Abstract
The porcine CD3 specific monoclonal antibody 898H2-6-15 has been used in allo- and xeno-transplantation studies as a porcine CD3 marker and as an effective T cell depletion reagent when conjugated to the diphtheria toxin mutant, CRM9. A recombinant anti-porcine CD3 immuntoxin was recently developed using single-chain variable fragments (scFv) derived from 898H2-6-15. In this study, using published sequence data, we have expressed the porcine CD3 ectodomain molecules in E. coli through inclusion body isolation and in vitro refolding approach. The expressed and refolded porcine CD3 ectodomain molecules include CD3ε, CD3γ, CD3δ, CD3εγ heterodimer, CD3εδ heterodimer, CD3εγ single-chain fusion protein and CD3εδ single-chain fusion protein. These refolded porcine CD3 ectodomain molecules were purified with a strong anion exchange resin Poros 50HQ. ELISA analysis demonstrated that only the porcine CD3εγ ectodomain single-chain fusion protein can bind to the porcine CD3 specific monoclonal antibody 898H2-6-15. The availability of this porcine CD3εγ ectodomain single-chain fusion protein will allow screening for affinity matured variants of scFv derived from 898H2-6-15 to improve the recombinant anti-porcine CD3 immunotoxin. Porcine CD3εγ ectodomain single-chain fusion protein will also be a very useful reagent to study the soluble phase interaction between porcine CD3εγ and porcine CD3 antibodies such as 898H2-6-15.
Keywords: Porcine CD3, 898H2-6-15 monoclonal antibody, T cell, Immunotoxin
1. Introduction
The TCR–CD3 complex consists of one clonotypic antigen-binding αβ or γδ TCR heterodimer and three conserved signal transducing modules: CD3εγ and CD3εδ heterodimers and a TCRζ homodimer. TCR–CD3 chains assemble into a minimal eight-subunit complex in the endoplasmic reticulum through a series of dimeric and trimeric interactions with a stoichiometry of one TCRαβ or TCRγδ heterodimer, one CD3εγ heterodimer, one CD3εδ heterodimer, and one TCRζ homodimer [1,11,13].
The anti-porcine CD3 monoclonal antibody 898H2-6-15 was generated by immunizing mice with porcine peripheral blood monoclonal cells [7]. It has been used as a porcine CD3 marker in our allo- and xeno-transplantation models as well as a T cell depletion reagent by conjugating with a diphtheria toxin binding mutant CRM9. We have demonstrated that the chemically conjugated anti-porcine CD3 immunotoxin is a very effective T cell depletion reagent in pigs [8] and this reagent has played a role in the maintenance of long-term hematopoietic stem cell transplants in the absence of graft versus host disease [3,4,6]. Recently using the single-chain variable fragment (scFv) derived from 898H2-6-15 monoclonal antibody (mAb) we have developed an anti-porcine CD3 recombinant immunotoxin [16]. Our interest to express the porcine CD3 molecules arose from the need to prepare a biotin-labeled porcine CD3 molecule to conduct the affinity maturation of this immunotoxin using a yeast display approach [2]. Our goal is to isolate the high affinity scFv (2-6-15) to improve the binding of our anti-porcine CD3 immunotoxin [15].
In this study, using the published sequence data we have synthesized and expressed the porcine CD3 ectodomain molecules in Escherichia coli. We isolated the inclusion bodies and refolded them in vitro [9].We expressed and refolded following porcine CD3 ectodomain molecules: CD3ε, CD3γ, CD3δ, CD3εγ heterodimer, CD3εδ heterodimer, CD3εγ single-chain fusion protein and CD3εδ single-chain fusion protein. These refolded porcine CD3 ectodomain molecules were purified with a strong anion exchange resin Poros 50HQ. The binding reactivity to 898H2-6-15 mAb was analyzed by ELISA. The results demonstrated that only the porcine CD3εγ ectodomain single-chain fusion protein binds to the 898H2-6-15 mAb.
2. Materials and methods
2.1. Plasmid construction
We designed 8 overlapping PCR primers covering the entire ε, or γ, or δ ectodomain respectively (Table 1) based on the DNA sequence of porcine CD3 ectodomain molecules (GenBank ID: S82909 for porcine CD3ε, AB190229 for porcine CD3γ and NM_213775 for porcine CD3δ, [12]. The first sense primer contained a 5′ NdeI restriction site; therefore an N-terminal methionine residue was added to each of the porcine CD3 ectodomain chains. The next primer was antisense and overlapped by 21 bp at both ends. Alternating overlapping sense and antisense primers were continued until the ectodomain was covered. Six histadine (6x-His) residues were added to the C terminus of each construct to facilitate the purification. A stop codon was inserted after the last ectodomain amino acid codon followed by an EcoRI site. The synthesis was conducted by PCR with cloned pfu polymerase (Stratagene) to increase the fidelity. The PCR program is 95 °C for 5 min, 25 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 1 min, extension for another 10 min at 72 °C. In the 50 μl PCR reaction, 10 pmol was added for the 5′ and 3′ end primers, and 2 pmol was added for the rest of the primers. After running the agarose gel, the correct DNA band was cut out of a 1% agarose gel, extracted with QIAquik gel extraction kit (QIAGEN, 28704), digested with NdeI + EcoRI, and cloned in frame into pET17b. All of the plasmid clones were confirmed by DNA sequencing.
Table 1.
PCR primers used to synthesize the porcine CD3 molecules.
| For porcine CD3ε ectodomain |
| pEP1 |
| 5′G GAA TTC CAT ATG GAG GAC ATT GAG AGA CCA GAC GAG GAC ACT CAA AAG ACT TTC AAG 3′ |
| pEP2 |
| 5′ACA AGT CAA CTC AAC CTT GTC ACC AGA AAT AGA AAC CTT GAA AGT CTT TTG AGT GTC 3′ |
| pEP3 |
| 5′GAC AAG GTT GAG TTG ACT TGT CCA GAG GAC CCA GAG TCT GAG AAG ATG ACT TGG AAG 3′ |
| pEP4 |
| 5′GTT GTC GTA AGA CTC GTA AAT TTG CAT GTC GTT TCT CTT CCA AGT CAT CTT CTC AGA 3′ |
| pEP5 |
| 5′ATT TAC GAG TCT TAC GAC AAC TAC ATG TTG TTG GAG AGA TTC TCT GAG GTT GAG AAC 3′ |
| pEP6 |
| 5′GTG CTT CTC ACC AAC AGT ACA AGT GTA GTA ACC AGA GTT CTC AAC CTC AGA GAA TCT 3′ |
| pEP7 |
| 5′TGT ACT GTT GGT GAG AAG CAC ACT CAC AGA TTG TAC TTG AAG GCT AGA GTT TGT GAG 3′ |
| pEP8 |
| 5′CCG GAA TTC TTA GTC AAC CTC AAC ACA GTT CTC ACA AAC TCT AGC CTT CAA 3′ |
| For porcine CD3γ ectodomain |
| pGA1 |
| 5′G GAA TTC CAT ATG CAA TTG AAG GAG GGT AAG CAC TCT GTT TTG TTG GAC GAC AAC 3′ |
| pGA2 |
| 5′CAA ACC ACA AGT CAA CAA AAC AGA ACC GTC CTC TCT GTT GTC GTC CAA CAA AAC AGA 3′ |
| pGA3 |
| 5′GTT TTG TTG ACT TGT GGT TTG CCA GAC CAA AAC ATT AGA TGG TTC AAG GAC GGT AAG 3′ |
| pGA4 |
| 5′AGT AGA TCT AGA GTT GTT CAA AGA ACA AAT CTC CTT ACC GTC CTT GAA CCA TCT 3′ |
| pGA5 |
| 5′TTG AAC AAC TCT AGA TCT ACT TGT AAC TTG GGT TCT TCT TCT AAG GAC CCA AGA 3′ |
| pGA6 |
| 5′GTT CTC CTT AGA ACC CTC ACA CCA GTA AAT ACC TCT TGG GTC CTT AGA AGA AGA 3′ |
| pGA7 |
| 5′TGT GAG GGT TCT AAG GAG AAC TCT AAG AGA TTG CAA GTT TAC TAC AGA ATG TGT 3′ |
| pGA8 |
| 5′CCG GAA TTC TTA GTT CAA CTC AAT ACA GTT TTG ACA CAT TCT GTA GTA AAC TTG 3′ |
| For porcine CD3δ ectodomain |
| pDE1 |
| 5′G GAA TTC CAT ATG GCT TTC TTG TCT AGA GTT TCT CCA TAC GAG GTT GAG ATG GAG 3′ |
| pDE2 |
| 5′GTT ACA AGA AAC GAA AAC CTT GTC CTC CAA CTC CTC CAT CTC AAC CTC GTA TGG 3′ |
| pDE3 |
| 5′AAG GTT TTC GTT TCT TGT AAC ACT TCT ATT ATT TGG TTG CAA GGT ACT GAG GGT 3′ |
| pDE4 |
| 5′ACC CAA GTC AAT CTT CTT GTC AGA CAA CAA CTC ACC CTC AGT ACC TTG CAA CCA 3′ |
| pDE5 |
| 5′GAC AAG AAG ATT GAC TTG GGT AAG AGA ATT TTG GAC CCA AGA GGT TTG TAC AAG 3′ |
| pDE6 |
| 5′AGA GTT AGA GTC TTG CTC CTT TGG AGC GTT ACA CTT GTA CAA ACC TCT TGG GTC 3′ |
| pDE7 |
| 5′AAG GAG CAA GAC TCT AAC TCT AAG ATT TTC TTG CAA GTT TAC TAC AGA ATG TGT 3′ |
| pDE8 |
| 5′CCG GAA TTC TTA GTC CAA CTC AAC ACA GTT TTG ACA CAT TCT GTA GTA AAC TTG 3′ |
| For porcine CD3εγ or εδ ectodomain single-chain fusion construct |
| Ep-NdeI |
| 5′G GAA TTC CAT ATG GAG GAC ATT GAG AGA CCA GAC 3′ |
| Epsilon R |
| 5′CGC GGA TCC ACC ACC ACC AGA ACC ACC ACC ACC GTC AAC CTC AAC ACA GTT CTC 3′ |
| Gamma F1 |
| 5′CGC GGA TCC GGT GGT GGT GGT TCT CAA TTG AAG GAG GGT AAG CAC 3′ |
| Gamma Rhis |
| 5′CCG GAA TTC TTA GTG GTG GTG GTG GTG GTG GTT CAA CTC AAT ACA GTT TTG 3′ |
| Delta F |
| 5′ GGA AGA TCT GGT GGT GGT GGT TCT GCT TTC TTG TCT AGA GTT TCT 3′ |
| Delta Rhis |
| 5′ CCG GAA TTC TTA GTG GTG GTG GTG GTG GTG GTC CAA CTC AAC ACA GTT TTG 3′ |
In order to build the porcine CD3 ectodomain εγ single-chain fusion construct (Fig. 1), the porcine CD3 ectodmain ε moiety was PCR amplified using primers Ep-NdeI + Epsilon R and the porcine CD3 ectodomain ε in pET17b was used as template. After running the agarose gel, the correct DNA band was cut out, extracted with QIAquik gel extraction kit, then digested with NdeI + BamHI labeled as insert I. The porcine CD3 ectodomain γ moiety was PCR amplified using Gamma F1 + Gamma Rhis using the porcine CD3 ectodomain γ in pET17b as template. After running the agarose gel, the correct DNA band was cut out, extracted with QIAquik gel extraction kit, then digested with BamHI + EcoRI as insert II. The insert I + insert II were cloned together into the NdeI–EcoRI digested pET17b and sequencing confirmed. The fusion construct was transformed into E. coli BL21 star (DE3) competent cell (Invitrogen). The porcine CD3 ectodomain εδ single-chain fusion construct was built with the same method as described above with the PCR primer Delta F replacing the Gamma F1 and the Delta Rhis replacing the Gamma Rhis. The porcine CD3 ectodomain δ in pET17b was used as template for amplifying the porcine CD3 ectodomain δ moiety.
Fig. 1.
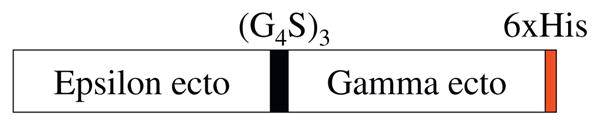
Schematic representation of the porcine CD3εγ ectodomain single-chain fusion protein construct: ε ecto-(G4S)3–γ ecto–6xHis.
2.2. E coli expression, inclusion body preparation and solubilization
Insoluble inclusion body protein was prepared using a protocol based on what is described by [5], with modifications. The characterized plasmid DNA was transformed into E. coli BL21 star (DE3). To prepare the seed culture, a single colony was inoculated into 25 ml LB containing 100 μg/ml ampicillin and cultured overnight at 37 °C with shaking at 250 rpm. The above seed culture was inoculated at 2.5% final concentration into 800 ml LB (in four 1 L flasks) containing 100 μg/ml ampicillin and cultured at 37 °C with shaking at 250 rpm until the OD600 reached 0.8–1.0. IPTG was added at 1 mM final concentration to induce the protein expression for 3 h at 37 °C with shaking at 250 rpm. The cells were harvested by centrifugation at 3000g for 10 min. The cell pellets were stored at −80 °C for later use.
The cell pellets from an 800 ml culture were suspended in 10 ml of 50 mM Tris HCl, pH 8.0, 25% sucrose, 1 mM EDTA, 0.1% sodium azide, 10 mM DTT. Lysozyme (1 mg/ml), DNase I (375 μg/ml), and 5 mM MgCl2 was added. Lysis buffer was added at 2.5 ml per ml of the suspension containing 50 mM Tris HCl, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate, 100 mM NaCl, 0.1% sodium azide, 10 mM DTT, 10 mM EDTA, pH 8.0. The suspension was frozen and thawed for one cycle then 10 mM MgCl2 was added for aiding DNase I activity. The cell debris was centrifuged at 13,000g at 4 °C for 50 min. The cell pellets were washed four times by centrifugation at 13,000g at 4 °C for 10 min with 50 mM Tris HCl, 0.5% (v/v) Triton X-100, 100 mM NaCl, 1 mM EDTA, 0.1% sodium azide and 1 mM DTT, pH 8.0. Then the inclusion bodies were suspended in 50 mM Tris HCl, 1 mM EDTA, 0.1% sodium azide, and 1 mM DTT, pH 8.0; centrifuged as above; pellets were then dissolved in 3 ml of 25 mM 2-(N-morpholino)ethanesulfonic acid, 8 M urea, 10 mM EDTA, and 0.1 mM DTT, pH 6.0 for 10 min at room temperature; centrifuged at 13,000 °g at 4 °C for 10 min. The supernatant was stored at −80 °C. The protein concentration of the supernatant was determined with BCA protein assay kit using a BSA standard (Pierce, cat# 23225).
2.3. In vitro refolding of the porcine CD3 molecules
The inclusion body solution [ε (8 μM), γ (8 μM), δ (8 μM), ε (6 μM) + γ (18 μM), ε (6 μM) + δ (18 μM), εγ single-chain fusion protein (8 μM), εδ single-chain fusion protein (8 μM)] was directly injected into refolding buffer containing 100 mM Tris HCl, 1 M L-arginine– HCl, 2 mM EDTA, pH 8.2, 1 mM oxidized glutathione, 0.33 mMreduced glutathione using a 18 gauge needle with stirring in a cold room at 4 °C. The reaction was stirred for approximately 3 min to completely mix the protein. The reaction was then kept in a 10 °C cabinet for 48–72 h without stirring.
2.4. Purification of the refolded porcine CD3 molecules
Ni–Sepharose, fast flow 4 mL resin (GE Healthcare Cat. #17-5318-02) equilibrated with 10 column volumes (CV) of 20 mM Tris–HCl, pH 7.4, 0.5 M NaCl, 5 mM imidazole was used for the first step purification. The refolded protein product was transferred into wet dialysis tubing with 3.5 kDa cutoff (Spectrum Laboratories, Inc. Cat# 132725), dialyzed for 24 h against 20 mM Tris– HCl, pH 7.4, 0.5 M NaCl, 5 mM Imidazole with one complete buffer exchange and constant stirring. The dialyzed samples were loaded onto the equilibrated Ni–Sepharose column and the flow through was collected. The column was then washed with six CVs of 20 mM Tris–HCl, pH 7.4, 0.5 M NaCl, 5 mM imidazole. Protein was eluted with six CVs of 20 mM Tris–HCl, pH 7.4, 0.5 M NaCl, 100 mM imidazole then six CVs of 20 mM Tris–HCl, pH 7.4, 0.5 M NaCl, 500 mMimidazole. Fractions containing the protein of interest were then dialyzed against 20 mM Tris HCl, pH 8.0, 1 mM EDTA, 5% glycerol for 24 h with one complete buffer exchanged and constant stirring. Poros 50 HQ, strong anion exchange 10 mL resin, (Applied Biosystems, Cat# 1-2559-11) was used in a 0.7 cm dia × 1.5 cmhigh column equilibrated with 10 column volumes (CV) 20 mMTris HCl, pH 8.0, 1 mMEDTA, 5% glycerol (10 CVs) for the second purification. The dialyzed samples, 40–60 ml, were loaded onto the column. The column was washed with 20 mM Tris–HCl, pH 8.0, 1 mM EDTA, 5% glycerol (6 CVs), eluted with six CVs of 20 mM Tris HCl, pH 8.0, 1 mMEDTA, 5% glycerol, with 150, then 200 mMNaCl. For each concentration of the NaCl, 6 fractions of 1 CV each were collected. The purified protein concentrations were determined by BCA protein assay kit using a BSA standard.
2.5. Elisa
The porcine CD3 ectodomain proteins were adsorbed at 20 μg/ml in PBS to Nunc immuno 96-well solid flat-bottom plate (Fisher, cat#12-565-136) overnight at 4 °C or 2 h at room temperature and then washed three times with 1 x DPBS containing 1 mM CaCl2 (Mediatech Inc, Cat# 21-030-CV) + 0.1%Tween 20. Plates were then incubated for 1 h at room temperature with 150 μl/well of 1%BSA + 1x DPBS containing 1 mM CaCl2 to prevent nonspecific binding to the plate. The wells were then washed as previously described, and incubated for 1 h with 100 μl of 2-6-15 mAb (0.2 μg/ml) in 1%BSA + 1x DPBS containing 1 mM CaCl2 + 0.1%Tween-20. Wells were washed and incubated for another hour with 100 μl goat anti mouse IgG polyclonal antibody (H + L)-HRP (1:2000, 500 ng/ml in 1%BSA + 1x DPBS containing 1 mM CaCl2 + 0.1%Tween-20) (GenScript, Cat# A00160). Wells were washed and 100 μl of ABTS solution/well (Southernbiotech, cat# 0303-01) was added. After 5 min, color development was stopped with 50 μl/well of 1% SDS and the optical density (OD) was measured with an ELISA reader using a 405 nm filter. Each sample was performed in doublet. Soluble competition ELISA was performed by binding the porcine CD3εγ single-chain fusion protein (20 μg/mL) to the plastic as above and blocking and washing as above. 898H2-6-15 mAb at 0.1 μg/ml was mixed with various concentrations competitor (100, 50, 0 μg/mL). Detection of 898H2-6-15 mAb binding to the solid phase was done as above. Solid phase competition (dose dependent) ELISA was performed by mixing various ratios of the porcine CD3εγ single-chain fusion protein and attaching these mixtures to the plates. 898H2-6-15 mAb binding to the solid phase was detected as before.
3. Results
3.1. Expression and purification of the porcine CD3 ectodomain molecules in E. coli
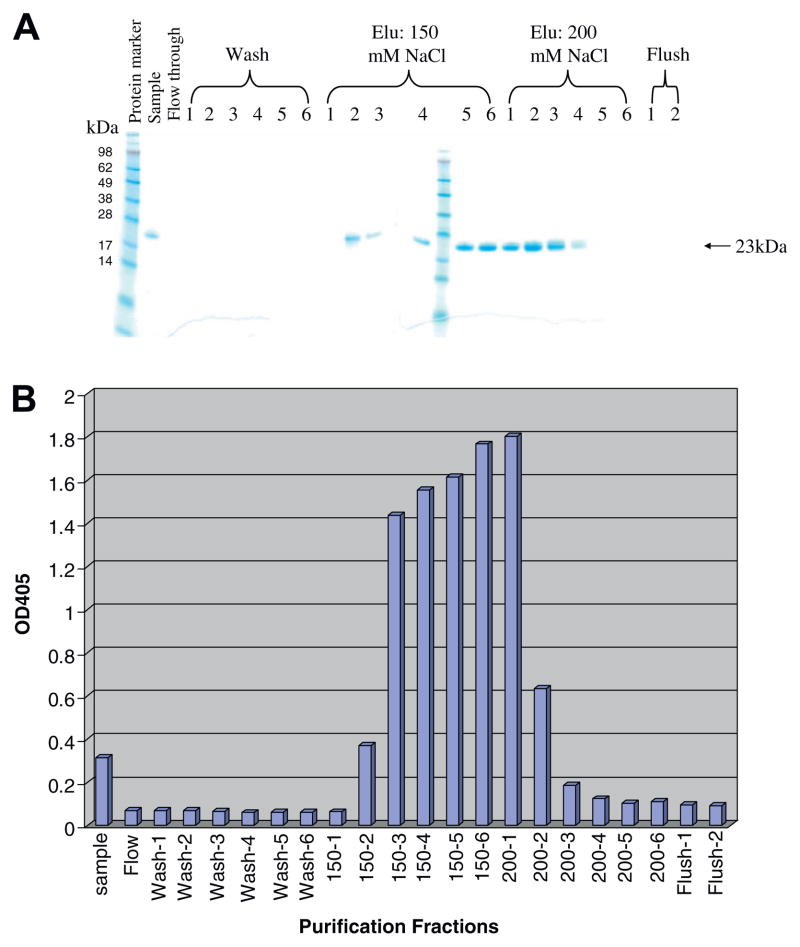
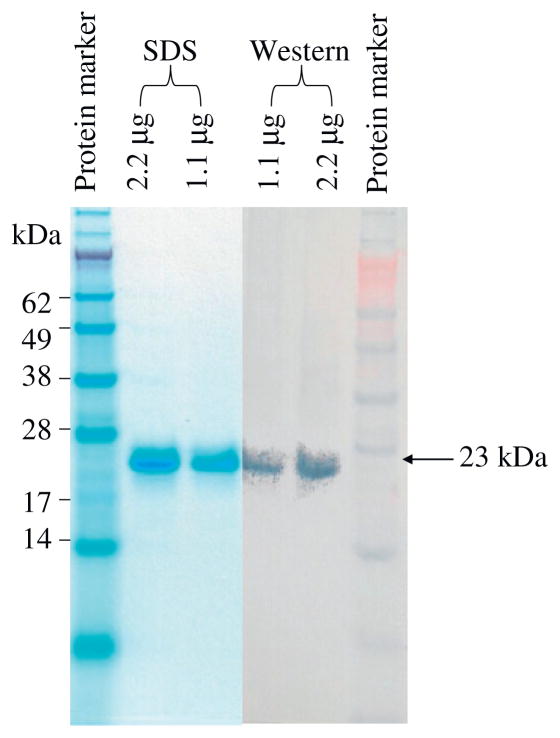
As shown in Fig. 1, the synthesized porcine CD3ε ectodomain and γ ectodomain DNA were linked together with a (G4S)3 linker to form a single-chain fusion construct using the published DNA sequences. The construct was cloned into E. coli expression vector pET17b, expressed in E. coli BL21 Star (DE3) strain and the inclusion body was isolated then refolded in vitro. As shown in Fig. 2A, the refolded porcine CD3εγ single-chain fusion protein (23 kDa) was purified using a strong anion exchange Poros 50 HQ resin. The purified porcine CD3εγ single-chain fusion protein was confirmed by Western blot analysis using anti-6xhis mAb (Fig. 3).
Fig. 2.
Purification and ELISA analysis of porcine CD3εγ single-chain fusion protein. (A) Purification of the refolded porcine CD3εγ single-chain fusion protein using strong anion exchange resin Poros 50HQ; (B) Porcine CD3 mAb 898H2-6-15 binding reactivity to the chromatographed fractions of the refolded porcine CD3εγ single-chain fusion protein. ELISA OD values are on the vertical axis, purification fractions on the horizontal axis. The peak reactivity corresponds to the peak of the porcine CD3εγ single-chain fusion protein elution as shown in Fig. 2A. Data representative of three experiments, p < 0.005.
Fig. 3.
Porcine CD3εγ single-chain fusion protein analysis with NuPAGE 12% Bis–Tris gel and Western blot using anti-6xHis mAb.
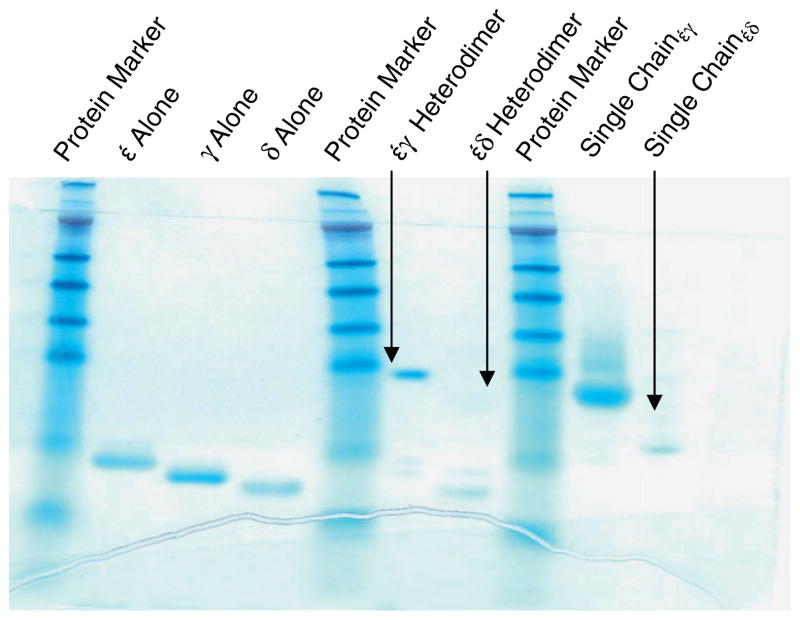
In order to map the epitope containing subunit(s) of 898H2-6-15 mAb from porcine CD3 molecules, we also expressed and refolded other porcine CD3 molecules including ε ectodomain alone, γ ectodomain alone, δ ectodomain alone, εγ ectodomain heterodimer, εδ ectodomain heterodimer, εδ ectodomain single-chain fusion protein using the same approach described above for the porcine CD3εγ ectodomain single-chain fusion protein (Fig. 4).
Fig. 4.
SDS non-reducing gel showing the anion-exchange resin purified porcine CD3 molecules.
3.2. ELISA analysis of the refolded porcine CD3 ectodomain molecules
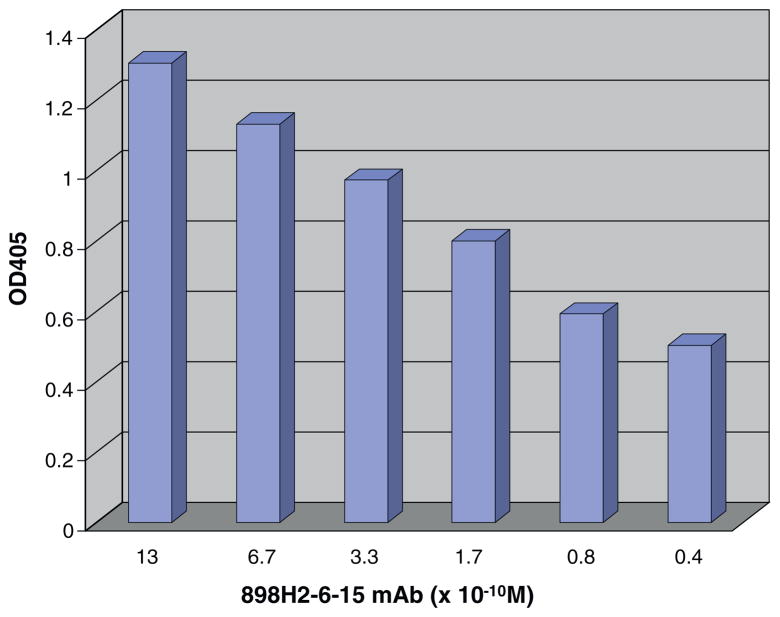
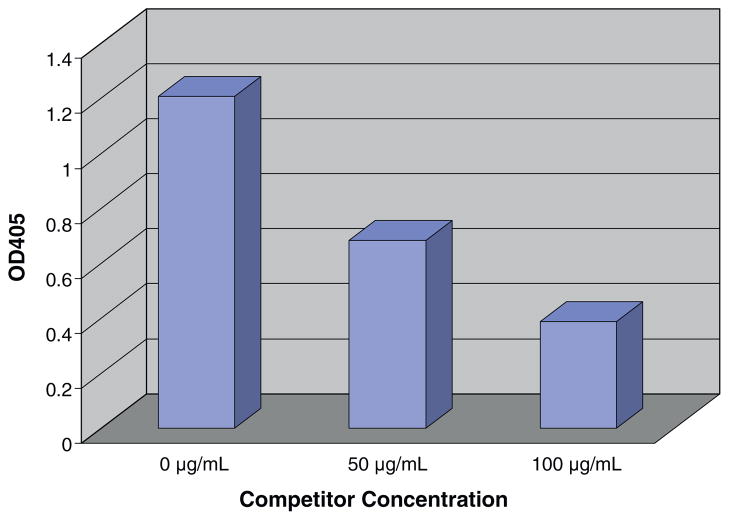
Anti-porcine CD3 mAb 898H2-6-15 is a conformational antibody [7], thus, ELISA was utilized to assess the binding activity of the porcine CD3 ectodomain molecules to 898H2-6-15 mAb. The ELISA analysis demonstrated that the porcine CD3εγ ectodomain single-chain fusion protein showed binding reactivity to the mAb 898H2-6-15 (Fig. 2B). None of the other refolded porcine CD3 ectodomain molecules, including ε ectodomain alone, γ ectodomain alone, δ ectodomain alone, εγ ectodomain heterodimer, εδ ectodomain heterodimer and εδ ectodomain single-chain fusion protein, bound to 898H2-6-15 (data not shown). As shown in Fig. 2B, 150 mM NaCl elution fraction 150–2 to 150–6 and the 200 mM NaCl elution fraction 200–1 and 200–2 bound well to the mAb 898H2-6-15. The observed binding for 200–1 and 200–2 is due to the purification system transition between the two different NaCl elution concentrations. Considering that the 200–1 fraction and 200–2 fraction are still partially under 150 mM NaCl conditions, we speculated that the conformation of the 200 mM NaCl eluted porcine CD3εγ single-chain fusion protein was not optimal for the 898H2-6-15 mAb binding. Additionally, the binding between the porcine CD3εγ single-chain fusion protein and the 898H2-6-15 mAb is calcium-dependent, which is consistent with how its parent porcine CD3 molecules behave on the porcine T cell surface during the FACS analysis using 898H2-6-15 mAb [7]. As shown in Fig. 5, the binding between the porcine CD3εγ single-chain fusion protein and 898H2-6-15 mAb is dose dependent. We also performed a competition ELISA to assess the binding under soluble conditions for the porcine CD3εγ single-chain fusion protein. As shown in Fig. 6, porcine CD3εγ single-chain fusion protein, under soluble conditions, successfully competed for the binding between the porcine CD3εγ single-chain fusion protein attached to the solid surface and the 898H2-6-15 mAb.
Fig. 5.
ELISA binding reactivity of dilutions of 898H2-6-15 mAb to porcine CD3εγ single-chain fusion protein coated plates. Porcine CD3 εγ single-chain fusion protein binding reactivity spans almost 2 log range of 898H2-6-15 mAb dilutions. Data representative of three experiments.
Fig. 6.
Competition ELISA. 898H2-6-15 binding to porcine CD3εγ single-chain fusion protein coated plates can be competitively inhibited by addition of soluble porcine CD3εγ single-chain fusion protein. 898H2-6-15 mAb is pre-incubated with the porcine CD3εγ single-chain fusion protein at the indicated concentrations and then added to the plate. Data representative of three experiments.
4. Discussion
We have successfully expressed and purified a functional soluble recombinant porcine CD3εγ ectodomain single-chain fusion protein. Either in solid phase or soluble phase it is a valid artificial probe of the 898H2-6-15 mAb-porcine CD3εγ interaction. It is possible that the addition of a flexible linker (G4S)3 helped the CD3εγ ectodomain to form the required epitope conformation for 898H2-6-15 mAb binding. The results indicate that 898H2-6-15 mAb only binds to porcine CD3εγ heterodimer in vivo similar to its counter partners anti-monkey CD3 mAb FN18 [14]. However we could not rule out the possibility that the in vivo conformation of the porcine CD3 molecules were different from our E. coli expressed and refolded ectodomain molecules.
Recombinant high affinity anti-T cell immunotoxins rather than chemical conjugates are required to insure homogeneity and elimination of Fc interactions. This has been achieved for anti-human and anti-monkey CD3 T-cell immunotoxins [10,17]. Recently using scFv derived from 898H2-6-15 mAb we have developed an antiporcine CD3 immunotoxin [16]. However the CD3 T cell depletion is not as great as with the conjugated version. We hypothesized that the main reason is the affinity loss of the antibody moiety scFv (2–6–15). Our goal is to perform the affinity maturation to increase the binding affinity of the scFv (2–6–15) by yeast display to improve the immunotoxin. We will build a random mutagenesis library of scFv (2–6–15) and screen the high affinity scFv (2–6–15) mutant using yeast display technology [2,15]. The availability of the soluble dye labeled porcine CD3εγ ectodmain single-chain fusion protein will facilitate this approach.
Acknowledgments
The authors would like to acknowledge Drs. Josef Kurtz and Vimukthi Pathiraja for critical review of the manuscript and J. Scott Arn for generation of the monoclonal antibody 898H2-6-15. This work was supported by National Institutes of Health (R01AI084657-02S1 to CAH and R01AI084657-02S2 to CAH) and Dana Farber/Harvard Cancer Center Core development grant.
References
- 1.Arnett KL, Harrison SC, Wiley DC. Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment. Proc Natl Acad Sci USA. 2004;101:16268–16273. doi: 10.1073/pnas.0407359101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boder ET, Wittrup KD. Yeast surface display for directed evolution of protein expression, affinity, and stability. Methods Enzymol. 2000;328:430–434. doi: 10.1016/s0076-6879(00)28410-3. [DOI] [PubMed] [Google Scholar]
- 3.Cina RA, Wikiel KJ, Lee PW, Cameron AM, Hettiarachy S, Rowland H, Goodrich J, Colby C, Spitzer TR, Neville DM, Jr, Huang CA. Stable multilineage chimerism without graft versus host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation. 2006;81:1677–1685. doi: 10.1097/01.tp.0000226061.59196.84. [DOI] [PubMed] [Google Scholar]
- 4.Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB, Ferrara V, Murphy MC, Sykes M, White-Scharf M, Neville DM, Jr, Sachs DH. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garboczi DN, Utz U, Ghosh P, Seth A, Kim J, VanTienhoven EA, Biddison WE, Wiley DC. Assembly, specific binding, and crystallization of a human TCR-αβ with an antigenic Tax peptide from human T lymphotropic virus type 1 and the class I MHC molecule HLA-A2. J Immunol. 1996;157:5403–5410. [PubMed] [Google Scholar]
- 6.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CA, Lorf T, Arn JS, Koo GC, Blake T, Sachs DH. Characterization of a monoclonal anti-porcine CD3 antibody. Xenotransplant. 1999;5:201–212. doi: 10.1034/j.1399-3089.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang CA, Yamada K, Murphy MC, Shimizu A, Colvin RB, Neville DM, Jr, Sachs DH. In vivo T cell depletion in miniature swine using the swine CD3 immunotoxin, pCD3–CRM9. Transplantation. 1999;68:855–860. doi: 10.1097/00007890-199909270-00019. [DOI] [PubMed] [Google Scholar]
- 9.Kastrup J, Pedersen LØ, Dietrich J, Lauritsen JP, Menné C, Geisler C. In vitro production and characterization of partly assembled human CD3 complexes. Scand J Immunol. 2002;56:436–442. doi: 10.1046/j.1365-3083.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim GB, Wang Z, Liu YY, Stavrou S, Mathias A, Goodwin KJ, Thomas JM, Neville DM. A fold-back single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng Des Sel. 2007;20:425–432. doi: 10.1093/protein/gzm040. [DOI] [PubMed] [Google Scholar]
- 11.Kim KS, Sun ZY, Wagner G, Reinherz EL. Heterodimeric CD3 epsilongamma extracellular domain fragments: production, purification and structural analysis. J Mol Biol. 2000;302:899–916. doi: 10.1006/jmbi.2000.4098. [DOI] [PubMed] [Google Scholar]
- 12.Kirkham PA, Takamatsu H, Yang H, Parkhouse RM. Porcine CD3 epsilon: its characterization, expression and involvement in activation of porcine T lymphocytes. Immunology. 1996;87:616–623. doi: 10.1046/j.1365-2567.1996.498566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law CL, Hayden-Ledbetter M, Buckwalter S, McNeill L, Nguyen H, Habecker P, Thorne BA, Dua R, Ledbetter JA. Expression and characterization of recombinant soluble human CD3 molecules: presentation of antigenic epitopes defined on the native TCR–CD3 complex. Int Immunol. 2002;14:389–400. doi: 10.1093/intimm/14.4.389. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Neville DM., Jr Expression and characterization of recombinant soluble monkey CD3 molecules: mapping the FN18 polymorphic epitope. Mol Immunol. 2004;40:1179–1188. doi: 10.1016/j.molimm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Kim GB, Woo JH, Liu YY, Mathias A, Stavrou S, Neville DM., Jr Improvement of a recombinant anti-monkey anti-CD3 diphtheria toxin based immunotoxin by yeast display affinity maturation of the scFv. Bioconjug Chem. 2007;18:947–955. doi: 10.1021/bc0603438. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Duran-Struuck R, Crepeau R, Matar A, Hanekamp I, Srinivasan S, Neville DM, Jr, Sachs DH, Huang CA. Development of a diphtheria toxin based antiporcine CD3 recombinant immunotoxin. Bioconjugate Chem. 2011;22:2014–2020. doi: 10.1021/bc200230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z, Thompson J, Neville DM., Jr Gene optimization is necessary to express a bivalent anti-human anti-T cell immunotoxin in Pichia pastoris. Protein Expr Purif. 2002;25:270–282. doi: 10.1016/s1046-5928(02)00009-8. [DOI] [PubMed] [Google Scholar]