane torque and position signals were filtered with a second-order Butterworth filter with a 4-HZ cut-off frequency. Passive valgus stiffness was determined at 6° as the slope of the line tangent to the loading portion of the torque–angle relationship.9
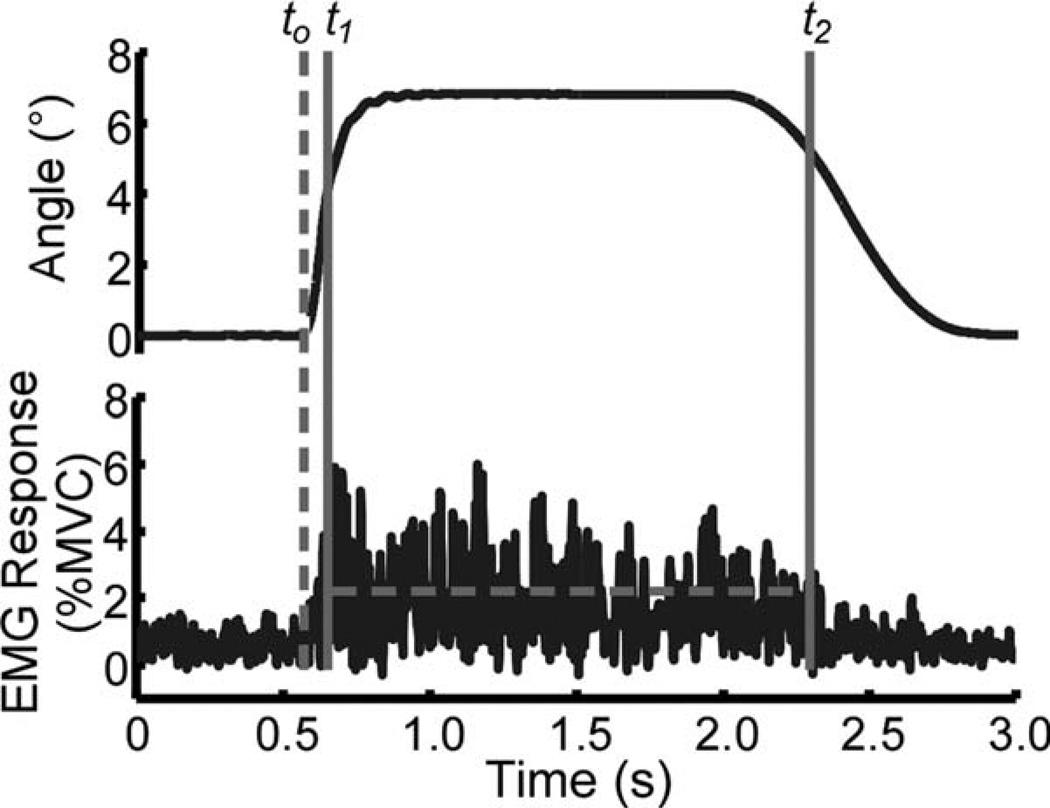
The knee was preloaded in the valgus direction (~3° to 4°) to insure initial stretch of the medial aspect of the joint’s periarticular tissues. Preloading insured an instantaneous resistance by these tissues to an additional mechanical perturbation, eliminating the potential for mechanical delays.31 From this position, multiple valgus ramp-and-hold positional perturbations (at least 4) were applied at the knee, consisting of a ramp-up loading phase at 60°/s, a constant position section of 7° held for 0.5 s, and an unloading phase (Fig. 2). The 7° amplitude positional perturbation was chosen because it is similar to the range of motion reported by LaFortune et al.35 during level walking. Subjects were asked to report any discomfort or pain during these initial movements to reduce the possibility of eliciting reflex responses from nociceptors (Aδ-fibers or C-fibers).
FIGURE 2.
Representative position input (top) and resulting EMG recording (bottom) during a “do not intervene” trial. The dashed vertical line represents the onset of movement, to. Muscle activation onset and offset (t1 and t2) are depicted for the EMG signal. The dashed horizontal line between t1 and t2 represents the intensity of the neuromuscular response.
To examine differences between volitional and reflex muscular control, the positional perturbations were applied under “do not intervene” and “intervene” testing conditions.19,23,24 Under the “do not intervene” condition, subjects were instructed not to activate their knee muscles or react during the application of the mechanical perturbation. During the “intervene” testing condition, subjects were instructed to resist the valgus perturbation by generating an opposing varus torque as soon as they detected movement. Post hoc analysis of surface EMG data was performed to identify trials with muscle activation prior to the mechanical perturbation to insure that subjects sustained the required relaxed state of knee muscles. Trials showing unacceptable EMG activity before the perturbation were rejected. As the valgus positional perturbation used in this study represented a novel loading paradigm, the “do not intervene” protocol was always performed first during the experimental sessions. In this context, we assumed that this allowed both males and females a short period of familiarization with the loading protocol prior to being asked to resist it.
Data Analysis
To eliminate high-frequency noise (>250 HZ) associated with the servomotor system and to prevent aliasing, the EMG and load-cell signals were filtered online with an eighth-order, low-pass, zero-phase digital Butterworth filter with a 220-HZ cut-off frequency and then sampled at 1 kHZ. For data processing and analysis, EMG signals were rectified and filtered offline using an eighth-order, low-pass Butterworth filter with a 120-HZ cut-off frequency.
Movement onset (to) of the valgus perturbation was defined as the time at which the loading velocity reached 10% of the desired maximum velocity (60°/s). The onset of EMG activity during the “do not intervene” protocol was identified starting 40 ms after to. Specifically, the EMG signal was searched for the onset of muscle activation (t1), which was defined as the point when the signal remained above the threshold, or 3 SDs above the mean background activity 100 ms prior to to, for 5 ms. The latency of the muscle response was computed for each muscle in each subject from the mean of all trials. Similarly, the offset of muscle activation (t2) was determined when the EMG signal dropped below the 3-SD threshold level for 5 ms. The onset of EMG activity during the “intervene” protocol was identified by first scanning the EMG signal starting at to for the largest muscle activity averaged over 100 ms. Once the largest mean and its corresponding SD were found, the EMG was scanned backward until the signal remained below the 3-SD threshold for 5 ms to identify EMG onset (t1). Likewise, EMG offset (t2) was found by scanning forward until the signal dropped below the 3-SD threshold. All EMG signals were also visually inspected to confirm the correct determination of the window of muscle firing. Figure 2 shows both the position stimulus signal and a representative record of EMG activity.
To quantify the intensity of the muscular activity, a normalized response (NR) measure was used for all muscles as follows30,31:
| (1) |
where was the time average of the rectified and filtered EMG activity during the activity period (from t1 to t2), was the time average of the baseline activity calculated over 100 ms before the onset of the mechanical perturbation, and was the time average of the EMG activity over 100 ms of the MVC data. Based on experimental condition, the NR was either classified as the normalized reflex response (NRR) or the normalized volitional response (NVR). A muscle response was significant if the NR was greater than the SD of the background activity for that trial. In the “do not intervene” condition, for each muscle, the probability that a perturbation could elicit muscle activation, or firing probability, was computed as the ratio of trials with a significant NRR to the total trials performed. In addition, an overall firing probability was computed as the ratio of trials with a significant NRR in at least one muscle to the total trials performed.
EMG signals recorded during the tendon tap protocol were filtered using the same filter settings used for the EMG data obtained in the valgus perturbation protocol. Tap response latencies were computed using a threshold criterion similar to that of Zhang et al.36 and Dhaher et al30 and were compared with the latency of the reflex responses elicited by valgus perturbation to determine if these responses were of comparable latency to monosynaptic stretch reflexes.
Four outcome measures were used to quantify the effect of gender and trial type on the neuromuscular response: firing probability; muscle latency; activation intensity; and activation pattern. Firing probability was compared between genders for the “do not intervene” condition using a two-sample t-test. A two-factor analysis of variance (ANOVA) was used to compare the effect of gender and experimental condition on muscle latency and activation intensity (NRR and NVR). Tukey–Kramer multiple comparisons were performed post hoc to examine the interactions of gender and trial type. Statistical significance was set a priori at P = 0.05.
In addition to describing the individual muscle activation intensity, a six-dimensional activation pattern unit vector was created to describe the coordinated muscle activation under both testing conditions. The response (NRR or NVR) for each muscle was divided by the Euclidian magnitude (square root of the sum of squared elements) of all muscle activation for that trial to create the activation pattern vector. The correlation between two vectors was assessed using the square root of the dot product between the vectors.12,37 This alignment coefficient has a magnitude of between 0 and 1, with 1 indicating perfect alignment between vectors.
Given previously reported differences in passive frontal-plane stiffness between genders,9 it is probable that the 7° valgus perturbation resulted in different levels of resistive torque across groups. To determine how frontal-plane loading varied between genders during the 7° perturbation, the filtered (second-order Butterworth filter with a 4-HZ cut-off frequency) load-cell signal corresponding to moments about the anteroposterior axis was examined. To compensate for inertial contributions, each subject’s lower limb inertia was estimated using the regression equations proposed by Zatsiorsky and Seluyanov38 as a function of subjects’ lower leg dimensions. The product of inertia and angular acceleration (in rad/s2) was subtracted from the recorded frontal-plane torque, leaving only the component of the torque signal resulting from resistance of subjects’ soft tissues.31 The amplitude of frontal-plane torque produced by the valgus perturbation was computed as follows:
| (2) |
where Tbackground was the mean frontal-plane torque signal over 100 ms prior to the onset of movement (to), and Tloading represented the torque produced during the loading portion of the perturbation. Because the latency of the reflex response was, on average, 95 ms, Tloading was calculated as the mean torque signal between 50 ms and 60 ms after to. Estimating Tloading at this time-point, which is significantly prior to the onset of muscle activity (<t1), insures that Tloading only reflects the resistance of the passive tissues of the joint.
RESULTS
Firing Probability
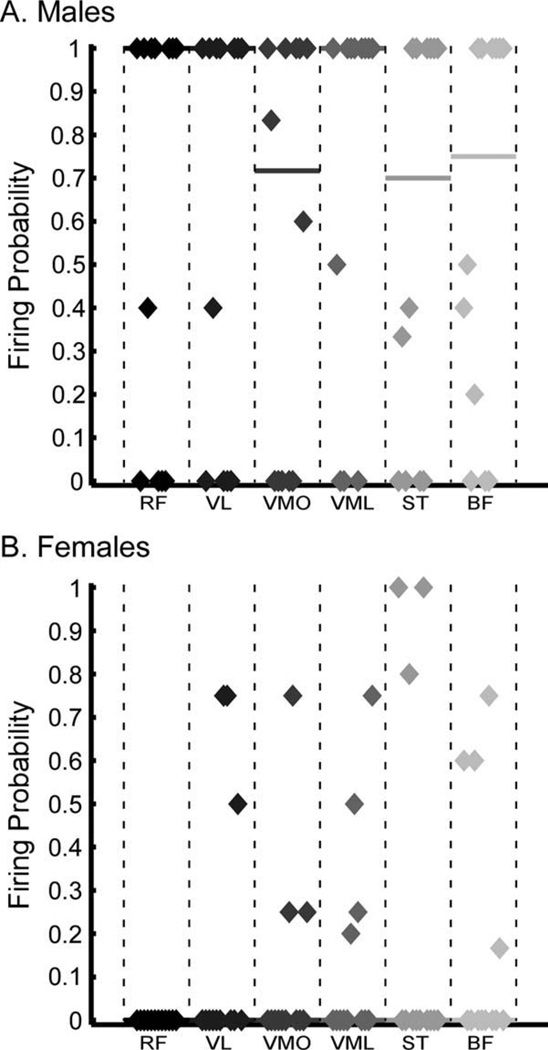
Under the “do not intervene” testing condition, reflex muscle activation was observed much more frequently in male than in female subjects (Fig. 3). Overall firing probability, or the proportion of trials in which at least one muscle was activated, was 0.81 for males, but only 0.43 for females. Indeed, 7 of the 12 female subjects had an overall firing probability of <0.5, including 6 subjects with no significant muscle activity in response to perturbation. On the other hand, 9 of 12 male subjects had an overall probability >0.5. Furthermore, the firing probability of each muscle was significantly greater in males than females (P < 0.001). As shown in Figure 3, within the female population who demonstrated muscle activity in response to the valgus perturbation, firing probability for each muscle was low and inconsistent across trials. For example, 2 female subjects demonstrated very consistent (firing probability = 1.0) activation of ST, but insignificant activation of the other muscles tested. Similarly, most female subjects displayed activation in only one or two muscles in each trial. Conversely, a majority of male subjects demonstrated activity across nearly all muscles tested.
FIGURE 3.
Firing probability of each muscle during the “do not intervene” testing condition for (A) males and (B) females. The firing probability was calculated as the ratio of trials with a significant NRR for that muscle to the total trials performed. The distribution of firing probabilities across subjects is represented, and the median firing probability is plotted as a straight line. Five female subjects demonstrated significant muscle activation in response to the perturbation for at least 50% of trials. Thus, the median firing probability across all muscles was 0. Nine male subjects demonstrated significant muscle activation in at least 50% of trials, and firing probability in males was much higher and more consistent than for the females across all of the muscles tested. Note that, for the RF, VL, and VML muscles, the median firing probability was 1.0 for the male subjects.
Latency
Muscle latencies were found to be significantly greater in the “intervene” (volitional) condition than in the “do not intervene” (reflex) condition (Table 2). A two-factor (gender and testing condition) ANOVA was performed to determine the effect of gender and experimental condition on muscle latency. A significant effect of testing condition (P < 0.05), but no significant effect of gender (P > 0.05), was found, with latencies significantly greater in the volitional case than in the reflex case. Further, no significant variations in latency across muscles were noted for either testing condition. In addition, reflex muscle latencies from the “do not intervene” testing condition were significantly greater than those obtained for the quadriceps muscles during the tendon tap protocol (Table 2).
Table 2.
Muscle latencies observed across testing conditions (in milliseconds).
| “Intervene” | “Do not intervene” | Tendon tap | ||||
|---|---|---|---|---|---|---|
| Muscles | Males | Females | Males | Females | Males | Females |
| RF | 193 (60) | 212 (32) | 88 (12) | NA* | 38 (3) | 35 (3) |
| VL | 198 (72) | 201 (29) | 96 (11) | 98 (12) | 39 (4) | 37 (4) |
| VMO | 182 (66) | 197 (26) | 99 (16) | 82 (5) | 38 (4) | 36 (3) |
| VML | 204 (66) | 205 (33) | 89 (20) | 89 (16) | 40 (3) | 36 (4) |
| ST | 161 (23) | 179 (30) | 84 (14) | 96 (9) | ||
| BF | 158 (37) | 197 (34) | 102 (21) | 104 (26) | ||
| Mean† | 184 (57) | 197 (32) | 93 (16) | 95 (16) | ||
Values presented as mean (SD). See text for abbreviations of muscles. NA, not available.
No reflex responses were recorded in the RF muscle for females.
Mean across all muscles tested.
Muscle Activation Intensity and Pattern
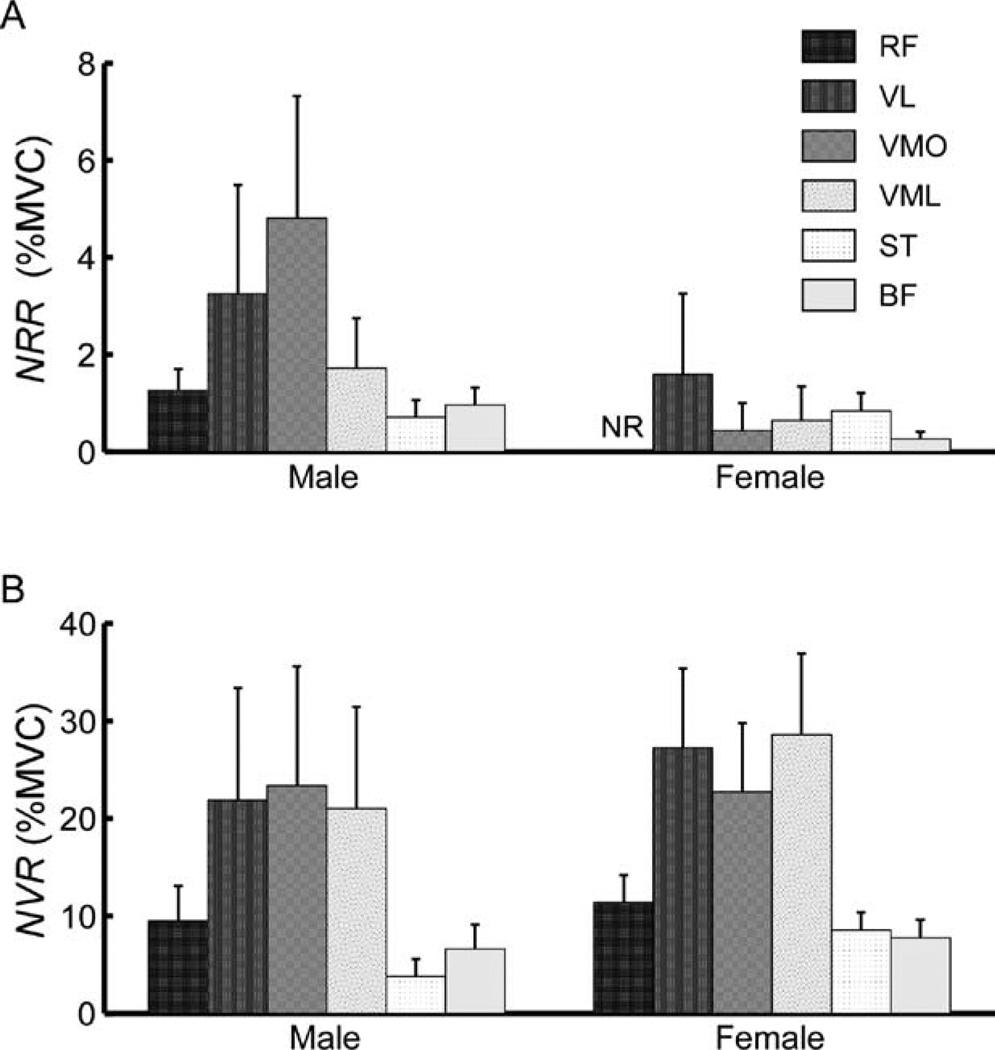
The intensity of muscle activation was found to be significantly greater under the “intervene” condition than the “do not intervene” condition (Fig. 4). A two-factor ANOVA was performed to compare the amplitude of response (NRR vs. NVR) in each muscle across testing condition. Reflex data from the “do not intervene” test condition was only considered from subjects who demonstrated significant muscle activation in at least 50% of trials (5 females and 9 males). A significant (P < 0.05) effect of experimental condition was found for all muscles, as the normalized muscular response was significantly greater in the “intervene” than the “do not intervene” condition. A significant effect of gender (P < 0.05) was found in the ST muscle, with females displaying greater activation intensity than males. No significant gender effects (P > 0.05) were found for the remaining muscles.
FIGURE 4.
Mean activation intensity (NR) across genders and testing conditions. Error bars represent standard error. (A) Responses are in the “do not intervene” condition. Shown is the mean NRR for the 5 of 12 females and 9 of 12 males who demonstrated significant muscle activation in at least 50% of trials. There was no response recorded in the rectus femoris (RF) muscle across females. (B) Muscle activation for the “intervene” condition for males and females (NVR). Muscle abbreviations are as previously defined.
For both genders and experimental conditions, the mean activation pattern vector was determined by averaging across subjects who demonstrated significant muscle activation (9 males and 5 females in “do not intervene” and all subjects for “intervene”). Similarity between pairs of activation pattern unit vectors was assessed as the square root of the dot product between them.12,37 To judge the significance of alignment coefficients, a bootstrap analysis was performed.39 The alignment coefficients of samples drawn at random from individual trials across both genders and testing conditions were computed for 5000 bootstrap samples. Based on the resulting bootstrap distribution, a threshold of 0.80 was used to judge similarity at the α = 0.05 level. Under the “intervene” testing condition, all subjects, both males and females, employed a generalized activation of the targeted leg muscles, resulting in similar volitional activation patterns with an alignment coefficient of 0.85. Activation patterns between testing conditions (NRR vs. NVR) were different for both males (0.77) and females (0.67). Finally, male and female activation patterns in the “do not intervene” condition were the least similar, with an alignment coefficient of 0.61.
To assess statistical differences between components of the activation pattern unit vector (i.e., relative weighting of each muscle’s activity), a two-factor (gender and experimental condition) ANOVA was performed. This analysis revealed that RF activation varied significantly between genders (P < 0.001) and across experimental conditions (P < 0.001). In particular, RF activation was significantly greater in the “intervene” than in the “do not intervene” trials, and there was significantly greater activation of RF in males than females for both testing conditions. VL activity was significantly greater (P = 0.03) in males than in females. There was also a significant interaction effect found (P < 0.02) between gender and trial type for VMO and VML, as females demonstrated less activation than males in the reflex response, but greater in the volitional response. Activation of the ST muscle was gender-specific in the “do not intervene” trials (P = 0.006), as females demonstrated greater activation than males. Finally, BF activation did not differ between genders or across testing conditions.
Frontal-Plane Mechanics
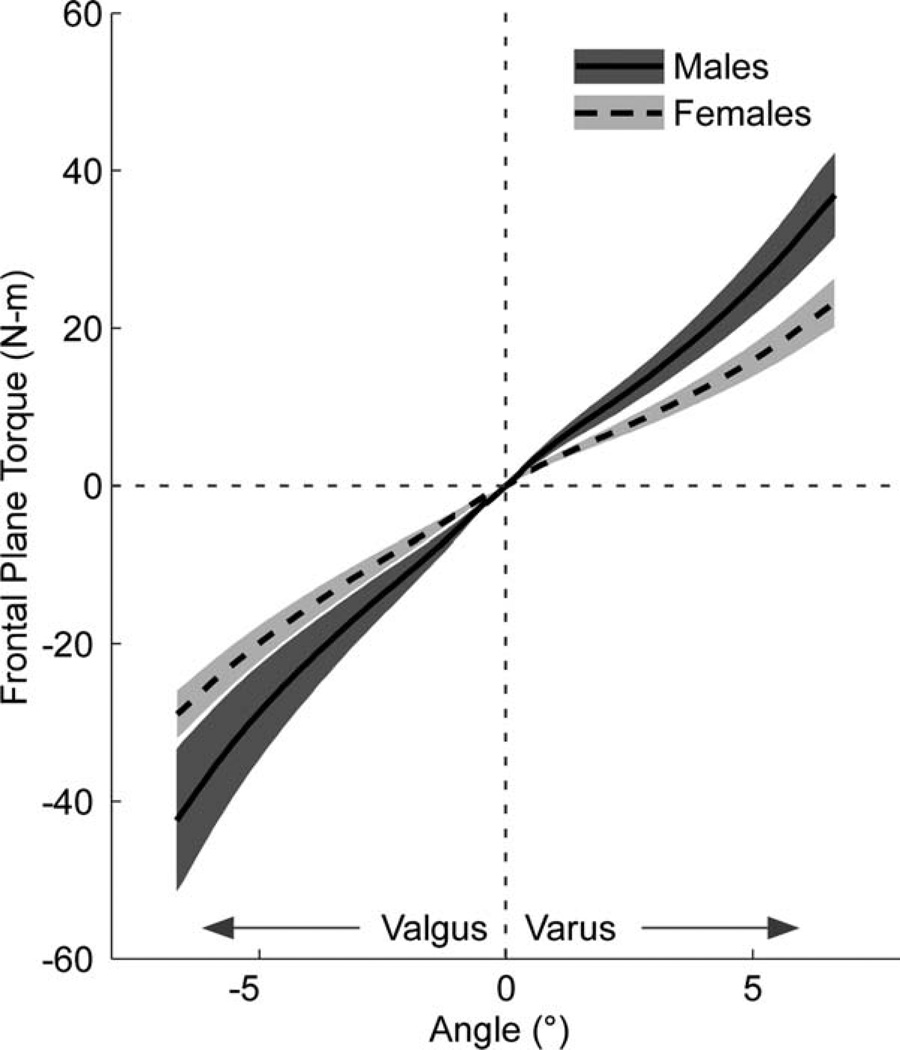
Figure 5 displays 95% confidence intervals of the mean frontal-plane torque–angle relationships for males and females. Passive valgus stiffness at 6° was significantly greater (P = 0.001, two-sample t-test) in male subjects, with a mean (SD) stiffness of 7.07 (2.31) N·m per degree, than in female subjects, with a mean (SD) stiffness of 4.48 (0.69) N·m per degree. Analysis of the frontal-plane torque resulting from the rapid valgus perturbation also revealed a significant effect of gender (Table 3). For the same 7° valgus perturbation, it was found that both Tbackground and ΔT were significantly greater in males than females (P < 0.05, two-sample t-test), indicating that the positional perturbation had different effects on the loading of the joint in the frontal plane.
FIGURE 5.
Mean frontal-plane torque versus angular displacement from the passive stretching trials. The solid black line represents males, and the dashed line represents females. The shaded regions represent the 95% confidence intervals for each subject group.
Table 3.
Frontal-plane torque resulting from the 7° rapid valgus perturbation (in N·m).
Values expressed as mean (SD).
Mean resistive torque in male subjects was significantly greater than in females (P < 0.05, two-sample t-test).
DISCUSSION
In this study, we sought to explore gender differences in volitional and reflex neuromuscular responses to a rapid valgus perturbation at the knee when applied under “do not intervene” and “intervene” testing conditions. Our results indicate a within-group difference in the neuromuscular behaviors during each testing condition23 in terms of both the response latency and activation patterns. Volitional neuromuscular control strategies did not vary between groups; however, gender significantly affected reflex responses observed under the “do not intervene” paradigm. Although the latency of the reflex response was similar across groups, the valgus perturbation elicited consistent muscle activation in a majority of male subjects, but in less than half of the female subjects. Further, firing probability for each individual muscle was higher in males than females. Last, when considering the population subset demonstrating reflex activity, the muscle activation pattern was gender-specific. These gender differences may provide new insights into neurophysiological mechanisms contributing to the mediolateral stability of the knee joint.
Gender differences in reflex control of the knee have been examined previously in response to a rapid anterior/posterior (AP) translation19 and an internal/external (IE) rotation of the weight-bearing tibia.17,18 The main experimental outcomes of these studies were to quantify muscle latency and the temporal pattern of muscle activation. For both perturbation directions, males and females employed a similar sequence of muscle activation, characterized by an initial activation of the gastrocnemius with a latency of 52–59 ms, followed by hamstrings and quadriceps activation after 10–40 ms. Although no significant gender differences in reflex muscle latency were found in the AP loading paradigm, females recruited the quadriceps faster than males in the IE loading protocol.17,18 One could argue that these loading protocols may have elicited reflexes mediated by muscle spindles. The reported latency of gastrocnemius responses is consistent with monosynaptic muscle reflexes,24,40 whereas the slightly longer latency (~70 ms) of the hamstring and quadriceps could potentially be heteronymous in nature.41 In contrast to the sequential muscle activation latencies reported in the aforementioned studies, the reflex latency in this study did not vary across muscles or genders and was considerably longer (~95 ms). This longer latency is consistent with reflexes mediated by joint soft-tissue afferents.30,31,42,43 Thus, these differences in muscle latency suggest that the various loading directions (AP, IE, or valgus) may engage different reflex pathways.
One of the most intriguing results of this study is the significant effect of gender on the reflex response to valgus perturbation under a relaxed state. Although differences in central neural mechanisms, such as motoneuron and/or corticomotor excitability, may have contributed to this bias, there is no evidence in the literature to suggest that either of these parameters vary with gender.44,45 Alternatively, we argue that the observed gender differences in reflex behavior may have been attributed primarily to peripheral neural mechanisms. Although the 7° valgus perturbation produced similar displacement at the knee, it resulted in decreased resistive torque in female subjects (Table 3), presumably due to gender-based differences in joint laxity (Fig. 5). If this reflex was mediated by muscle spindles, one would expect to observe similar muscular reflex activations corresponding to the same perturbation-induced muscle displacement. However, if this reflex response was mediated by soft-tissue resistive torque rather than displacement, the diminished reflex responses in the female population may indicate that the 7° position perturbation produced a subthreshold mechanical stimulus. Further, it has been noted that 3 of the 12 male participants failed to produce reflex responses. Thus, if this reflex response is mediated by resistive torque, one would hypothesize that the 7° positional perturbation produced decreased joint torque in these male participants. Indeed, upon closer examination, two-factor ANOVA revealed a significant effect of both gender (P < 0.05) and the presence of a significant reflex response (overall firing probability > 0.5) on the observed frontal-plane joint mechanics. Post hoc Tukey–Kramer comparisons showed that the resistive torque (ΔT) in the male subjects without significant reflexes [N = 3; mean (SD): 13.65 (2.56) N·m] was significantly decreased compared to males with reflex responses [19.15 (2.22) N·m], but it was not different from that of female subjects. Further, although not significant, the mean ΔT observed in the female population with reflex responses [N = 6; mean (SD): 12.41 (4.54) N·m] was greater than ΔT in females without reflex responses [11.11 (1.88) N·m]. These observations suggest that the observed reflex responses may be correlated with joint frontal-plane mechanics and the corresponding periarticular tissue stiffness. Combined with data from reduced preparations indicating mechanoreceptor stress sensitivities,46–48 we argue that the observed gender differences may provide indirect evidence that these reflexes are indeed mediated by periarticular mechanoreceptors.30,31,49 Nonetheless, further investigations using torque-based perturbations are necessary to fully describe the relationship between soft-tissue intrinsic stress levels, gender, and the observed reflex intensity.
In contrast to reflex behaviors, both males and females employed similar volitional neuromuscular strategies when resisting the valgus perturbation, which resembled a generalized coactivation of the knee muscles. These findings are consistent with the agonist/antagonist coactivation observed during an isokinetic knee extension task, which is presumably employed by the motor control system to help protect the joint passive tissues.50 One could also argue that the observed volitional activation patterns may be related to subjects’ unfamiliarity with the loading condition. Indeed, when learning to perform a novel task, subjects may initially adopt a coactivation strategy; however, as familiarity with the task increases, the neuromuscular strategies become more targeted and efficient.51 In the upper extremity, such targeted motor control strategies can emerge over a relatively short time-course (one to three trials).51 As such, it is possible that motor adaptation occurred throughout our experimental session. However, statistical analysis revealed consistent activation patterns across trials, with intrasubject alignment coefficients >0.9 across all subjects. Given the novel valgus loading paradigm, it is possible that the number of trials performed in the current study was insufficient to capture any potential adaptations. Further, given the non-random experimental protocol (i.e., the “do not intervene” trials were always performed first), there is a possibility that learning effects may have played a role in the observed volitional neuromuscular strategies in both populations. We argue, however, that, because both males and females underwent the same number of “do not intervene” trials, any motor adaptation would have been similar across groups. To explore the potential effect of learning across gender on the volitional motor templates, future investigations should employ a randomized “intervene/do not intervene” treatment protocol.
It has been demonstrated that the integration of sensory feedback is highly state- and task-dependent52; therefore, our choice of loading paradigm may have influenced the neuromuscular strategies observed in the current study. The testing posture used herein (knee in 0° flexion and non–weight-bearing) does not replicate conditions experienced during everyday tasks. For example, frontal-plane loading typically occurs under weight-bearing conditions with the knee in a flexed position.3,35 Although performing the perturbations in a flexed-knee position would have more closely resembled natural loading, it is extremely difficult to isolate and control valgus movement as the knee is flexed.9 Thus, use of this loading paradigm allowed us to more precisely control the input to the neuromuscular system and provided an experimental platform to evaluate gender differences in volitional and reflex neuromuscular control. However, direct application of these results to the behavior of the neuromuscular system during functional tasks should be approached with caution.
The reflex responses to valgus loading were of relatively small amplitude (~1–5% MVC), which may seem insignificant to increase joint stability. However, these values may have been biased by the normalization method used in this study. The collection of MVC data and the valgus loading protocol were performed with the knee in a posture of 60° knee flexion and 0° flexion, respectively. As the amplitude of a maximal volitional quadriceps EMG signal at 60° knee flexion is significantly greater than that produced at 0° knee flexion,53 normalizing by these MVC values may have systematically decreased the amplitude of the reflex response obtained at the 0° knee flexion posture. Further, in this study, reflex responses were recorded in an isolated setting with quiescent knee muscles. However, the contribution of these reflexes to joint stability may be substantially different during functional tasks in the presence of descending drive and potentially heightened motoneuron excitability. Indeed, it has been demonstrated that the reflex contribution to joint stiffness increases with increasing muscle preactivation (or increased motoneuron excitability).31,54 In addition, sensory feedback from periarticular mechanoreceptors may contribute to joint stability by modulating muscle stiffness via reflex action on γ-motoneurons.55
An alternative hypothesis for the functional significance of these reflexes is that afferent information from periarticular mechanoreceptors may contribute to the development of predictive motor templates during everyday tasks.31,43 In fact, periarticular afferents have been demonstrated to project to a number of supraspinal structures, including the cerebellum, which has been linked to motor learning.55 If such neural mechanisms exist, exposing the neuromuscular system to loading in the frontal plane could potentially enhance awareness of joint loading in these directions and lead to the development of appropriate motor control strategies. Indeed, this hypothesis may be supported by the success of neuromuscular training programs in reducing the incidence of anterior cruciate ligament (ACL) injuries.56,57
Our comparison of neuromuscular responses in male and female subjects to a valgus perturbation provides new insight into the control of frontal-plane knee joint loading. In particular, our results may suggest that this class of reflexes is mediated by intrinsic stress levels, as opposed to stretch, in joint soft tissues. Thus, a larger angular joint excursion may be necessary to elicit protective reflex responses in females, an excursion that may play a significant role in increased risk of joint injury.3 In addition, future investigations are necessary to describe the input–output relationship between joint tissue stress and the intensity of reflex response. Such investigations may elucidate the neuromechanical underpinnings associated with neuromuscular control in functional tasks.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIAMS RO1 AR049837 and NICHD T32 HD07418). The authors thank T. DeMott, A. Tsoumanis, and C. Stanford for their help with data collection and analysis.
Abbreviations
- ACL
anterior cruciate ligament
- ANOVA
analysis of variance
- AP
anterior/posterior
- BF
biceps femoris
- EMG
electromyography
- IE
internal/external
- MVC
maximum voluntary contraction
- NR
normalized response
- NRR
normalized reflex response
- NVR
normalized volitional response
- RF
rectus femoris
- ST
semitendinosus
- VL
vastus lateralis
- VML
vastus medialis longus
- VMO
vastus medialis oblique
REFERENCES
- 1.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 2.Hewett TE, Zazulak BT, Myer GD, Ford KR. A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br J Sports Med. 2005;39:347–350. doi: 10.1136/bjsm.2005.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 4.Ford KR, Myer GD, Toms HE, Hewett TE. Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc. 2005;37:124–129. [PubMed] [Google Scholar]
- 5.Zeller BL, McCrory JL, Kibler WB, Uhl TL. Differences in kinematics and electromyographic activity between men and women during the single-legged squat. Am J Sports Med. 2003;31:449–456. doi: 10.1177/03635465030310032101. [DOI] [PubMed] [Google Scholar]
- 6.Horton MG, Hall TL. Quadriceps femoris muscle angle: normal values and relationships with gender and selected skeletal measures. Phys Ther. 1989;69:897–901. doi: 10.1093/ptj/69.11.897. [DOI] [PubMed] [Google Scholar]
- 7.Roberts VI, Mereddy PK, Donnachie NJ, Hakkalamani S. Anatomical variations in vastus medialis obliquus and its implications in minimally-invasive total knee replacement. An MRI study. J Bone Joint Surg Br. 2007;89:1462–1465. doi: 10.1302/0301-620X.89B11.18636. [DOI] [PubMed] [Google Scholar]
- 8.Wojtys EM, Huston LJ, Schock HJ, Boylan JP, Ashton-Miller JA. Gender differences in muscular protection of the knee in torsion in size-matched athletes. J Bone Joint Surg Am. 2003;85-A:782–789. doi: 10.2106/00004623-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Cammarata ML, Dhaher YY. The differential effects of gender, anthropometry, and prior hormonal state on frontal plane knee joint stiffness. Clin Biomech (Bristol, Avon) 2008;23:937–945. doi: 10.1016/j.clinbiomech.2008.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu WH, Fisk JA, Yamamoto Y, Debski RE, Woo SL. Differences in torsional joint stiffness of the knee between genders: a human cadaveric study. Am J Sports Med. 2006;34:765–770. doi: 10.1177/0363546505282623. [DOI] [PubMed] [Google Scholar]
- 11.Field EF, Pellis SM. The brain as the engine of sex differences in the organization of movement in rats. Arch Sex Behav. 2008;37:30–42. doi: 10.1007/s10508-007-9270-4. [DOI] [PubMed] [Google Scholar]
- 12.Cheung VC, d’Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci. 2005;25:6419–6434. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol Rev. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Toussaint HM, Michies YM, Faber MN, Commissaris DA, van Dieen JH. Scaling anticipatory postural adjustments dependent on confidence of load estimation in a bi-manual whole-body lifting task. Exp Brain Res. 1998;120:85–94. doi: 10.1007/s002210050380. [DOI] [PubMed] [Google Scholar]
- 15.Li CS, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuroimage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Gorbet DJ, Sergio LE. Preliminary sex differences in human cortical BOLD fMRI activity during the preparation of increasingly complex visually guided movements. Eur J Neurosci. 2007;25:1228–1239. doi: 10.1111/j.1460-9568.2007.05358.x. [DOI] [PubMed] [Google Scholar]
- 17.Shultz SJ, Perrin DH, Adams MJ, Arnold BL, Gansneder BM, Granata KP. Neuromuscular response characteristics in men and women after knee perturbation in a single-leg, weight-bearing stance. J Athlet Train. 2001;36:37–43. [PMC free article] [PubMed] [Google Scholar]
- 18.Carcia CR, Shultz SJ, Granata KP, Perrin DH, Martin RL. Females recruit quadriceps faster than males at multiple knee flexion angles following a weight-bearing rotary perturbation. Clin J Sport Med. 2005;15:167–171. doi: 10.1097/01.jsm.0000164042.76540.e5. [DOI] [PubMed] [Google Scholar]
- 19.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 20.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech (Bristol, Avon) 2004;19:828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Dordevic GS, Shadmehr R. Learning the dynamics of reaching movements results in the modification of arm impedance and long-latency perturbation responses. Biol Cybern. 2001;85:437–448. doi: 10.1007/s004220100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen JB, Cohen LG. The Olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J Physiol. 2008;586:65–70. doi: 10.1113/jphysiol.2007.142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol. 1956;132:17P–18P. [PubMed] [Google Scholar]
- 24.Berardelli A, Hallett M, Kaufman C, Fine E, Berenberg W, Simon SR. Stretch reflexes of triceps surae in normal man. J Neurol Neurosurg Psychiatry. 1982;45:513–525. doi: 10.1136/jnnp.45.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piva SR, Fitzgerald K, Irrgang JJ, Jones S, Hando BR, Browder DA, et al. Reliability of measures of impairments associated with patello-femoral pain syndrome. BMC Musculoskel Disord. 2006;7:33. doi: 10.1186/1471-2474-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66:1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 27.Crotts D, Thompson B, Nahom M, Ryan S, Newton RA. Balance abilities of professional dancers on select balance tests. J Orthop Sports Phys Ther. 1996;23:12–17. doi: 10.2519/jospt.1996.23.1.12. [DOI] [PubMed] [Google Scholar]
- 28.Shultz SJ, Gansneder BM, Sander TC, Kirk SE, Perrin DH. Absolute serum hormone levels predict the magnitude of change in anterior knee laxity across the menstrual cycle. J Orthop Res. 2006;24:124–131. doi: 10.1002/jor.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss JF, Barbieri RL, Yen SSC. Yen and Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. Philadelphia: Elsevier/Saunders; 2004. p. 1042. [Google Scholar]
- 30.Dhaher YY, Tsoumanis AD, Rymer WZ. Reflex muscle contractions can be elicited by valgus positional perturbations of the human knee. J Biomech. 2003;36:199–209. doi: 10.1016/s0021-9290(02)00334-2. [DOI] [PubMed] [Google Scholar]
- 31.Dhaher YY, Tsoumanis AD, Houle TT, Rymer WZ. Neuromuscular reflexes contribute to knee stiffness during valgus loading. J Neurophysiol. 2005;93:2698–2709. doi: 10.1152/jn.00921.2004. [DOI] [PubMed] [Google Scholar]
- 32.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 33.Fuglevand AJ, Winter DA, Patla AE, Stashuk D. Detection of motor unit action potentials with surface electrodes: influence of electrode size and spacing. Biol Cybern. 1992;67:143–153. doi: 10.1007/BF00201021. [DOI] [PubMed] [Google Scholar]
- 34.Õunpuu S, DeLuca PA, Bell KJ, Davis RB. Using surface electrodes for the evaluation of the rectus femoris, vastus medialis and vastus lateralis muscles in children with cerebral palsy. Gait Posture. 1997;5:211–216. [Google Scholar]
- 35.Lafortune MA, Cavanagh PR, Sommer HJ, III, Kalenak A. Three-dimensional kinematics of the human knee during walking. J Biomech. 1992;25:347–357. doi: 10.1016/0021-9290(92)90254-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang LQ, Huang H, Sliwa JA, Rymer WZ. System identification of tendon reflex dynamics. IEEE Trans Rehabil Eng. 1999;7:193–203. doi: 10.1109/86.769410. [DOI] [PubMed] [Google Scholar]
- 37.Valero-Cuevas FJ. Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol. 2000;83:1469–1479. doi: 10.1152/jn.2000.83.3.1469. [DOI] [PubMed] [Google Scholar]
- 38.Zatsiorsky V, Seluyanov V. Estimation of the mass and inertia characteristics of the human body by means of the best predictive regression equations. In: Winter DA, Norman RW, Well RP, Hayes KC, Patla AE, editors. Biomechanics IX-B. Champaign, IL: Human Kinetics; 1985. pp. 233–239. [Google Scholar]
- 39.Efron B, Tibshirani R. Statistical data analysis in the computer age. Science. 1991;253:390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb GL, Agarwal GC. Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol. 1979;42:91–106. doi: 10.1152/jn.1979.42.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Marque P, Nicolas G, Marchand-Pauvert V, Gautier J, Simonetta-Moreau M, Pierrot-Deseilligny E. Group I projections from intrinsic foot muscles to motoneurones of leg and thigh muscles in humans. J Physiol. 2001;536:313–327. doi: 10.1111/j.1469-7793.2001.t01-1-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dyhre-Poulsen P, Krogsgaard MR. Muscular reflexes elicited by electrical stimulation of the anterior cruciate ligament in humans. J Appl Physiol. 2000;89:2191–2195. doi: 10.1152/jappl.2000.89.6.2191. [DOI] [PubMed] [Google Scholar]
- 43.Krogsgaard MR, Dyhre-Poulsen P, Fischer-Rasmussen T. Cruciate ligament reflexes. J Electromyogr Kinesiol. 2002;12:177–182. doi: 10.1016/s1050-6411(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 44.Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20:570–576. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman M, Harter RA, Hayes BT, Wojtys EM, Murtaugh P. The interrelationships among sex hormone concentrations, motoneuron excitability, and anterior tibial displacement in women and men. J Athlet Train. 2008;43:364–372. doi: 10.4085/1062-6050-43.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalsa PS, Hoffman AH, Grigg P. Mechanical states encoded by stretch-sensitive neurons in feline joint capsule. J Neurophysiol. 1996;76:175–187. doi: 10.1152/jn.1996.76.1.175. [DOI] [PubMed] [Google Scholar]
- 47.Raunest J, Sager M, Burgener E. Proprioceptive mechanisms in the cruciate ligaments: an electromyographic study on reflex activity in the thigh muscles. J Trauma. 1996;41:488–493. doi: 10.1097/00005373-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Grigg P, Hoffman AH. Properties of Ruffini afferents revealed by stress analysis of isolated sections of cat knee capsule. J Neurophysiol. 1982;47:41–54. doi: 10.1152/jn.1982.47.1.41. [DOI] [PubMed] [Google Scholar]
- 49.Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Bojsen-Moller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10:58–67. doi: 10.1034/j.1600-0838.2000.010002058.x. [DOI] [PubMed] [Google Scholar]
- 51.Hinder MR, Milner TE. Rapid adaptation to scaled changes of the mechanical environment. J Neurophysiol. 2007;98:3072–3080. doi: 10.1152/jn.00269.2007. [DOI] [PubMed] [Google Scholar]
- 52.Hultborn H. State-dependent modulation of sensory feedback. J Physiol. 2001;533:5–13. doi: 10.1111/j.1469-7793.2001.0005b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worrell TW, Karst G, Adamczyk D, Moore R, Stanley C, Steimel B, et al. Influence of joint position on electromyographic and torque generation during maximal voluntary isometric contractions of the hamstrings and gluteus maximus muscles. J Orthop Sports Phys Ther. 2001;31:730–740. doi: 10.2519/jospt.2001.31.12.730. [DOI] [PubMed] [Google Scholar]
- 54.Carter RR, Crago PE, Keith MW. Stiffness regulation by reflex action in the normal human hand. J Neurophysiol. 1990;64:105–118. doi: 10.1152/jn.1990.64.1.105. [DOI] [PubMed] [Google Scholar]
- 55.Sjolander P, Johansson H, Djupsjobacka M. Spinal and supraspinal effects of activity in ligament afferents. J Electromyogr Kinesiol. 2002;12:167–176. doi: 10.1016/s1050-6411(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 56.Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34:490–498. doi: 10.1177/0363546505282619. [DOI] [PubMed] [Google Scholar]
- 57.Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Rel Res. 2002:76–94. doi: 10.1097/00003086-200209000-00008. [DOI] [PubMed] [Google Scholar]