Abstract
Immune tolerance is instituted early in life, during which time regulatory T (Treg) cells have an important role. Recurrent infections with respiratory syncytial virus (RSV) in early life increase the risk for asthma in adult life. Repeated infection of infant mice tolerized to ovalbumin (OVA) through their mother’s milk with RSV induced allergic airway disease in response to OVA sensitization and challenge, including airway inflammation, hyper-reactivity and higher OVA-specific IgE, as compared to uninfected tolerized control mice. Virus infection induced GATA-3 expression and T helper type 2 (TH2) cytokine production in forkhead box P3 (FOXP3)+ Treg cells and compromised the suppressive function of pulmonary Treg cells in a manner that was dependent on interleukin-4 receptor α (IL-4Rα) expression in the host. Thus, by promoting a TH2-type inflammatory response in the lung, RSV induced a TH2-like effector phenotype in Treg cells and attenuated tolerance to an unrelated antigen (allergen). Our findings highlight a mechanism by which viral infection targets a host-protective mechanism in early life and increases susceptibility to allergic disease.
Mucosal tolerance is induced early in life and is an important mechanism of protection from diseases such as asthma. However, this early bias toward immune tolerance renders newborns more vulnerable to infections by pathogens such as RSV1. There is a strong association between recurrent RSV infections requiring hospitalization in early life and the development of asthma in subsequent years2–6. Independent prospective studies have documented that 40–50% of children who experience severe RSV-mediated bronchiolitis are eventually diagnosed with asthma7,8. In addition, neonatal infection of mice with RSV causes more aggressive airway inflammation when the mice were reinfected as adults than when the initial virus infection was delayed9. Despite these associations, there is a gap in our knowledge regarding the mechanisms whereby RSV infection in early life adversely affects the immune system and renders the host more susceptible to allergic asthma in adult life.
Recent investigations in humans have highlighted the presence of Treg cells as early as the embryonic stage10,11. Treg cells are important for immune tolerance, serving as a safeguard against a host of self and foreign antigens from the antenatal to the postnatal stage by suppressing unwarranted immune responses to these antigens. Studies in both humans and mice suggest that Treg cell–mediated protection from asthma is initiated at the neonatal stage. Exposure of nursing mice to the model allergen OVA induced tolerance in the newborns as a result of maternal transfer of OVA and transforming growth factor β (TGF-β) through breast milk12,13. Because RSV compromises immunoregulatory mechanisms in humans and mice, we hypothesized that repeated RSV infections result in Treg cell dysfunction, which impairs maternally transferred tolerance, thereby increasing the risk for allergic disease. Our studies show the ability of a pathogen to target a fundamental immunoregulatory mechanism in early life with an effect on subsequent disease development.
ONLINE METHODS
Mice
BALB/c, C57BL/6, FOXP3-eGFP knock-in (stock number 006769), IL-4Rα–null (stock number 003514) and CD4-TGF-β DNRII (stock number 005551) mice were purchased from The Jackson Laboratory. The DO11.10 × Rag2−/− (model number 4091) strain of mice was purchased from Taconic. DO11.10 transgenic mice, originally provided by K. Murphy at Washington University, St. Louis, were bred in the Division of Laboratory Animal Resources, University of Pittsburgh. Spleens of OT-II × FOXP3-eGFP knock-in mice were a gift from E. Shevach. Mice were housed under pathogen-free conditions and used between 3 (newborns) and 8 weeks of age. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. For experiments involving maternally transferred tolerance, both males and females were used. For all other experiments, males were mostly used.
Antibodies and flow cytometry
Antibodies to CD4 (CD4–allophycocyanin (APC), 1:500) or CD4-phycoerythrin (PE) (RM4-5, BD Biosciences, 1:500), FOXP3-FITC (FJK-16S, eBiosciences, 1:250) and IL-13–Alexa Fluor 647 (ebio13A, eBioscience, 1:200), GATA-3–PE or GATA-3–Alexa Fluor 647 (L50-823, BD Biosciences, 15 µl per reaction), CD11c-APC (HL3, BD Biosciences,1:200), CD86 (GL1, BD Biosciences, 1:200), CD40-PE (3/23, BD Biosciences, 1:200), MHC II–PE (NIMR4, Southern Biotech, 1:200), CD127-PerCP Cy5.5 (A7R34, eBioscience, 1:200), CD25-PE (PC61, BD Biosciences, 1:200), IL-4 receptor α chain–PE (mIL4R-M1, BD Biosciences, 1:200), GITR-PE (DTA-1, BD Biosciences, 1:200) were used in our flow cytometry experiments. Single-cell suspensions were stained with antibodies and examined on a FACSCalibur flow cytometer (BD Immunocytometry Systems), and the data were analyzed using FlowJo software (Tree Star).
Virus culture, titration, quantification of viral RNA and ultraviolet radiation inactivation
RSV line 19 or A2 was cultured in the HEp-2 cell line, as described previously47. The virus titration was performed as described previously48 with minor modifications. Briefly, Vero 81 cells were grown in six-well plates until they were 75% confluent. Before infection, the culture medium was removed from the wells, and the viral stock was serially diluted and added to each well in duplicate. The viral stock in PBS was allowed to adsorb to the cell monolayer for 2 h, and the plate was placed on a shaker during this time. The PBS was then aspirated off and replaced with cell culture medium, and the culture was harvested after 24 h. The harvested cells were cytospun onto glass slides and fixed with acetone. The cells were then stained with antibody to RSV-FITC (5022;Millipore). The stained slides were visualized using fluorescence microscopy, and the number of fluorescent cells was counted. The virus was routinely tested by measuring IL-6 production from infected bone marrow–derived dendritic cells by ELISA and also by assessing the ability of the virus to induce IL-13 (by qRT-PCR) and mucus (by PAS staining) production in the lungs after infection. RSV replication in the lungs was detected by qRT-PCR of the RSV large polymerase (L) gene using forward primer (5′-GAACTCAGTGTAGGTAGAATGTTTGCA-3′), reverse primer (5′-TTCAGCTATCATTTTCTCTGCCAAT-3′) and probe (5′-TTTGAACCT GTCTGAACATTCCCGGTT-3′). The level of mRNA was calculated relative to the expression of the housekeeping gene GUS, and the results were analyzed using the 2−ΔΔCt method. For virus inactivation, the virus was placed on ice 8 in below an ultraviolet lamp and exposed to the light for 30 min.
Tolerization of pups
We tolerized pups to OVA indirectly through their mother’s breast milk as previously described12. Briefly, 24 h after the birth of the pups, the mothers were exposed to 1% OVA aerosol for 20 min every other day based on the original protocol of tolerance by inhaled antigen (OVA)12,20,21,49. The mothers were exposed to aerosolized OVA ten times over the course of 20 d. During this time, the pups were nursed by either tolerized or nontolerized mothers (controls). After 20 d, the pups were weaned. For each experiment, three or four mothers were used, and after weaning, the pups were pooled together and segregated into groups to ensure that there was no bias in the selected mice within the groups.
Infection and immunization of pups
The pups were infected with RSV line 19 or A2 at weeks 3, 4 and 5. In weeks 3 and 4, the pups received 5 × 104 PFU of RSV line 19 by the intranasal route, and in week 5, the pups received 1 × 105 PFU. One week after the last infection, the pups were immunized with OVA/CT, followed by seven consecutive challenges with 1% OVA aerosol.
Adoptive transfer of TH2 cells
For the data shown in Figure 3, FOXP3+CD4+ T cells were sorted based on eGFP expression from tolerized mice with or without RSV infection after the last infection in week 5. From both groups, 1.5 × 105 eGFP+ (FOXP3+) T cells were adoptively transferred intravenously into BALB/c mice that had been sensitized to OVA/CT. In addition, one group of BALB/c mice received twice the number of eGFP+ T cells from the RSV-infected group. Twenty-four hours after the adoptive transfer of cells, the mice were exposed to OVA aerosols for 7 d and then euthanized after the last challenge. For the data shown in Figure 4, 4 × 106 TH2 cells from DO11.10 × Rag2−/− mice were adoptively transferred into recipient mice intravenously. Twenty-four hours after cell transfer, the mice were exposed to OVA aerosols on 3 consecutive days to recruit the cells into the lungs.
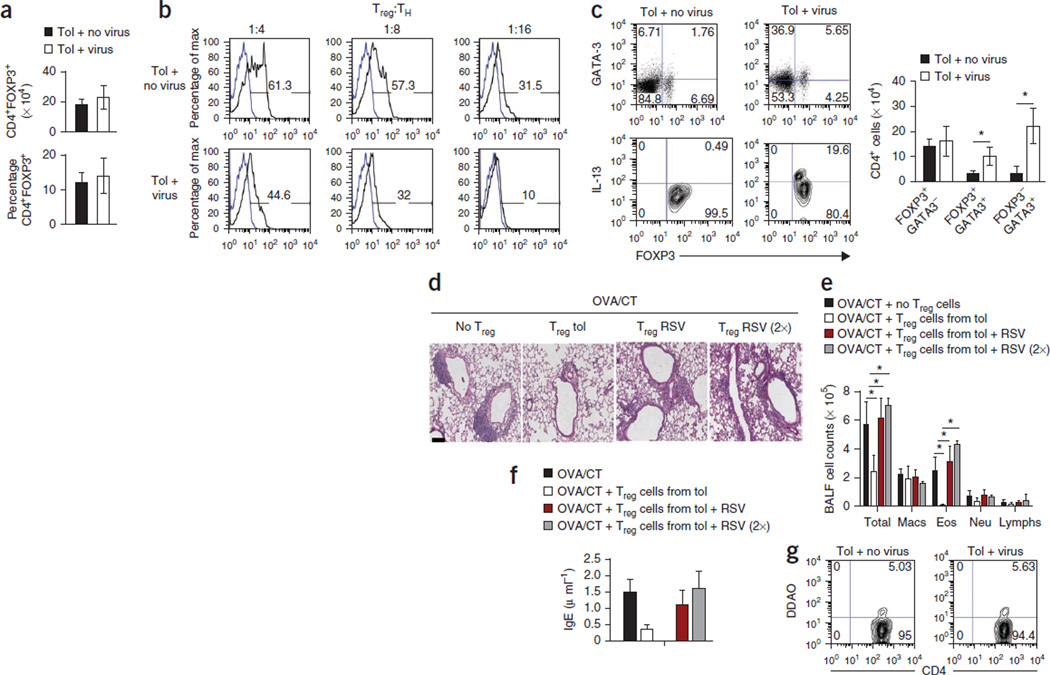
Figure 3. RSV infection alters the phenotype and function of Treg cells in the lung.
(a) The numbers (top) and the frequencies (bottom) of Treg (CD4+FOXP3+) cells in the lungs of tolerized FOXP3-eGFP knock-in pups in the presence or absence of virus infection. (b) Proliferation of naive responder T cells (TH) cocultured with different ratios of Treg (FOXP3+) cells isolated from uninfected and infected DO11.10 pups in the presence of OVA (100 µg/ml), as determined by the dilution of DDAO-labeled cells. (c) The expression of GATA-3, IL-13 and FOXP3 in CD4+ T cells isolated from the lungs of tolerized mice with and without RSV infection after stimulation with PMA and ionomycin. Cells shown in the upper images were gated on CD4 expression, and cells shown in the lower images were gated on FOXP3 expression. The numbers in the corners of the FACS dot plots are the percentage of each cell population within that quadrant. (d) PAS staining of lung sections of mice that received adoptively transferred eGFP+CD4+ T cells from tolerized mice that were uninfected or infected by RSV and then were sensitized to OVA/CT and challenged by OVA aerosols. (e) Number of immune cells in BALF recovered from different groups of mice. RSV (2×) indicates mice that received double the number of eGFP+CD4+ cells as compared to the RSV group (third group). (f) Serum IgE concentrations in different groups measured by ELISA. All data in a,c,e and f are means ± s.d. n = 4–5 mice per group (a–f). *P < 0.01 by Student’s unpaired two-tailed t test (c) or two-way ANOVA with Bonferroni’s post-hoc test (e). (g) Frequency of DDAO-labeled CD4+FOXP3+ (eGFP+) cells isolated from uninfected and RSV-infected mice in the lungs of OVA/CT-immunized recipient mice challenged with OVA. Data shown are representative of two independent experiments. The numbers in the corners of the FACS dot plots are the percentage of each cell population within that quadrant.
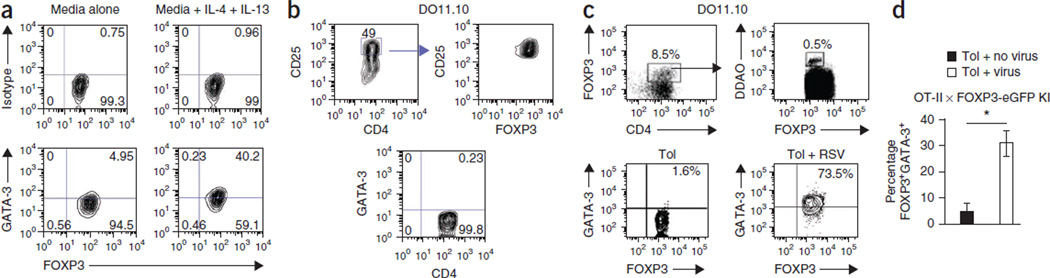
Figure 4. Treg cells acquire a TH2-like effector phenotype in tolerized RSV-infected mice.
(a) Expression of FOXP3 and GATA-3 in CD4+FOXP3+ T cells isolated from tolerized mice and cultured with OVA and antibodies to CD28 with or without IL-4 and IL-13 for 48 h. (b) FOXP3 expression (top) and the absence of GATA-3 expression (bottom) in in vitro–generated Treg cells sorted on high CD25 expression. (c) Gating strategy (top) and GATA-3 expression in FOXP3+ cells isolated from tolerized uninfected and infected mice adoptively transferred with in vitro–generated DDAO-labeled Treg cells. (d) Percentage of FOXP3+GATA-3+ Treg cells isolated from the lungs of tolerized C57BL/6 mice with or without RSV infection. Treg cells were generated in vitro from splenocytes of OT-II × FOXP3-eGFP knock-in (KI) mice and labeled with DDAO dye prior to transfer. Total DDAO+ cells within the CD4 gate is shown. *P < 0.05 by Student’s two-tailed t test. Data are the means ± s.d. The experiments were performed twice with similar results and used a minimum of three mice in each group.
Generation of induced Treg cells in vitro
Single-cell suspension of splenocytes was prepared and cultured in the presence of CD3-specific antibody (145-2c11, BD Biosciences, 1 µg/ml), TGF-β (3 ng/ml; Peprotech) and all-trans retinoic acid (Sigma; 1 nM). The cultures were supplemented with retinoic acid daily, and at the end of 5 d, more than 90% of the CD4+ T cells were FOXP3+, and the CD25hi cell population was almost exclusively FOXP3+ (>98%).
Adoptive transfer of Treg cells into BALB/c or OT-II × FOXP3-eGFP knock-in mice
In vitro–generated Treg cells were labeled with DDAO-SE dye (as described below in the Suppression Assay section) and adoptively transferred into 3-week-old tolerized BALB/c mice with or without RSV infection. Mice were infected with RSV 3 d before the transfer. Five-hundred thousand labeled cells were transferred, and 24 h after the transfer, the mice were administered OVA aerosols for 2 consecutive days and then euthanized. The lungs cells from both the groups were first enriched for CD4 expression by magnetic bead selection (CD4+ T cell isolation kit II, Miltenyi Biotech). Nearly all of the resulting cell population was positive for CD4 expression by flow cytometry, less than 10% of which (7.6% and 8.5% for uninfected and RSV-infected, respectively) coexpressed FOXP3 (Fig. 4c, upper left). A gate was drawn around CD4 and FOXP3 coexpressing cells, and then a 1 million cellular events were collected to visualize adoptively transferred, DDAO-expressing cells within this population (Fig. 4c, upper right). The proportion of FOXP3+DDAO+CD4+ T cells was comparable between uninfected and RSV-infected mice. The CD4+FOXP3+DDAO+ T cells were further selected (as shown by the gate drawn in Fig. 4c, upper right) for analysis of GATA-3 expression.
Cell isolation and sorting
The lungs were perfused with sterile PBS, removed and digested as described previously50,51. Lungs were then dissociated on a gentle MACS Dissociator (Miltenyi Biotech) according to the manufacturer’s protocol. Single-cell suspensions were obtained by passing the dissociated tissue through a 70-µm cell strainer (BD Falcon). Anti-CD4 microbeads (Miltenyi Biotech) were used to enrich for CD4 T cells. Treg cells were sorted using a FACSAria cell sorter based on CD4 and eGFP (FOXP3) expression.
Suppression assay
The suppression assay was set up as described previously26. Naive CD4+ T cells from DO11.10 TCR transgenic mice were labeled with CellTrace Far Red DDAO-SE (C34553), and Treg cells from FOXP3-eGFP knock-in mice were sorted and cultured in round-bottom, 96-well plates 7 d after the last infection. Cells were stimulated with OVA (100 µg/ml) and 5 × 104 γ-irradiated APCs per well, and freshly isolated Treg cells were added at various ratios to the DDAO-labeled cells. After 72 h in culture, cells were harvested, and cell proliferation was assessed by examination of DDAO-SE fluorescence.
ELISA, ELISPOT assay, BALF cell numbers, lung histology and OVA-specific serum IgE
ELISA for cytokines, quantifying cells in the BALF, lung histology and quantification of OVA-specific IgE in the sera were performed as previously described50,51. Cytokine production in CD4+ T cells were assayed using ELISPOT kits (eBioscience), as previously described50.
Pulmonary function (airway hyper-reactivity testing)
Mice were examined for respiratory mechanics using the forced oscillation technique52. To determine the difference in airway hyper-responsiveness between the experimental groups, mice were treated with ascending doses (3.125, 12.5 and 25 mg/ml) of methacholine. Each mouse was challenged with 10 s of aerosol followed by input impedance measurements, with Newtonian resistance (Rn) representing central airway caliber.
qRT-PCR
Tissues or cells were treated with TRIzol (Invitrogen). RNA was isolated using RNeasy kit (Qiagen) and treated with RNase-free DNase (Qiagen). Complementary DNA was synthesized and used for qRT-PCR using TaqMan Gene Expression Assays (Applied Biosystems) according to the manufacturer’s instructions. The level of mRNA was normalized to GUS expression, and the results were analyzed using the 2−ΔΔCt method.
Statistical analyses
After testing for normal distribution of the populations, two-way ANOVA with Bonferroni’s post-hoc test was used to compare differences between multiple groups. Student’s unpaired two-tailed t test was used for all other statistical analyses. Differences between groups were considered significant when P < 0.05. All statistical analyses were performed using GraphPad Prism software.
RESULTS
Infection with RSV breaches maternally transferred tolerance
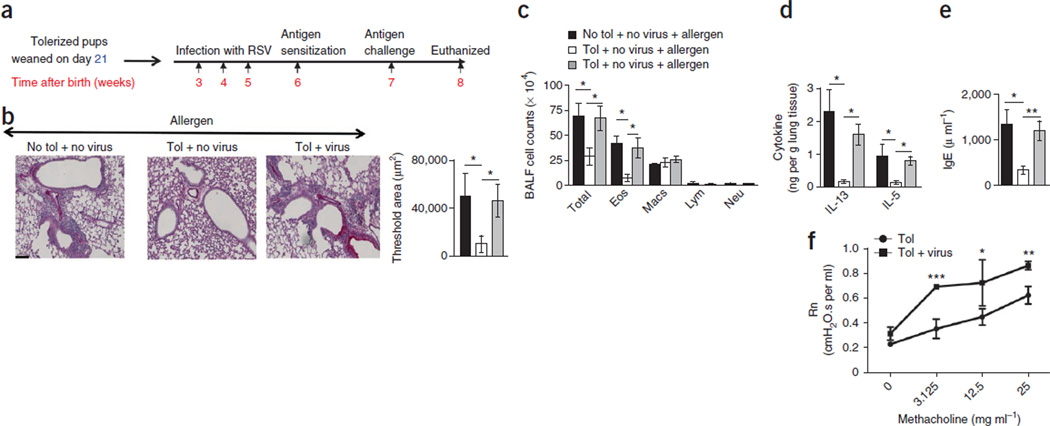
To study the impact of RSV infection on immune tolerance in early life, we first tolerized newborn mice using breast milk12,13. Twenty-four hours after the birth of the pups, we exposed their mothers to OVA every other day for 10 d and weaned the pups at the end of this regimen (day 21). Immediately after weaning, we infected the tolerized pups with RSV (line 19) during weeks 3, 4 and 5 to mimic recurrent infections in humans. Of note, for reasons that are unclear, despite induction of humoral responses to RSV infection through the nasal mucosa, newborn humans remain susceptible to repeated infections, and the virus also causes severe illness in the elderly14,15. In week 6, we tested for the establishment of tolerance in the pups by immunizing them with OVA plus a mucosal adjuvant (cholera toxin) (OVA/CT) and then challenging them with OVA aerosol (Fig. 1a). Uninfected pups from tolerized mothers did not mount an allergic response to OVA, as determined by periodic-acid Schiff (PAS) staining for mucus, inflammatory cell numbers in the bronchoalveolar lavage fluid (BALF), the IL-13 and IL-5 protein levels in the lung tissue and the concentration of OVA-specific IgE in the serum (Fig. 1). In contrast, the pups that were nursed and tolerized but repeatedly infected with RSV had increased cellular infiltration in the airways and increased mucus production in response to OVA (Fig. 1b,c). The concentrations of the TH2 cytokines IL-5 and IL-13 in lung homogenates of the tolerized infected mice and the concentration of OVA-specific IgE in their serum were higher than in tolerized, uninfected mice (Fig. 1d,e). Airway hyper-reactivity in response to methacholine was higher in tolerized infected mice compared to tolerized uninfected mice (Fig. 1f).
Figure 1. Recurrent RSV infection compromises maternally transferred tolerance to inhaled allergen.
(a) Experimental scheme for measuring the effects of tolerization and RSV infection in mice. (b) Histology, as evaluated by PAS staining. Scale bar, 100 µm. The threshold values shown indicate the quantification of the number of inflammatory cells and PAS staining in the different groups using MetaMorph. Tol, tolerization. (c) Quantification of total cell count, eosinophils (Eos), macrophages (Macs), lymphocytes (Lym) and neutrophils (Neu) in BALF cytospins. (d) Concentration of cytokines in the lung homogenates by ELISA. (e) Concentration of OVA-specific IgE in the serum. For b–e, the results shown are from an experiment performed five times. All data in b–e are means ± s.d. n = 4–5 mice per group. *P < 0.05, **P < 0.01 by two-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test. (f) Central airway resistance (Newtonian resistance, Rn) in response to increasing doses of methacholine in uninfected and infected mice tolerized mice. Data are means ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 by Student’s unpaired two-tailed t test.
We next examined whether recurrent RSV infection triggers inflammation in tolerized lungs in the absence of antigen challenge. We used the same protocol of tolerance outlined above and infected the tolerized pups with RSV; we then euthanized the pups in week 7. In the absence of antigen challenge, recurrent RSV infection induced inflammation in the lungs of these mice (Supplementary Fig. 1a–c). Viral replication in the lungs of 3-week-old mice was followed by assaying the mRNA level of the RSV large polymerase (L) gene over time (Supplementary Fig. 1e). The transcript level of this gene continued to increase until day 4 and then decreased by day 9. This expression pattern mimicked the profile reported in previous studies9. The more commonly used strain of RSV, A2, also induced airway inflammation and breached maternally transferred tolerance (Supplementary Fig. 2a–c) similar to line 19. Treatment of the virus with ultraviolet radiation abrogated its ability to promote eosinophilic airway inflammation (Supplementary Fig. 2d,e).
Next, to address the state of tolerance in the infant mice at the time of weaning, we immediately sensitized and challenged weaned mice with OVA/CT at 21 d (Supplementary Fig. 3a). When the pups were nursed by tolerized mothers during the first 21 days, they were also indirectly efficiently tolerized through the breast milk (Supplementary Fig. 3a–c). These results suggest that RSV breaches tolerance that has already been established in the neonates. Newborns expressing TGF-β DNRII (the dominant-negative receptor) on their CD4+ T cells were not tolerized and mounted an allergic airway response to OVA as compared to the pups of wild-type (WT) mice (Supplementary Fig. 4a,b), confirming that in the absence of TGF-β signaling, tolerance cannot be transferred to pups12.
Reduced suppression by Treg cells from RSV-infected mice
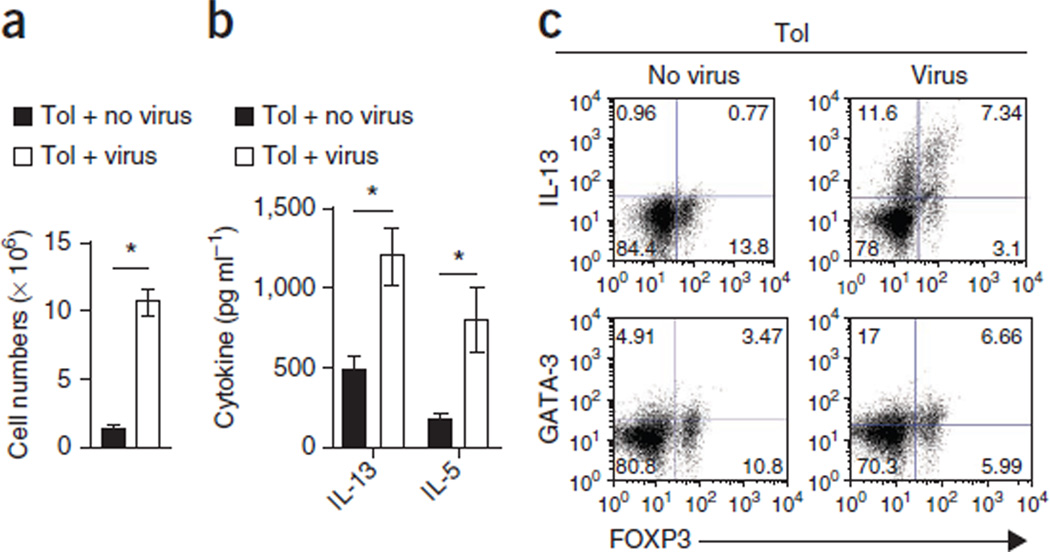
RSV infection elicits a TH2 cell response against specific epitopes of the G protein of RSV16–18. To examine whether the breach of tolerance is initiated during the priming phase in the lung-draining lymph nodes, we tolerized pups to OVA, subjected them to recurrent RSV infection and harvested the lymph nodes from the mice 1 week after the last infection. A visual inspection revealed substantially larger lung-draining lymph nodes in the RSV-infected tolerized mice than in the tolerized uninfected control mice (Supplementary Fig. 5), and the lymph nodes from the infected mice were populated with more cells (Fig. 2a). Lung draining lymph node cells from the RSV-infected mice secreted higher amounts of the TH2 cytokines IL-13 and IL-5 ex vivo when they were stimulated with either OVA (Fig. 2b) or PMA and ionomycin (Fig. 2c and Supplementary Fig. 5) as compared to tolerized uninfected mice.
Figure 2. RSV infection triggers inflammation in lung-draining lymph nodes.
(a) The total number of cells in the pooled lymph nodes of uninfected and infected mice tolerized as shown in Figure 1a, as determined by light microscopy after discounting dead cells on the basis of trypan blue exclusion. (b) Cytokine concentrations in the supernatant from lymph node cell cultures after stimulation with OVA (100 µg/ml) for 3 d as determined by ELISA. (c) Expression of GATA-3, FOXP3 and IL-13 on lymph node cells isolated from tolerized mice that were uninfected or infected with RSV after stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 6 h. Cells were gated on CD4. The numbers in the corners of the FACS dot plots are the percentage of each cell population within that quadrant as a fraction of the total CD4 cells. All data are means ± s.d. and are representative of two independent experiments. n = 4–5 mice per group. *P < 0.05 by Student’s unpaired two-tailed t test.
As Treg cells have a crucial role in immune tolerance in the airways19–21 and in inflammatory conditions can secrete cytokines characteristic of a distinct helper T cell lineage that can aggravate pathology22,23, we examined whether IL-13–expressing FOXP3+ cells were induced in the lymph nodes of RSV-infected mice. We found a distinct population of FOXP3+IL-13+CD4+ T cells in the lymph nodes of the RSV-infected pups that was not present in the uninfected mice (Fig. 2c). GATA-3 is the transcription factor that defines the TH2 cell subset24,25. Virus infection promoted an increase in the frequency of GATA-3+FOXP3+CD4+ T cells in the lymph nodes of the virus-infected mice. Although GATA-3+FOXP3+CD4+ T cells were also present in the lymph nodes of the uninfected mice, there were very few IL-13–expressing FOXP3+ T cells in this context.
The total numbers of CD4+ T cells (data not shown) and the frequency of FOXP3+ cells were similar in the lungs of tolerized mice whether they were infected with RSV or not (Fig. 3a). CD4+FOXP3-eGFP+ Treg cells were isolated 24 h after the last RSV infection from the lungs of infected tolerized FOXP3–enhanced GFP (eGFP) mice and used in a suppression assay with DO.11 T cells expressing an OVA-specific T cell receptor (TCR) and OVA. Because the Treg cells were eGFP+, we could not use carboxyfluorescein succinimidyl ester to label the responder cells, and so we instead used the dye 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one) succinimidyl ester (DDAO-SE)26. Treg cells from the uninfected mice efficiently inhibited the proliferation of the naive CD4+ T cells, whereas Treg cells from the infected mice had compromised suppressive function (Fig. 3b). We al so found an increase in the frequency of TH2 cytokine–secreting CD4+FOXP3+ and CD4+FOXP3− cells in the lungs of the RSV-infected mice (Supplementary Fig. 6). Similar to the effects in the lung-draining lymph nodes (Fig. 2d), RSV infection resulted in an increase in the percentage of GATA-3+FOXP3+CD4+ T cells in the lungs, with 20% of the FOXP3+ cells expressing IL-13 (Fig. 3c). There were higher total numbers of GATA-3+FOXP3+ and GATA-3+FOXP3− cells in the lungs of the tolerized mice as a result of RSV infection (Fig. 3c).
We next adoptively transferred Treg cells isolated from either virus-infected or uninfected tolerized infant mice into mice immunized with OVA/CT but before challenge with OVA. eGFP+CD4+ (FOXP3+CD4+) T cells from the uninfected mice potently suppressed airway inflammation and OVA-specific IgE levels in response to OVA challenge, an effect that was absent when we transferred similar or twice the numbers of Treg cells from the lungs of virus-infected tolerized mice (Fig. 3d–f). Notably, when we adoptively transferred double the number of eGFP+FOXP3+ Treg cells from the infected mice, airway inflammation was exacerbated, suggesting the suppressive activity of FOXP3+CD4+ T cells in the lungs of virus-infected mice is dysfunctional (Fig. 3d–f). The functional difference we observed in the two groups of recipients was not a result of differences in trafficking of the adoptively transferred cells (Fig. 3g). When we compared FOXP3+ T cells isolated from the lungs of infected and uninfected mice with respect to their expression of cell surface markers such as CD25 and glucocorticoid-induced tumor necrosis factor receptor–related protein (GITR), we found no marked difference, although the cells from the virus-infected mice had higher expression of IL-4Rα (Supplementary Fig. 7). In uninfected mice, ~59% of the CD4+FOXP3+ cells were CD127lo/−, a common feature of human Treg cells27, and only 1% expressed low amounts of GATA-3. In infected mice, we found that 79% of the CD4+FOXP3+ cells were CD127lo/−, 12% of which were GATA-3+. In naive mice, >90% of all CD4+FOXP3+ cells were CD127lo/− (Supplementary Fig. 7).
Treg cells acquire a TH2 phenotype in infected mice
We next studied the influence of the TH2-type inflammatory environment in the lung induced by virus infection on the phenotype and function of Treg cells. Exposure of CD4+FOXP3+ T cells to the TH2-type cytokines IL-4 and IL-13 promoted GATA-3 expression (Fig. 4a). We generated CD4+FOXP3+GATA-3− T cells in vitro by culturing splenocytes from DO11.10 TCR transgenic mice specific for the OVA323–339 peptide with TGF-β and retinoic acid28–30 (Fig. 4b). We labeled CD4+FOXP3+GATA-3− cells expressing high amounts of CD25 (Fig. 4b) with DDAO and adoptively transferred them into tolerized uninfected or infected 3-week-old BALB/c recipient mice, which we then exposed to aerosolized OVA to recruit the cells to the lung. We found negligible expression of GATA-3 in FOXP3+DDAO+CD4+ T cells isolated from the lungs of tolerized uninfected mice, whereas 73.5% of these cells coexpressed GATA-3 when we recovered them from tolerized RSV-infected mice (Fig. 4c). We also performed a similar experiment using splenocytes from OT-II mice crossed to FOXP3-eGFP mice (OT-II × FOXP3-eGFP mice) and, as shown in Figure 4d, ~30% of the transferred Treg cells recovered from 3-week-old recipient tolerized infected C57BL/6 mice coexpressed GATA-3.
Treg cells have been shown to attenuate the immunogenicity of pulmonary dendritic cells by downregulating the expression of major histocompatibility complex (MHC) class II and co-stimulatory molecules on dendritic cells31. Pulmonary dendritic cells from uninfected tolerized mice had lower expression of MHC class II, CD40 and CD86 as compared to dendritic cells from RSV-infected mice (Supplementary Fig. 8).
Host IL-4Rα signaling is important to breach tolerance
The data obtained thus far suggested that viral infection abrogates maternally transferred tolerance by inducing the expression of TH2-type cytokines in the lung and promoting a phenotypic switch of Treg cells to a TH2-like phenotype. Indeed, the concentrations of IL-13 and IL-5 were higher in the BALF recovered from virus-infected lungs as compared to uninfected lungs (Fig. 5a). Lack of IL-4Rα signaling significantly attenuated GATA-3 expression in both FOXP3+ and FOXP3− cells after RSV infection (Fig. 5b).
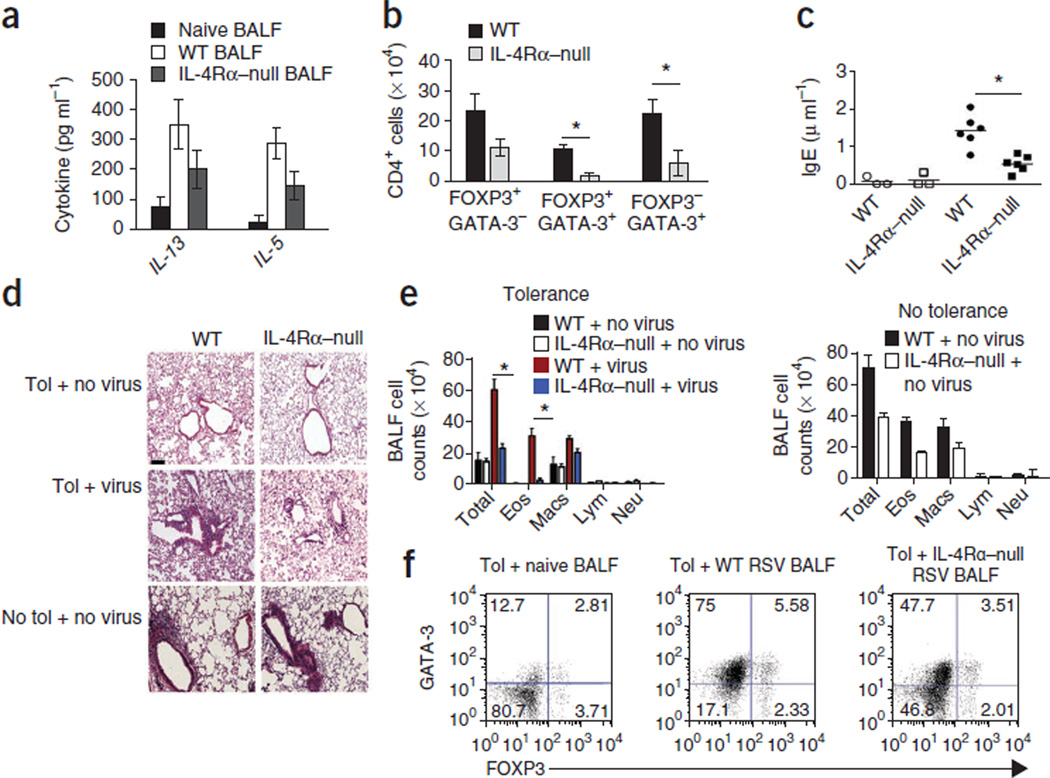
Figure 5. Host IL-4Rα signaling is important to breach tolerance.
(a) Cytokine concentrations determined by ELISA 8 d after RSV infection in the BALF from naive, RSV-infected WT or IL-4Rα–null mice. Data are means ± s.d. n = 4–5 mice per group. Data are representative of two independent experiments. (b) Number of CD4+ cells expressing FOXP3 and/or GATA-3 in the lungs of WT and IL-4Rα–null mice (both on (BALB/c background) tolerized to OVA and infected with RSV. Data are the mean of values pooled from individual mice of the number of CD4+ T cells within each population ± s.d. n = 4–5 mice per group. *P < 0.05 by Student’s unpaired two-tailed t test. (c) Serum concentration of OVA-specific IgE in WT and IL-4Rα–null mice after transfer of 3 × 106 CD4+ T cells sorted from DO11.10 × Rag2−/− mice and exposed to OVA aerosol. *P < 0.05. Open symbols denote the transfer of TH2 cells into tolerized uninfected mice, and closed symbols indicate transfer into tolerized infected mice. (d,e) H&E-stained lung sections (d) and cell counts in the BALF (e) of the recipient mice described in (c). *P < 0.05. Scale bar, 100 µm. (f) FOXP3 and GATA-3 expression in CD4+ T cells from tolerized mice cocultured with dendritic cells, OVA and cell-free BALF from naive mice or from WT or IL-4Rα–null mice infected by RSV. The experiment was performed twice with similar results, and the results of one representative experiment are shown. The numbers in the corners of the FACS dot plots are the percentage of each cell population within that quadrant.
To assess the contribution of host IL-4 signaling, we adoptively transferred TH2-skewed cells isolated from DO11.10 × Rag2−/− mice (devoid of Treg cells) into WT and Il4ra−/− tolerized mice, which we then infected with RSV and challenged with OVA. Transfer of OVA-specific, TH2 cells induced airway inflammation and an increase in the concentration of IgE in the serum of WT tolerized, virus-infected mice, whereas transfer into WT tolerized, uninfected mice did not promote these responses (Fig. 5c–e). Transfer of these cells into Il4ra−/− mice that had been tolerized to OVA by WT mothers elicited minimal lung inflammation and low titers of serum IgE (Fig. 5c–e). Transfer of TH2 cells into nontolerized and uninfected WT and Il4ra−/− mice induced significant allergic airway inflammation in both groups, demonstrating that Il4ra−/− mice can mount an inflammatory response in the absence of maternally transferred tolerance (Fig. 5d,e). CD4+ T cells isolated from tolerized mice were cultured with cell-free BALF from uninfected WT, WT infected and Il4ra−/− infected mice. BALF from the RSV-infected mice increased GATA-3 expression in both FOXP3−CD4+ and FOXP3+CD4+ T cells, which was significantly attenuated when the BALF was derived from infected Il4ra−/− mice (Fig. 5e,f). These results suggest that IL-4Rα signaling in the host is required for the impairment in maternally transferred tolerance induced by RSV infection.
DISCUSSION
Our study suggests that RSV infection induces TH2-like inflammation in the lung, which promotes a TH2-like effector phenotype in Treg cells and a loss of suppressive function. It is becoming increasingly evident that Treg cells have phenotypic plasticity in different inflammatory environments. Treg cells expressing transcription factors and cytokines that are characteristic of effector T cells are detectable at sites of inflammation22,23,32–35. These Treg cells express the same chemokine receptors that are present on the coexisting T helper cells, allowing them to home to the site of inflammation and suppress tissue-resident effector T cells. At sites of inflammation, Treg cells also lose their suppressive function, which can be restored with blocking antibodies to the specific cytokines present in the inflammatory milieu35. This may serve as a protective mechanism whereby an adaptive immune response develops, and after effector cell death, Treg suppressor function is restored as the concentration of inflammatory cytokines is reduced35. To the best of our knowledge, what had not previously been shown is that by inducing an inflammatory response, a pathogen can attenuate the suppressive function of Treg cells against an unrelated antigen (allergen) that even a strong adjuvant such as alum (shown in our previous studies) or cholera toxin cannot20.
In general, a TH2 immune response is favored in neonates36. RSV-induced pulmonary inflammation in mice was previously found to cause a shift from TH1 to TH2 cell inflammation37. RSV uses multiple mechanisms to induce a TH2 cell response in the host, including RSV G protein–mediated effects16,17,38, increasing IL-4 production from basophils39 and induction of alternatively activated macrophages40. These multiple mechanisms of inducing a TH2 response along with the ability of RSV to upregulate expression of IL-4Rα on Treg cells may underlie the ability of the virus, but not of adjuvants, to affect Treg cell phenotype and function even in tolerized hosts. Although more confirmatory studies are needed in humans, the bias toward TH2 cells with aging that has been noted in mouse studies41 might also explain why RSV causes serious illness in the elderly15. Prophylactic therapy with antibodies to G protein or therapeutics such as pitrakinra, an IL-4 variant that improves symptoms in individuals with asthma42, may be useful to blunt the inflammatory cascade triggered by RSV.
Recent studies have reported GATA-3 with FOXP3 expression during both human and mouse Treg cell development43,44. However, in those studies, dual GATA-3+FOXP3+ T cells were detectable only at low levels in the lungs, although they were readily detected in other organs44. Neither of the previous studies showed that FOXP3-expressing cells were able to produce TH2 cytokines43,44. Also, neither of the previous studies examined the effect of TH2 cell inflammation on Treg cells in the lung43,44. GATA-3 is involved in multiple stages of T cell development and is expressed at low levels in naive CD4+ T cells45. Therefore, it is not entirely surprising that at some level it would also regulate the development of Treg cells. GATA-3, which is upregulated in Treg cells after RSV infection, and signal transducer and activator of transcription 6 (STAT6) have been shown to inhibit the expression of FOXP3 (refs. 30,46). As IL-4 signaling has been shown to be important for the upregulation of GATA-3 in T cells24,45, this may account for the preservation of maternally transferred tolerance in infected Il4ra−/− mice.
In conclusion, our study provides evidence of pathogen-driven instability of Treg cells that breaches a robust form of maternally transferred tolerance. These findings suggest that increased susceptibility to asthma later in life as a result of recurrent RSV infection may be the result of an effect of RSV on the lung microenvironment, impairing Treg function and tolerance to inhaled allergens.
Supplementary Material
Acknowledgments
We thank E. Shevach and G. Chattopadhyay (Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, US National Institutes of Health (NIH)) for providing spleens of OT-II × FOXP3-eGFP knock-in mice and J. Zhu (Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, NIH) for his efforts in identifying the source of these mice. DO11.10 transgenic mice were originally provided by K. Murphy (Washington University, St. Louis). This work was supported by NIH grants HL060207 and AI093116 (to P.R.), HL 077430 and AI048927 (to A.R.) and U19 AI095227 (to R.S.P.).
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS
N.K. designed and performed experiments, analyzed data and wrote the manuscript. A.K. and T.B.O. performed cell sorting and analyzed flow cytometry data. M.R. performed qRT-PCR, C.M. performed lung function tests and assisted in the analysis of ELISPOT data, and M.Y. performed mouse surgeries. M.L.M. and R.S.P. provided stocks of RSV line 19, as well as guidance regarding virus propagation and use in mice. S.E.W. analyzed data and edited the manuscript. A.R. designed experiments, analyzed data and wrote the manuscript. P.R. conceived of the study, designed experiments, analyzed data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 2.Noble V, et al. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch. Dis. Child. 1997;76:315–319. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 5.Wu P, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am. J. Respir. Crit. Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J. Allergy Clin. Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Pullan CR, Hey EN. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br. Med. J. (Clin. Res. Ed.) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacharier LB, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012:91–100. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell–mediated disease during reinfection in adulthood. J. Exp. Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhasselt V, et al. Breast milk–mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 13.Polte T, Hansen G. Maternal tolerance achieved during pregnancy is transferred to the offspring via breast milk and persistently protects the offspring from allergic asthma. Clin. Exp. Allergy. 2008;38:1950–1958. doi: 10.1111/j.1365-2222.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 14.Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J. Virol. 2003;77:11303–11311. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 16.Johnson TR, et al. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs NW, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 2006;169:977–986. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curotto de Lafaille MA, et al. Adaptive Foxp3+ regulatory T cell–dependent and –independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Ostroukhova M, et al. Treg-mediated immunosuppression involves activation of the Notch-HESI axis by membrane-bound TGF-β. J. Clin. Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostroukhova TY, Kulikov AV, Rozenkrants AA, Smirnova OV. Overexpression of prolactin receptors during intrahepatic transplantation of RS1 rat cholangiocellular carcinoma cells. Bull. Exp. Biol. Med. 2006;141:364–367. doi: 10.1007/s10517-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine TH1 and TH2 cells and controls TH2-specific expression of the interleukin-5 gene. J. Biol. Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 25.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya D, et al. Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J. Immunol. 2007;179:6808–6819. doi: 10.4049/jimmunol.179.10.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 29.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaki H, et al. STAT6 inhibits TGF-β1–mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J. Biol. Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewkowich IP, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 37.Openshaw PJ. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am. J. Respir. Crit. Care Med. 1995;152:S59–S62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 38.Graham BS, Johnson TR, Peebles RS. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology. 2000;48:237–247. doi: 10.1016/s0162-3109(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 39.Moore ML, et al. STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J. Immunol. 2009;183:2016–2026. doi: 10.4049/jimmunol.0803167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirey KA, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R α-, TLR4-, and IFN-β–dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008;41:329–335. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wohlfert EA, et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray A, Cohn L. TH2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Invest. 1999;104:985–993. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantel PY, et al. GATA3-driven TH2 responses inhibit TGF-β1–induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore ML, et al. Cutting edge: oseltamivir decreases T cell GM1 expression and inhibits clearance of respiratory syncytial virus: potential role of endogenous sialidase in antiviral immunity. J. Immunol. 2007;178:2651–2654. doi: 10.4049/jimmunol.178.5.2651. [DOI] [PubMed] [Google Scholar]
- 48.Domachowske JB, Bonville CA. Overnight titration of human respiratory syncytial virus using quantitative shell vial amplification. Biotechniques. 1998;25:644–647. doi: 10.2144/98254dt01. [DOI] [PubMed] [Google Scholar]
- 49.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 50.Krishnamoorthy N, et al. Activation of the c-kit–PI3 kinase axis induces the regulatory cytokine interleukin-6 in dendritic cells impacting allergic immune responses in the lung. Nat. Med. 2008;14:565–573. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oriss TB, et al. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J. Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 52.Xu H, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes TH2 responses and allergic inflammation. Proc. Natl. Acad. Sci. USA. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.