Abstract
Background
Gender differences in passive frontal plane knee stiffness may contribute to the increased anterior cruciate ligament injury rate in females. Gender-based stiffness differences have been attributed to anthropometric variations, but little data exist describing this relationship. Furthermore, sex hormone levels appear to influence joint stiffness, but the differential effects of instantaneous and prior hormonal concentrations remain unknown. This study sought to explore the effect of gender, prior hormonal status, and anthropometry on passive frontal plane knee joint stiffness.
Methods
Twelve males and 31 females participated. Females were grouped by hormonal contraceptive use (non users [n=11], monophasic contraceptive users [n=11], and triphasic contraceptive users [n=9]) and tested at the same point in the menstrual cycle. Subjects’ right knee was passively stretched ±7° in the frontal plane at 3°/s. Stiffness was estimated at three loading levels and normalized by body size to minimize anthropometric biases. A 4 (group) × 3 (load) repeated measures analysis of variance was performed for both raw and normalized stiffness. Linear regression analyses were preformed between stiffness estimates and knee diameter and quadriceps femoris angle.
Findings
Males displayed significantly greater (P<0.05) frontal plane stiffness than females. When normalized, males displayed significantly greater stiffness in valgus (P<0.05), but not varus (P>0.05) than females. No significant effect (P>0.05) of prior hormonal state was found; however, when normalized, varus stiffness was significantly less for triphasic contraceptive users than the other female groups (P<0.05). Quadriceps femoris angle was negatively correlated and knee diameter was positively correlated to knee stiffness.
Interpretation
Consistent with earlier in vitro findings, our data may indicate that ligament material properties are gender specific. A deficit in passive knee joint stiffness may place a larger burden on the neuromuscular system to resist frontal plane loading in females.
Key Terms: gender, hormones, contraceptives, knee stiffness, frontal plane
INTRODUCTION
Knee joint stability has classically been attributed to five major factors: bone/cartilaginous contact forces, ligament and capsule stiffness, intrinsic stiffness of active muscles, and reflexively mediated muscle stiffness (Dhaher et al., 2005). The ligament and capsular components potentially protect the joint in constrained degrees of freedom, such as varus/valgus loading. There is evidence to suggest that the role of these passive constraints is gender specific, as it has been demonstrated that females display increased laxity and decreased joint stiffness in many degrees of freedom at the knee compared to males (Bryant and Cooke, 1988, Hsu et al., 2006, Wojtys et al., 2003). Decreased passive stiffness may place the joint at a higher risk of injury and could be a factor in the observed gender difference in non-contact anterior cruciate ligament (ACL) injuries (Agel et al., 2005, Uhorchak et al., 2003). Gender differences in passive joint stiffness may in part be explained by variation in anthropometric and anatomical factors between genders, such as height, body mass, and joint alignment (Granata et al., 2002, Hsu et al., 2006). However, as of yet, few studies have sought to quantify the association between these anthropometric/anatomical factors and estimates of the joint passive stiffness, specifically in the frontal plane.
In addition to the potential effects of anthropometric factors, gender specificity in the contribution of passive tissue constraints may also be attributed to differences in sex hormone levels. Receptors specific to estrogen, progesterone, and testosterone have been isolated on the human ACL (Hamlet et al., 1997, Liu et al., 1996) and in vitro experiments have demonstrated a dependence of the cellular metabolism of collagen components on sex hormones (negatively associated with estrogen, positively associated with testosterone) (Abubaker et al., 1996, Yu et al., 2001). It has been proposed that these hormone dependent alterations in metabolism could potentially lead to alterations in the mechanical properties of the ligaments, although the exact translation from metabolic changes to mechanical changes remains unknown (Slauterbeck et al., 1999, Strickland et al., 2003). Nevertheless, evidence from in vivo experiments in females indicates that anterior-posterior (A-P) laxity varies significantly throughout the course of the menstrual cycle and seems to be mediated by fluctuations in sex hormone levels (Zazulak et al., 2006). Furthermore, the effects of sex hormone levels on joint laxity appear to be time delayed (3–5 days), indicating that there is a dynamic, history-dependent relationship between hormonal levels and overall joint stiffness (Shultz et al., 2004).
Potentially, the use of hormonal contraceptives (HCs), which decrease periodic fluctuations in hormone concentrations, could attenuate menstrual cycle-induced ligamentous laxity variations in females. To our knowledge, there are only two studies that have examined the effect of HC use on A-P knee joint laxity. Pokorny et al. (2000) found no significant differences between the two groups, but with low post-hoc statistical power. On the other hand, Martineau et al. (2004) found that HC users exhibit significantly decreased knee joint laxity compared to non-users. However, results from these studies may be confounded by the experimenters’ lack of control for menstrual cycle phase at the time of testing. As such, the difference observed by Martineau et al. (2004) could be the result of instantaneous hormone concentrations and/or an accumulative effect of prior HC usage and hormonal fluctuations (Strauss et al., 2004). The differential contributions of instantaneous and prior hormonal concentrations to joint stiffness remain unknown.
While the effects of gender and hormonal environment on A-P knee laxity have been investigated, there has been less attention paid to the contributions of the joint periarticular tissues in promoting knee stability in the frontal plane. It has been observed that ACL injuries can result from large valgus loading and internal/external rotation often encountered during rapid deceleration and cutting/jump-landing maneuvers in sports (Bahr and Krosshaug, 2005). Furthermore, a recent prospective study implicated abnormal abduction loading at the knee during jump-landing as the primary indicator of ACL injury risk in female athletes (Hewett et al., 2005). Accordingly, the goal of the current study was to examine potential contributors to frontal plane knee joint stiffness differences across genders. We estimated joint stiffness in males and females by passively adducting/abducting the lower limb via a servomotor system under no load bearing conditions at the neutral flexion/extension (0° knee flexion) posture. Consistent with earlier studies (Bryant and Cooke, 1988), we hypothesized that males exhibit increased joint stiffness as compared to females. Furthermore, we explored the association between joint stiffness estimates and anthropometric/anatomic parameters that systematically vary between genders. Namely, stiffness estimates were normalized by the product of body mass and height and compared among subject groups to examine the contribution of body size to joint stiffness. Also, a linear regression analysis was performed to determine the relationship between frontal plane knee stiffness and quadriceps femoris angle (Q angle) and knee diameter. We hypothesized that, while these factors are important in the determination of knee joint stiffness, they can not entirely explain stiffness variations between genders. Finally, a direct comparison of knee joint stiffness estimates from HC users and non-users at a fixed point in the menstrual cycle was conducted, allowing an isolation of the effect of the prior menstrual cycle hormonal profiles on current knee joint stiffness.
MATERIALS AND METHODS
Subjects
All experimental procedures were approved by the Institutional Review Board of Northwestern University and complied with the principles of the Declaration of Helsinki. Twelve male subjects and thirty-one female subjects participated in the experiment after giving informed consent. Subjects were excluded if they had a history of injuries to the knee or lower extremity. Lachman’s test was performed by a physical therapist as a simple screen for potential ACL and other ligament injuries, which was complemented by a questionnaire regarding subjects’ knee injury history. The rationale for performing Lachman’s test was to directly evaluate asymmetry between the left and right knees, which could be indicative of a ligament injury. A test that resulted in a soft end feel and dissimilar results at the right and left knee was positive and excluded subjects from participating in the study. All subjects reported a moderate level of physical activity, which mainly consisted of running/jogging or recreational sports.
Prior to testing, subjects were evaluated by a physical therapist to measure anthropometric factors and joint alignment. In addition to height and body mass, knee diameter was also measured across the femoral epicondyles with the leg extended using calipers. Body mass index (BMI) was calculated as the ratio of mass in kg to the square of height in m. Frontal plane joint alignment was assessed by determining the Q angle, defined as the acute angle between the line connecting the tibial tubercle and the middle of the patella, and the line extending from the middle of the patella to the anterior superior iliac spine (ASIS). For measurement of the Q angle, subjects were supine with the knee extended and leg muscles relaxed. Using a previously described procedure by Piva et al. (2006), a trained physical therapist palpated the tibial tubercle, center of the patella, and ASIS and marked their locations using washable ink. A universal goniometer was used to measure the acute angle formed by the lines connecting these bony landmarks. Table 1 presents a summary of subject demographics.
Table 1.
Subject demographics. Reported as mean (SD).
Subject demographics for males (M), female non HC users (F1), female monophasic HC users (F2), and female triphasic HC users (F3). Reported as mean (SD)
| M | F1 | F2 | F3 | |
|---|---|---|---|---|
| Age (yrs) | 25.8 (3.6) | 26.3 (3.8) | 24.4 (2.4) | 25.0 (2.4) |
| Weight (kg) | 79.0 (5.7) | 60.8 (6.1)* | 59.2 (4.3)* | 60.8 (6.6)* |
| Height (m) | 1.8 (0.07) | 1.7 (0.07)* | 1.6 (0.05)* | 1.7 (0.08)* |
| BMI (kg/m2) | 24.5 (1.9) | 22.3 (1.7)* | 22.2 (1.5)* | 22.4 (3.3) |
| Q Angle (°) | 7.5 (3.7) | 10.6 (3.0)# | 11.5 (4.8)# | 13.1 (3.3)# |
| Knee Diameter (cm) | 10.8 (1.2) | 9.7 (0.6)* | 9.6 (0.3)* | 9.4 (0.5)* |
Males significantly greater than females (P<0.05)
Females significantly greater than males (P<0.05)
Female subjects were divided into three groups based on their use of HCs: non-users (n = 11, group F1), monophasic HC users (n = 11, F2), and triphasic HC users (n = 9, F3). Classification of HCs as monophasic or triphasic was based on the dosing of exogenous hormones over the course of one cycle. Monophasic HCs deliver a constant level of exogenous estrogen and progesterone in each dose, whereas triphasic HCs supply three doses of hormones in three different phases of one cycle (Strauss et al., 2004) (Figure 1). HC users were included in the study if they had been using contraceptives for at least three months (range: F2: 5–95 months, F3: 17–120 months). To explore the effects of short term hormonal history, female non-users were tested during the early follicular phase and HC users tested during their “off-week” of contraceptive use (Figure 1), which is similar to the early follicular phase in non-users (Schlaff et al., 2004). Testing of female non-users was scheduled for within 3 days (mean 2.1 (SD 1) days) of the subjects’ self-reported start of menstrual bleeding when estrogen and progesterone levels are lowest (Strauss et al., 2004).
Figure 1.
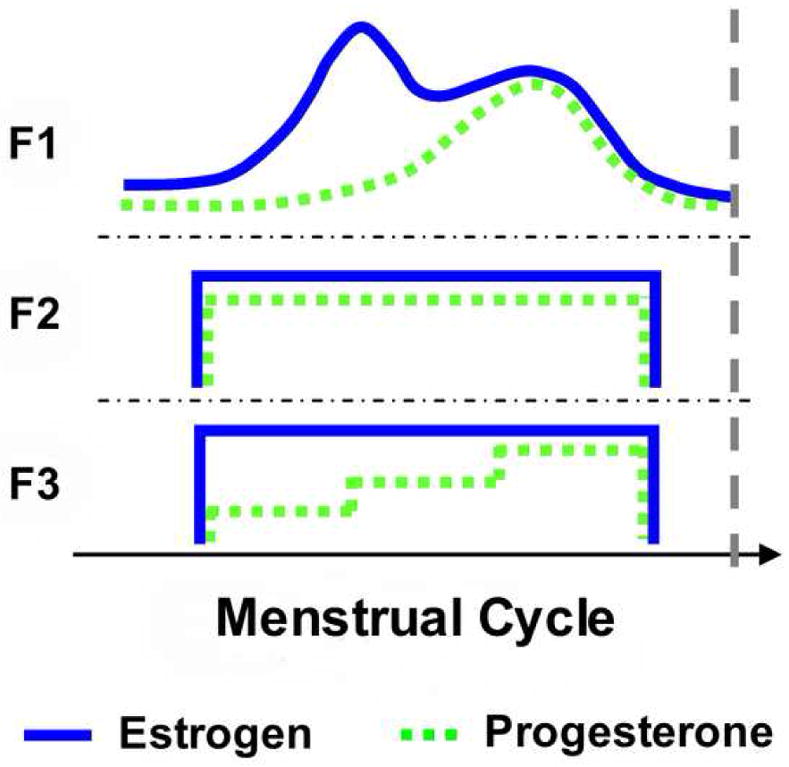
Schematic of estrogen and progesterone profiles over the course of the menstrual cycle in the three female testing groups: non HC users (F1), monophasic HC users (F2), and triphasic HC users (F3). Profiles for groups F2 and F3 represent synthetic hormone concentrations. The dashed vertical line represents the time point at which all females were tested.
Experimental Protocol
Following the initial physical evaluation, subjects were seated in an experimental chair with the right knee at neutral flexion/extension (0° knee flexion) (Figure 2). The zero flexion angle was measured using a universal goniometer with the fulcrum at the lateral epicondyle and the goniometer arms aligned with the greater trochanter and the apex of the lateral malleolus. A neutral flexion/extension posture corresponded to a zero goniometer reading (Dhaher and Francis, 2006). The subject’s right ankle was placed in a cast and then secured within a coupling ring, which fixed the limb to a servomotor actuator, via a rigid cantilever beam. The beam was visually aligned with the subject’s lower leg and the subject was allowed to assume their natural frontal plane knee joint alignment. The servomotor was equipped with a precision potentiometer and tachometer, as well as a six degrees-of-freedom load cell (JR3, Inc. Woodland, CA, USA) to record the position, force, and torque signals during each experiment. Brackets were securely fastened around the knee joint at the femoral epicondyles to prevent medial/lateral translation of the knee during testing and a strap was placed over the right thigh to prevent movement of the proximal limb (Dhaher et al., 2005, Dhaher et al., 2003).
Figure 2.
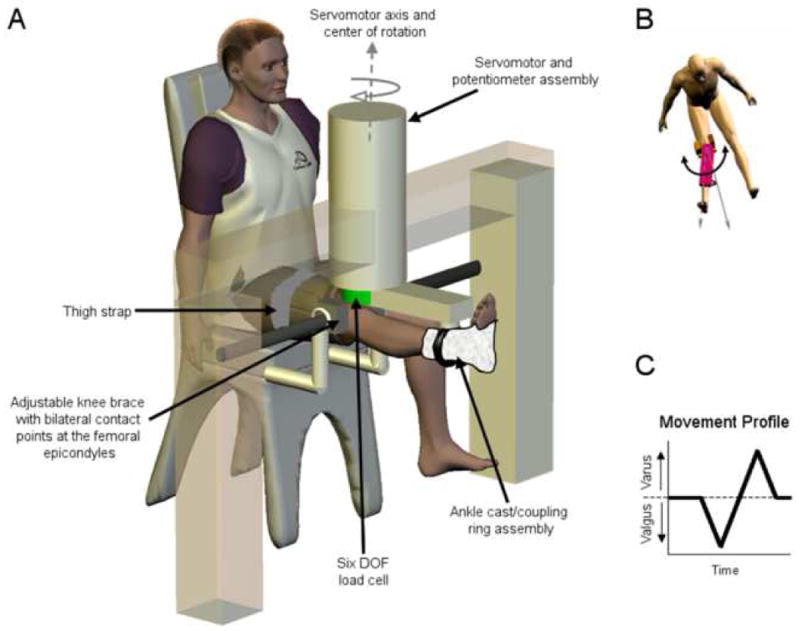
Experimental set-up. A) Subjects were seated in a biodex chair with the right leg extended. The apex of the patella was aligned with the center of rotation of the servomotor. The right leg was fixed within a coupling ring with a cast placed around the ankle joint. The coupling ring was fixed to a servomotor actuator with a precision potentiometer and tachometer. The knee was fitted within a bracket mounted firmly at the medial and lateral femoral epicondyles. Together with a thigh strap, the brackets isolated the knee adduction–abduction movement from the abduction–adduction movement of the hip joint. B) The servomotor acted to move the lower limb into varus and valgus. C) The movement profile used in the experiment.
The loading protocol consisted of applying a quasi-static frontal plane stretch to the knee joint at a constant velocity (3°/s). Starting from the neutral position the servomotor rotated the subjects’ knee to 7° of valgus, then to 7° of varus, and then back to the neutral position (see movement profile, Figure 2c). The 7° amplitude varus/valgus stretch was chosen because it is consistent with the apparent varus/valgus rotations reported by Byrant and Cooke (Bryant and Cooke, 1988) using similar experimental protocols and it is also within the range of motion reported by LaFortune et al (Lafortune et al., 1992) during level walking. To ensure that subjects were comfortable with this movement, several smaller stretches were applied at the beginning of the experimental session. Starting at 4°, the stretches were incrementally increased by 1° to the desired 7° stretch and subjects were asked to report any discomfort during these initial stretches. All study participants were comfortable with the desired 7° amplitude varus/valgus movement. Preliminary data showed very low intra-subject variability of the torque-angle relationship; therefore, the 7° loading protocol was performed only once or twice on a majority of subjects.
Subjects were instructed to remain relaxed during the loading protocol. Surface EMG electrodes recorded muscle activity in the semitendinosus, biceps femoris, rectus femoris, vastus medialis, and vastus lateralis prior to and during the mechanical perturbation to ensure that subjects maintained a relaxed state. Baseline EMG was collected 100 ms prior to the onset of the angular perturbation. Unacceptable EMG activity was identified when the mean EMG activity was greater than two standard deviations above the mean baseline activity. Trials that showed unacceptable EMG activity during the loading protocol were rejected.
Data analysis
To eliminate high-frequency noise associated with the servomotor system, the EMG and load cell signals were online filtered using an eighth order, zero-phase Butterworth filter at a 220 Hz cut-off frequency and then sampled at 1 kHz. Prior to data analysis, position and load cell signals were filtered off-line using a first order Butterworth filter with a 4 Hz cutoff. When multiple trials were performed on the same subject, the mean frontal plane load and position signals were used in the analysis. Only the loading phases of the hysteresis loop were considered for analysis. Previous in vitro work has attributed liagmentous loading to the non-linear portion of the torque- angle relationship. Thus, stiffness was estimated at several locations along the non-linear region as the slope of the line tangent to the torque-angle relationship (Dhaher et al., 2005). Specifically, stiffness was calculated at 50%, 70%, and 90% of the maximum torque produced in varus and valgus by each subject.
Statistical analysis was performed using the NCSS software suite (NCSS, Kaysville, UT, USA). A repeated measures analysis of variance (ANOVA) was performed with one between (group [M, F1, F2, F3]) factor and one within (load [50%, 70%, and 90% of maximum torque]) factor to determine the effect of gender and HC use on varus and valgus stiffness. Post-hoc Tukey-Kramer multiple comparisons and confidence intervals were used to assess stiffness differences between all pairs. This statistical model allowed comparisons among all testing groups, while maintaining a low family-wise Type I error rate, which was set a priori to P = 0.05.
To address the contribution of anthropometric factors, joint stiffness estimates were standardized across subjects. By experimental design, the amplitude of stretch was standard across all subjects (7° varus/valgus). To standardize the resulting frontal plane torque (N-m), stiffness estimates were normalized by the product of mass and height (N-m) for each subject. This standardized quantity was then compared across testing groups using the same repeated measures ANOVA. This normalization procedure allowed for examination of the effect of gender and HC usage on joint stiffness while minimizing anthropometric biases.
During rotation of the knee joint, the resulting stiffness is also a function of the effective moment arm of the structures resisting rotation (Hsu et al., 2006). In the frontal plane, joint alignment and knee diameter are two external parameters that could reflect the moment arm of resisting structures. Therefore, a linear regression analysis was performed between stiffness estimates and Q angle and knee diameter to assess the association between joint geometry and stiffness.
RESULTS
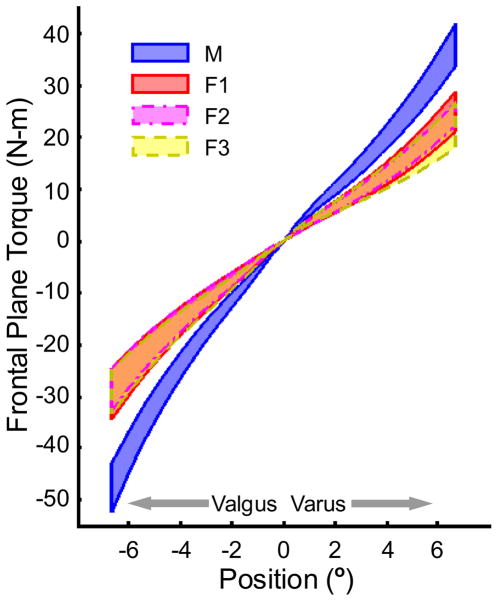
In response to mechanical loading, subjects displayed a non-linear torque-angle relationship. Figure 3 displays 95% confidence intervals of the mean of the hysteresis loop for each subject group. Frontal plane stiffness was estimated at 50%, 70%, and 90% of the maximum torque recorded in both varus and valgus for each subject and stiffness was found to increase with increasing external load (Figure 4). Statistical analysis indicated significant main effects of group (P < 0.001) and load level (P <0.001) for both valgus and varus stiffness estimates. Tukey-Kramer multiple comparisons revealed that males had greater stiffness than all female groups but no differences existed between the female subgroups for both varus and valgus stiffness at all loading conditions.
Figure 3.
95% confidence intervals of frontal plane torque versus angular displacement by testing group. M: males, F1: female non HC users, F2: female monophasic HC users, F3: female triphasic HC users.
Figure 4.
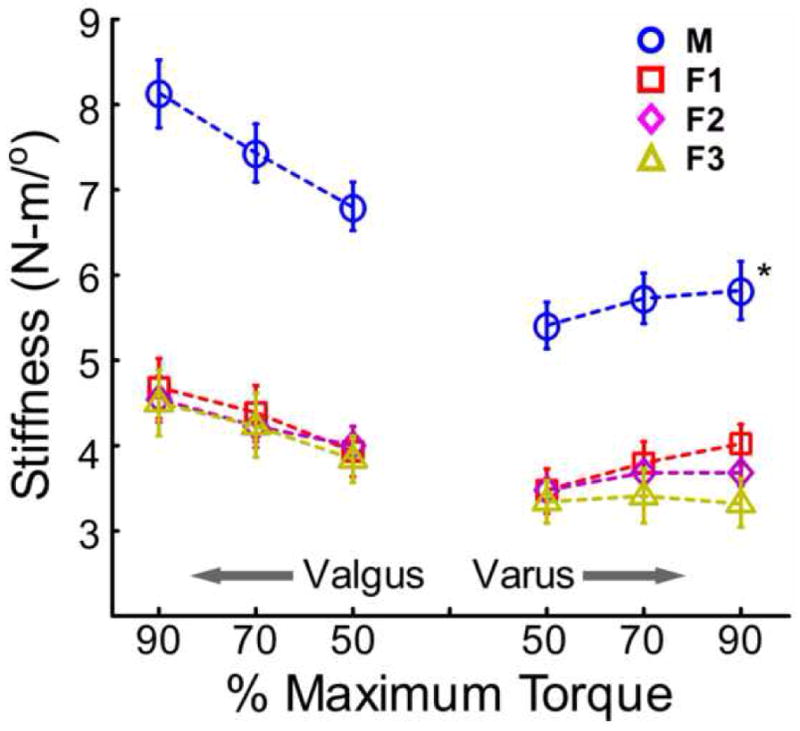
Frontal plane stiffness determined at 50%, 70%, and 90% of maximum varus/valgus torque. Error bars represent standard error. M: males, F1: female non HC users, F2: female monophasic HC users, F3: female triphasic HC users. *Males displayed significantly greater varus and valgus stiffness than all female groups (P<0.05).
To determine if these gender differences could be explained by anthropometric variations between the genders, stiffness was normalized by the product of body mass and height (Figure 5) and the same repeated measures ANOVA was performed to determine the differences between groups. For normalized valgus stiffness, significant effects were found for both group (P = 0.01) and loading level (P <0.001). Tukey-Kramer multiple comparisons and confidence intervals (CIs) revealed that males had significantly greater stiffness than all female groups (Tukey-Kramer CIs for the difference between group M and F1 (M-F1): [6.2 × 10−3, 1.3 × 10−2], [6.3 × 10−3, 1.3 × 10−2], [8.3 × 10−3, 1.5 × 10−2]; M-F2: [3.7 × 10−3, 1.1 × 10−2], [5.9 × 10−3, 1.3 × 10−2], [7.5 × 10−3, 1.4 × 10−2]; M-F3: [7.2 × 10−3, 1.5 × 10−2], [8.0 × 10−3, 1.5 × 10−2], [1.1 × 10−2, 1.8 × 10−2] at 50%, 70%, and 90% of maximum valgus torque, respectively). There was a significant difference for normalized stiffness at 50% maximum valgus torque between groups F2 and F3 (Tukey-Kramer CIs for F2-F3: [1.08 × 10−5, 7.6 × 10−3]), but none of the other comparisons between female groups were significant.
Figure 5.
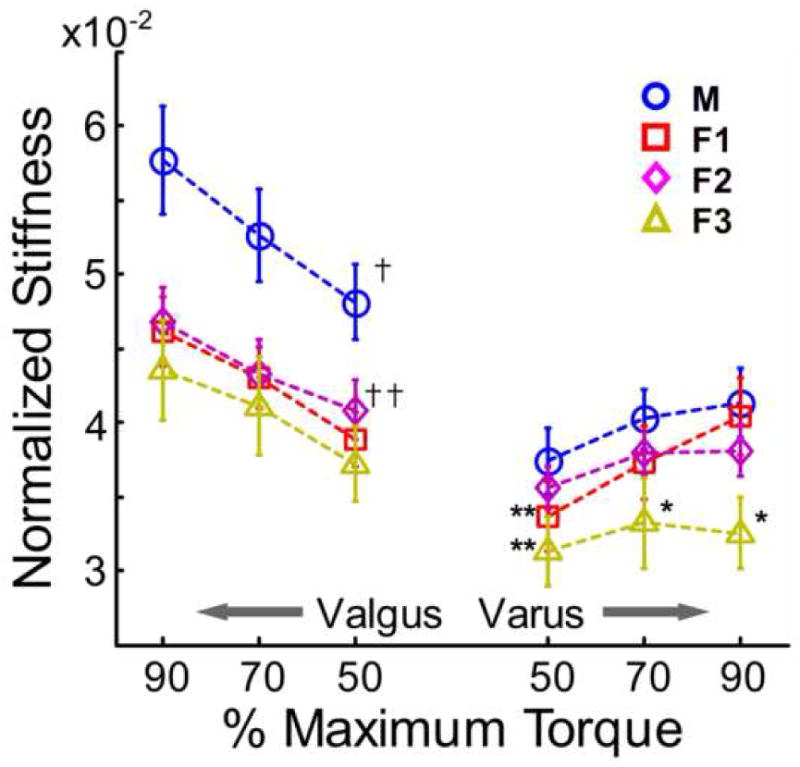
Normalized frontal plane stiffness at various percentages of maximum varus/valgus torque. †Males displayed significantly greater normalized valgus stiffness than all female groups. ††Group F3 displayed significant less valgus stiffness than group F2 at 50% maximum torque. **Males displayed significantly greater normalized stiffness at 50% maximum varus torque than groups F1 and F3. *Group F3 displayed significantly less normalized varus stiffness at 70% and 90% maximum torque than all other testing groups. (P<0.05)
For normalized varus stiffness, a significant effect of loading level (P < 0.001) and load by group interaction (P = 0.04) was found, but no significant main effect of group (P = 0.16). However, post-hoc multiple comparisons revealed that group F3 displayed significantly decreased stiffness than males at all loading levels (Tukey-Kramer CIs for M-F3: [1.9 × 10−3, 9.6 × 10−3], [3.4 × 10−3, 1.1 × 10−2], [5.0 × 10−3, 1.3 × 10−2] at 50%, 70%, and 90% of maximum varus torque, respectively). Group F1 also displayed significantly decreased stiffness than males at 50% maximum varus torque (Tukey-Kramer CIs for M-F1: [1.8 × 10−4, 7.2 × 10−3]). Further, there were differences between female subgroups: groups F1 and F2 had greater stiffness than group F3 at both 70% and 90% of maximum varus torque (Tukey-Kramer CIs for F1-F3: [1.4 × 10−3, 9.3 × 10−3], [4.1 × 10−3, 1.9 × 10−2]; F2-F3: [1.2 × 10−3, 9.1 × 10−3], [2.0 × 10−3, 9.8 × 10−3] at 70% and 90% of maximum varus torque, respectively) (See Figure 5).
Linear regression was performed to determine the relationship between frontal plane stiffness estimates and anatomical factors, knee diameter and Q angle. Knee diameter was found to be significantly correlated to all stiffness estimates (P < 0.05). Pearson correlation coefficients were 0.60, 0.53, and 0.54 for stiffness at 50%, 70%, and 90% of maximum valgus torque. For varus stiffness, Pearson correlation coefficients were 0.38, 0.37, and 0.35 for stiffness at 50%, 70%, and 90% of maximum torque. Q angle was also found to be significantly correlated to stiffness, with Pearson correlation coefficients of −0.49, −0.51, −0.49 for valgus stiffness and −0.39, −0.39, and −0.38 for varus stiffness at 50%, 70%, and 90% of maximum torque, respectively.
DISCUSSION
Consistent with previous studies (Bryant and Cooke, 1988), we found that males exhibit significantly greater frontal plane knee joint stiffness than females. Our data indicated that this gender difference was persistent even after adjustment for anthropometric factors, by normalizing stiffness by the product of body mass and height. To assess the contribution of a short term history of hormonal modification, we compared frontal plane knee joint stiffness between female users and non-users of HCs and found no differences in the raw stiffness estimates. However, when stiffness was normalized by body size, varus stiffness estimates differed between the female groups. Further, frontal plane stiffness estimates were found to be correlated to knee diameter and Q angle, measures of joint geometry. The results from this study elucidate the contributions of both intrinsic (gender, anthropometric) and extrinsic (hormonal contraceptive use) factors to frontal plane knee joint stiffness. Furthermore, the persistent gender bias in passive valgus stiffness suggests that females need to rely more on active stabilizers to maintain joint stability during activities that place medial-lateral loads on the knee.
Results from this study indicate that valgus stiffness estimates were, on average, roughly 20% higher than varus stiffness for all subjects, which is consistent with previous reports (Bryant and Cooke, 1988). These differences between varus and valgus stiffness may be due to a potential kinematic asymmetry in the joint response to frontal plane loading between the two directions. While it has been noted that the application of a valgus load at the unflexed knee seems to result in isolated valgus movement of the joint, the kinematics resulting from a varus load have been qualitatively described as more complex and coupled with movement in other degrees of freedom, such as knee flexion and/or internal/external rotation (Bryant and Cooke, 1988, Yu et al., 1997). Unfortunately, these investigations did not provide quantitative three dimensional kinematics data to substantiate these descriptions. Anecdotally, however, in the current study, it appeared that the joint response to the varus loading protocol was variable and often coupled with knee flexion or internal rotation, especially near terminal loading. On the other hand, the joint response to valgus loading appeared to be more consistent and consisted of motion only in the frontal plane. Potentially, this kinematic asymmetry may be the result of side-to-side differences in the contributions of resisting soft tissues. In a cadaver model, sectioning of the medial collateral ligament alone significantly reduced valgus stiffness, whereas a measurable reduction in varus stiffness was only achieved with sectioning of the lateral collateral ligament in conjunction with the ACL and/or posterior capsule (Markolf et al., 1976). The significant involvement of multiple soft tissue structures to resist varus loading may induce movement in other degrees of freedom. Further investigations are needed to document the differential varus and valgus 3-D kinematics and soft tissue stresses in response to frontal plane loading at the unflexed knee.
In this study passive frontal plane stiffness estimates were obtained with the knee at a posture of 0° flexion angle. In this position, while the joint’s soft tissues provide the primary resistance to frontal plane loading, there are also contributions from bony/meniscus congruity. Clinical varus/valgus stress tests are performed with the knee at 20° of flexion in order to solely evaluate the collateral ligaments. Further, during cutting/jump-landing maneuvers in sports knee varus/valgus loading often occurs with the knee in a flexed position (Bahr and Krosshaug, 2005, Hewett et al., 2005). However, frontal plane loading is extremely difficult to isolate and control when the knee is flexed (Dhaher and Francis, 2006). We used the zero knee flexion posture as an experimental model to evaluate differences between testing groups while isolating knee varus/valgus movement from movement at other joints (i.e. hip internal/external rotation).
It has been suggested that the gender difference in knee stiffness may in part be a consequence of anthropometric factors (Granata et al., 2002, Hsu et al., 2006). Indeed, the result of normalizing the stiffness estimates by body size was to decrease the gap between males and females. When averaged across all loading levels and female subgroups valgus stiffness in male subjects was 43% (SD 1.2%) greater than valgus stiffness in females. When stiffness estimates were normalized, however, this average difference dropped to 20% (SD 2.9%). Likewise, when varus stiffness was normalized, female stiffness estimates dropped from 37% (SD 3.5%) to 10% (SD 6.6%) less than male stiffness. However, while these anthropometric factors appear to contribute to passive frontal plane joint stiffness, they did not eliminate gender differences. Males still demonstrated significantly greater normalized valgus stiffness than all female groups. On the other hand, few significant differences were found between males and groups F1 and F2 for normalized varus stiffness. While one could speculate that gender differentially affects varus and valgus stiffness, it is possible that varus stiffness estimates were confounded by kinematic and soft tissue loading variability, as described above, which might have masked any lingering gender differences in varus stiffness.
Linear regression analysis revealed that knee diameter and Q angle were significantly correlated to both varus and valgus stiffness estimates. Both of these parameters are measures of joint geometry and indirectly represent the effective moment arms of resisting structures (Hsu et al., 2006). However, while statistically significant, Pearson correlation coefficients and corresponding R2 values were of relatively small magnitude and the meaning of the correlations should be interpreted cautiously. The poor correlation between Q angle and knee stiffness may be related to the less than perfect reliability of the method used for determining Q angle in this study (Piva et al., 2006). Though the procedure used in this study is simple to implement, assessing frontal plane joint alignment via radiographic techniques may lead to more accurate and reliable measurements (Rauh et al., 2007). As a posture of dynamic knee valgus could increase the risk of ACL injury in females (Chaudhari and Andriacchi, 2006, Hewett et al., 2005), we believe that further investigation of the relationship between Q angle and frontal plane joint stiffness on a larger sample size and utilizing more reliable measures of joint alignment is warranted. A large Q angle coupled with decreased passive valgus joint stiffness, as suggested by the preliminary results present here, may place an even larger burden on the neuromuscular system to maintain joint stability.
In this study, we sought to explore potential anthropometric/anatomical parameters which might contribute to the observed gender difference in passive knee stiffness. While our results indicate that body size, Q angle, and knee diameter are associated to frontal plane knee stiffness, gender remained a significant determinant. This finding is consistent with other in vivo and in vitro literature examining gender differences in joint properties. Wojtys et al. (2003) demonstrated a gender bias in passive torsional knee stiffness when comparing sized- matched males and females during in vivo testing. During in vitro mechanical testing, Chandrashekar et al. (2006) found persistent sex differences in human ACL tensile properties even when anthropometric covariates were taken into account. Further, in a sheep model, ultimate stress, a material property, was found to be greater in ram ligaments than in ewe ligament (Strickland et al., 2003). These results suggest that soft tissue material properties, in addition to structural properties, are gender specific.
Gender specific soft tissue material properties may be due to sex hormone mediated differences in the content and characteristics of the collagen within these tissues. In a rat model, collagen content, the main determinant of ligament strength, within the temporomandibular joint disc was found to be greater in males than females (Abubaker et al., 1996). This gender specificity was attributed to differences in the concentrations of sex hormones, as collagen content increased following ovariectomy and treatment with exogenous testosterone, but decreased following orchiectomy in male rats and treatment with exogenous estrogen (Abubaker et al., 1996). Given that frontal plane knee joint stiffness is mainly a reflection of the mechanical properties of ligament and capsule tissue (Markolf et al., 1976), hormone-mediated gender differences in collagen content could potentially alter overall knee joint stiffness. However, the precise translation of these biochemical properties to the biomechanical properties of knee joint soft tissues remains unknown (Slauterbeck et al., 1999, Strickland et al., 2003). Recent investigations, though, have correlated variations in estrogen, progesterone, and testosterone levels with menstrual cycle-dependent variations in A-P knee joint laxity obtained in vivo in females (Shultz et al., 2006), which suggests that sex hormone-mediated collagen content variations could lead to variations overall joint stiffness, although the exact nature of this association is not fully understood.
We sought to investigate the effect of the short term history of hormonal modification, via HC usage in the previous menstrual cycle, on frontal plane knee joint stiffness at the onset of the ensuing menstrual cycle. The rationale for this investigation was driven from earlier examinations of the dynamic relationship between joint laxity and hormonal concentrations, which suggest that knee laxity is a function of both instantaneous and prior serum hormone concentrations (Shultz et al., 2004). As such, hormonal and menstrual cycle status prior to testing has been used as inclusion/exclusion criteria in studies examining various biomechanical parameters throughout the menstrual cycle (Chaudhari et al., 2007). However, little data existed to describe the dependence of knee joint properties on previous menstrual cycle hormonal profiles. When considering only valgus stiffness, which was more kinematically consistent than varus stiffness, our data suggest that differences in hormonal environment from the previous menstrual cycle do not lead to differences in joint stiffness at the start of the next menstrual cycle.
In this study, all female subjects were tested at beginning of the menstrual cycle, defined as the onset of menses for group F1 and during the “off-week” of HC use for groups F2 and F3. We assumed that hormone levels were similar across all female subjects at the time of testing (Schlaff et al., 2004), but that prior hormonal history from the previous cycle varied among the three groups. While serum hormone concentrations were not obtained to verify this claim, the use of HC likely attenuated the hormonal fluctuations that are associated with the menstrual cycle, leading to differences between HC users and non-users (Strauss et al., 2004). Further, given the differences in hormonal dosing between monophasic and triphasic contraceptives, there were likely variations in average serum hormone concentrations between groups F2 and F3. It appears that these differences in hormonal history may have had an impact on varus stiffness, as our statistical analysis revealed that normalized varus stiffness in triphasic HC users (group F3) was significantly less than in the other two female groups. Nonetheless, the effect of prior hormonal state may be difficult to delineate for varus stiffness given the confounding factors mentioned previously. However, normalized valgus stiffness was also less in group F3 than the other female groups. While this difference was only statistically significant for the comparison between F3 and F2 at 50% maximum valgus torque, group F3 demonstrated less normalized valgus stiffness than groups F1 and F2 by 5% and 7%, respectively, averaged across all loading conditions. Taken together, these preliminary results may suggest that prior use of triphasic HCs leads to a decrease in frontal plane knee joint stiffness at the onset of the ensuing menstrual cycle. Nevertheless, due to the relatively small sample size used in this study and lack of quantitative measurement of prior serum hormone levels, additional investigation is necessary before a decisive statement regarding the effects of triphasic HC use on frontal plane stiffness can be made.
It remains to be seen if frontal plane knee joint stiffness varies between HC users and non-users at other points throughout the menstrual cycle due to the instantaneous and time-delayed effects of hormonal environment. Hence, future studies should further quantify the differential effects of hormonal contraceptive use on frontal plane knee joint stiffness by tracking knee joint stiffness and serum hormone levels throughout the menstrual cycle. To fully characterize the potential time-dependent properties of this relationship, daily measurements of stiffness and hormonal environment may be necessary (Zazulak et al., 2006). As the use of hormonal contraceptives has been suggested to reduce menstrual cycle-induced knee laxity variations and potentially reduce the risk of ACL injury in female athletes (Martineau et al., 2004), elucidation of this relationship may help to further our understanding of joint stability and injury risk factors.
CONCLUSIONS
As knee joint stiffness is a structural property, it is important to consider the contributions of variations in joint structure and anthropometry when evaluating stiffness in disparate groups. Indeed, it was found that Q angle and knee diameter are associated with knee joint stiffness. However, further examination of this relationship with a larger sample size and more reliable measurements is necessary to better understand the functional implications of these associations. Further, knee joint stiffness was affected by body size, as normalization by body mass and height decreased the stiffness differences between genders. Nonetheless, despite the influence of these anthropometric factors, gender remained a significant determinant of joint stiffness. This may suggest that the mechanical properties of the ligaments and soft tissues which resist frontal plane loading are influenced by gender. Gender differences in hormonal concentrations may help to explain variations in soft tissue mechanical properties (Abubaker et al., 1996). The concentration of sex hormones differs much more between the genders than between the female subgroups of this study, which could help to explain the observed gender difference in joint stiffness, but few differences between users and non-users of HCs. Future investigations should further quantify the relationship between hormonal concentrations and ligament mechanical properties in both males and females, as well as their role in determining overall knee joint stiffness.
Acknowledgments
This work was supported by grants from the National Institutes of Health: NIAMS RO1 AR049837 and NICHD T32 HD07418. The authors would like to thank Tobey DeMott, MS, PT, Jennifer Moore, MPT, and Anastasios Tsoumanis, MS, for their help with data collection and analysis.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no financial or personal relationships that may lead to a conflict of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abubaker AO, Hebda PC, Gunsolley JN. Effects of sex hormones on protein and collagen content of the temporomandibular joint disc of the rat. J Oral Maxillofac Surg. 1996;54:721–7. doi: 10.1016/s0278-2391(96)90690-4. [DOI] [PubMed] [Google Scholar]
- Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33:524–30. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- Bahr R, Krosshaug T. Understanding injury mechanisms: a key component of preventing injuries in sport. Br J Sports Med. 2005;39:324–9. doi: 10.1136/bjsm.2005.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JT, Cooke TD. Standardized biomechanical measurement for varus-valgus stiffness and rotation in normal knees. J Orthop Res. 1988;6:863–70. doi: 10.1002/jor.1100060610. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39:2943–50. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Chaudhari AM, Andriacchi TP. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J Biomech. 2006;39:330–8. doi: 10.1016/j.jbiomech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Chaudhari AM, Lindenfeld TN, Andriacchi TP, Hewett TE, Riccobene J, Myer GD, Noyes FR. Knee and hip loading patterns at different phases in the menstrual cycle: implications for the gender difference in anterior cruciate ligament injury rates. Am J Sports Med. 2007;35:793–800. doi: 10.1177/0363546506297537. [DOI] [PubMed] [Google Scholar]
- Dhaher YY, Francis MJ. Determination of the abduction-adduction axis of rotation at the human knee: helical axis representation. J Orthop Res. 2006;24:2187–200. doi: 10.1002/jor.20281. [DOI] [PubMed] [Google Scholar]
- Dhaher YY, Tsoumanis AD, Houle TT, Rymer WZ. Neuromuscular reflexes contribute to knee stiffness during valgus loading. J Neurophysiol. 2005;93:2698–709. doi: 10.1152/jn.00921.2004. [DOI] [PubMed] [Google Scholar]
- Dhaher YY, Tsoumanis AD, Rymer WZ. Reflex muscle contractions can be elicited by valgus positional perturbations of the human knee. J Biomech. 2003;36:199–209. doi: 10.1016/s0021-9290(02)00334-2. [DOI] [PubMed] [Google Scholar]
- Granata KP, Padua DA, Wilson SE. Gender differences in active musculoskeletal stiffness. Part II. Quantification of leg stiffness during functional hopping tasks. J Electromyogr Kinesiol. 2002;12:127–35. doi: 10.1016/s1050-6411(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Hamlet WP, Liu SH, Panossian V, Finerman GA. Primary immunolocalization of androgen target cells in the human anterior cruciate ligament. J Orthop Res. 1997;15:657–63. doi: 10.1002/jor.1100150505. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- Hsu WH, Fisk JA, Yamamoto Y, Debski RE, Woo SL. Differences in torsional joint stiffness of the knee between genders: a human cadaveric study. Am J Sports Med. 2006;34:765–70. doi: 10.1177/0363546505282623. [DOI] [PubMed] [Google Scholar]
- Lafortune MA, Cavanagh PR, Sommer HJ, 3rd, Kalenak A. Three-dimensional kinematics of the human knee during walking. J Biomech. 1992;25:347–57. doi: 10.1016/0021-9290(92)90254-x. [DOI] [PubMed] [Google Scholar]
- Liu SH, al-Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, Finerman GA, Lane JM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J Orthop Res. 1996;14:526–33. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–94. [PubMed] [Google Scholar]
- Martineau PA, Al-Jassir F, Lenczner E, Burman ML. Effect of the oral contraceptive pill on ligamentous laxity. Clin J Sport Med. 2004;14:281–6. doi: 10.1097/00042752-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Piva SR, Fitzgerald K, Irrgang JJ, Jones S, Hando BR, Browder DA, Childs JD. Reliability of measures of impairments associated with patellofemoral pain syndrome. BMC Musculoskelet Disord. 2006;7:33. doi: 10.1186/1471-2474-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny MJ, Smith TD, Calus SA, Dennison EA. Self-reported oral contraceptive use and peripheral joint laxity. J Orthop Sports Phys Ther. 2000;30:683–92. doi: 10.2519/jospt.2000.30.11.683. [DOI] [PubMed] [Google Scholar]
- Rauh MA, Boyle J, Mihalko WM, Phillips MJ, Bayers-Thering M, Krackow KA. Reliability of measuring long-standing lower extremity radiographs. Orthopedics. 2007;30:299–303. doi: 10.3928/01477447-20070401-14. [DOI] [PubMed] [Google Scholar]
- Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation of the pill-free interval in oral contraceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol. 2004;190:943–51. doi: 10.1016/j.ajog.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Shultz SJ, Gansneder BM, Sander TC, Kirk SE, Perrin DH. Absolute serum hormone levels predict the magnitude of change in anterior knee laxity across the menstrual cycle. J Orthop Res. 2006;24:124–31. doi: 10.1002/jor.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SJ, Kirk SE, Johnson ML, Sander TC, Perrin DH. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–74. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauterbeck J, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters the failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17:405–8. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- Strauss JF, Barbieri RL, Yen SSC. Yen and Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. Elsevier/Saunders; Philadelphia: 2004. [Google Scholar]
- Strickland SM, Belknap TW, Turner SA, Wright TM, Hannafin JA. Lack of hormonal influences on mechanical properties of sheep knee ligaments. Am J Sports Med. 2003;31:210–5. doi: 10.1177/03635465030310020901. [DOI] [PubMed] [Google Scholar]
- Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–42. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- Wojtys EM, Huston LJ, Schock HJ, Boylan JP, Ashton-Miller JA. Gender differences in muscular protection of the knee in torsion in size-matched athletes. J Bone Joint Surg Am. 2003;85-A:782–9. doi: 10.2106/00004623-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Yu B, Stuart MJ, Kienbacher T, Growney ES, An KN. Valgus-varus motion of the knee in normal level walking and stair climbing. Clin Biomech (Bristol, Avon) 1997;12:286–293. doi: 10.1016/s0268-0033(97)00005-3. [DOI] [PubMed] [Google Scholar]
- Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001:268–81. doi: 10.1097/00003086-200102000-00031. [DOI] [PubMed] [Google Scholar]
- Zazulak BT, Paterno M, Myer GD, Romani WA, Hewett TE. The effects of the menstrual cycle on anterior knee laxity: a systematic review. Sports Med. 2006;36:847–62. doi: 10.2165/00007256-200636100-00004. [DOI] [PubMed] [Google Scholar]