Abstract
Objective
Pregnancy is a time of rapidly changing demands on the thyroid axis, and knowledge of thyroid hormone levels, especially during the first trimester, is important for ensuring maternal and fetal health. The thyroid hormone assays currently in use become more inaccurate at extremes of binding protein concentrations and when heterophilic antibodies are present. Pregnancy is characterized by both these conditions, making accurate determination of free thyroid hormone levels by conventional direct analog immunoassay methods difficult. The objective of this study was to characterize the performance of a novel tandem mass spectrometric assay for free thyroxine during the physiologic conditions of pregnancy.
Design
Healthy women without a history of thyroid abnormalities were recruited from the obstetrics and gynecology and endocrinology clinics of a university medical center and their thyroid status was monitored. Free thyroxine levels were assessed by both immunoassay and tandem mass spectrometry during the course of their pregnancy. Serum thyrotropin levels were also measured. The distributions of free thyroid concentrations obtained by the two assays were compared.
Main outcome
The tandem mass spectrometry and immunoassay values did not correlate well with each other. However, tandem mass spectrometry values correlated well with the current gold standard equilibrium dialysis values. Moreover, the good agreement between equilibrium dialysis and tandem mass spectrometry was maintained across all weeks of gestation.
Conclusions
We conclude that tandem mass spectrometry has a superior performance to immunoassay for the measurement of free thyroxine during pregnancy. Furthermore, it is ideally suited to generating trimester-specific reference intervals for free thyroxine levels. Future studies will determine if it is a better assay to use in most clinical circumstances.
Introduction
The dramatic changes in thyroid physiology occurring during the pregnant state make it crucial to develop reliable trimester-specific intervals for thyroid analytes. Use of nonpregnant reference intervals could lead to erroneous assessment of thyroid status in this rapidly changing hormonal environment (1,2). Reliable trimester-specific reference intervals have not been established for free thyroxine (FT4), although immunoassay data do exist for small samples (1,3). Trimester-specific reference intervals are available for thyrotropin (TSH), total thyroxine (TT4), and total triiodothyronine (TT3) (3–12). One of these studies reports thyroid hormone reference intervals for both immunoassay and isotope dilution tandem mass spectrometry (12). Some prior studies are limited by the lack of first trimester data, the failure to exclude women with autoimmune thyroid disease, or the presence of iodine insufficiency. It is also possible that reference intervals for specific ethnic groups may be indicated (13–16).
Clearly, availability of accurate trimester-specific intervals for thyroid parameters benefits clinical decision-making. Knowledge of accurate trimester-appropriate TT4 levels may be particularly critical in patients whose TSH values do not reflect the clinical situation due to central hypothyroidism or steroid use. Similarly, there is a possibility that TSH levels may not fully reflect thyroid status in situations of iodine insufficiency (17,18), despite a low maternal thyroxine level. In such cases there might be some concern about TT4 levels during the latter half of pregnancy not rising to within the reference range for that trimester. A TT4 of less than 7.8 µg/dL by immunoassay was the cut-off selected to define maternal hypothyroxinemia in a 1999 study of maternal thyroid status and childhood development (19). Accurate measurement of FT4 in pregnancy and trimester-specific FT4 reference intervals may be critically important if there is merit to the theory that a decreased FT4 concentration, not an elevated TSH, specifically places a fetus at risk (20–22).
Studies employing direct analog immunoassays generally show that FT4 serum concentrations peak during the first trimester and subsequently fall (3,4,13,23–25). Values during the second and third trimesters may be lower than nonpregnant values (3,4,6,24–26). Protein-binding abnormalities may contribute to this phenomenon, but equilibrium dialysis assays also show lower values during the third trimester than in the nonpregnant state (27). Historically equilibrium dialysis has been considered to be the most reliable assay for use in unusual or extreme physiologic conditions (27), but it suffers from being cumbersome, time consuming, and costly.
Establishing pregnancy-specific reference ranges for FT4 is particularly challenging. Not only are gestation-related changes in binding proteins such as thyroxine-binding globulin and albumin substantial, but also each immunoassay used to measure FT4 is affected to a varying and unpredictable degree by protein binding (27–30). Therefore, different FT4 assays may produce discrepant results (9, 27–29,31–33). Thus, pregnancy reference intervals ideally should not only be trimester specific, but also method specific (9). Another potential source of error when measuring TT4, TT3, and FT4 levels during pregnancy is heterophilic antibodies. These may be present in 0.2–15% of the general population and are common in pregnant and multiparous women (34). They may also be present in autoimmune disease and in individuals receiving therapy with monoclonal antibodies. When present during thyroid hormone quantification, these heterophilic nonspecific antibodies compromise the veracity of thyroid hormone measurements obtained by immunoassay (34,35). Moreover, it is difficult to predict whether the interference will produce a falsely low or high hormone level. Furthermore, reference intervals need to be established with the most accurate assay. Immunoassay, unlike tandem mass spectrometry, does not perform well at extremes of thyroid hormone concentrations, potentially leading to missed diagnoses (5). Rigorously defined, accurately measured reference intervals in euthyroid pregnant women without autoimmune thyroid disease or iodine insufficiency would best serve the clinician who is attempting to diagnose and treat thyroid disorders during pregnancy.
We recently reported a novel technique for measuring FT4 employing a previously used procedure, ultrafiltration, followed by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) (36). We here report serial measurements of TSH, TT4, and FT4 in a cohort of 98 euthyroid pregnant women, with utilization of LC/MS/MS for the thyroid hormone measurements. This is the first report of the use of FT4 LC/MS/MS assays in a clinical situation.
Methods
Participant groups
Serum samples were obtained from 98 healthy pregnant and 29 healthy nonpregnant women from February 2003 to August 2004. These samples were assayed for TSH, FT4 by immunoassay and MS/MS, and TT4 by MS/MS. FT4 was also measured by equilibrium dialysis in a subset of the pregnant subjects. Sample size was insufficient to measure thyroid antibodies. Urine samples for assessment of iodine status were not collected. None of the women had a prior history of either hypothyroidism or hyperthyroidism, and all had serum TSH values ≤ 3 mIU/L at study initiation. Within the pregnant group 59, 35, and 26 women gave samples in the first, second, and third trimesters successively, yielding 120 samples obtained during pregnancy. The nonpregnant subjects comprised a completely separate group from the pregnant subjects. Permission to perform this investigation was granted by the Georgetown University Hospital Institutional Review Board. Written consent was obtained from all the participants. Volunteers were recruited from the obstetrics and gynecology and endocrine clinics at Georgetown University Hospital. Information about use of prenatal vitamins was recorded.
Chemicals, reagents, solutions, and standards
Thyroxine (T4) was purchased from Sigma (St Louis, MO). A stable deuterium-labeled internal standard, l-thyroxin-d2 (d2-T4) was synthesized for the purpose of these studies by the Georgetown University Chemistry Department. High performance liquid chromatography grade methanol was purchased from VWR Scientific (West Chester, PA). All other chemicals were of analytical grade and were purchased from Sigma. Stock solutions of T4 and internal standard were prepared separately to obtain a concentration of 10 mg/mL for each using 40% ammonium hydroxide (v/v) in methanol as a solvent. The analyte stock solutions were diluted with methanol to obtain the spiking solutions. The solutions were stored at −20°C and could be used for several months. Standards for the T4 calibration curve in the range of 2.5–50 pg/mL were prepared by adding the analytes to water. A solution of 0.05 ng/mL d2-T4 in methanol was used as an internal standard.
FT4 sample preparation
Samples were thawed at room temperature and 600-µL aliquots were filtered through Centrifree YM-30 ultrafiltration devices (30,000 MW cutoff, Millipore, Bedford, MA) by centrifugation employing the Eppendorf temperature-controlled centrifuge (model no. 5702 R, Eppendorf, AG, Hamburg) and using a fixed angle rotor at 2900 rpm at a temperature of 25°C for 1 hour. This ultrafiltration process replaced the dialysis step of the classic equilibrium dialysis method. One hundred eighty microliters of internal standard (0.05ng/mL) was added to 360 µL ultrafiltrate and a volume of 400 µL was injected onto the C-18 column of the liquid chromatography tandem mass-spectrometer system (LC/MS/MS).
LC/MS/MS setup and procedure
An API 4000 tandem mass-spectrometer (SCIEX, Toronto, Canada) equipped with TurboIonSpray (Applied Biosystems, Foster City, CA) and an Agilent 1100 (Agilent Technologies, Santa Clara, CA) high performance liquid chromatography system was used to perform the analysis. Negative ion multiple reaction-monitoring (MRM) mode was used. The transitions to monitor were selected and were m/z 775.9 → 126.9 for T4 and m/z 777.9 → 126.9 for d2-T4. Nitrogen served as auxiliary, curtain, and collision gas. Gas flow rates, source temperature, ion spray voltages, and collision energies were optimized for every compound by infusion of 1 µg/mL standards solutions in methanol at 20 µL/min and by flow-injection analysis at the liquid chromatography flow rate. The main working parameters of the mass spectrometer are summarized in a previous publication (36).
LC/MS/MS procedure is based on an online extraction/cleaning of the injected samples with subsequent introduction into the mass spectrometer by using a built-in Valco switching valve. Four hundred microliters of the sample were injected onto the Supelco LC-18-DB (3.3 cm × 3.0 mm, 3.0-µm particle size) chromatographic column equipped with a Supelco Discovery C-18 (3.0-mm) guard column (Sigma-Aldrich, St. Louis, MO), where it underwent cleaning with 20% (v/v) methanol in 5 mM ammonium acetate pH 4.0 at flow rate of 0.8mL/min. After 4 minutes of cleaning the switching valve was activated, the column was flushed with a water/methanol gradient at a flow rate of 0.6 mL/min and the samples were introduced into the mass spectrometer. The gradient parameters and FT4 chromatogram are shown in a recent publication (36). The between-day and within-day precision was assessed at three different concentrations and yielded CVs ≤ 6.6% and ≤ 7.1% respectively.
Direct analog FT4, TT4, and equilibrium dialysis measurement
The Dade Dimension RXL was used for both the TT4 and the FT4 direct analog immunoassay measurements (Dade-Behring Diagnostics, Glasgow, DE). Throughout the rest of this article direct analog immunoassay is abbreviated to simply immunoassay. Results on patient samples obtained during pregnancy were compared with values obtained using tandem mass spectrometry (n = 120). The Nichols FT4 kit (Nichols Institute Diagnostics, catalog no. 30–0652, San Clemente, CA) was used according to the directions provided by the manufacturer. This assay measures FT4 directly in the protein-free dialysate. A comparison between the equilibrium dialysis and the LC/MS/MS method was performed on a random subset of 51 samples from pregnant patients. There were insufficient sera remaining to measure FT4 by equilibrium dialysis in the nonpregnant subjects.
Statistical analysis
Descriptive statistics were calculated for each analyte in nonpregnant patients and in pregnant patients by trimester. Statistically significant changes in analytes for subjects during their successive trimesters of pregnancy were determined using linear regression models (p<0.05 was considered significant). At each of the collection points, values for FT4 were obtained via both immunoassay and LC/MS/MS. Additionally some samples were also assayed for FT4 by equilibrium dialysis. The trends for each analyte (TT4 by LC/MS/MS, FT4 by LC/MS/MS, FT4 by immunoassay, TSH) were graphed over the weeks of gestation, with typical nonpregnant clinical laboratory reference ranges shown as dashed lines. However, for the upper limit of TSH the value of 3.0 mIU/L was used instead. Some subjects were missing one analyte, so some graphs depict less than the full cohort of 98 pregnant patients. Immunoassay and LC/MS/MS results were compared using nonparametric correlation coefficients and simple x–y scatter plots. Results are presented for each trimester separately and for all trimesters together. A scatter plot was also used to display the comparison between FT4 by LC/MS/MS and equilibrium dialysis. Scatter plots utilized the same scale for the x and y axes that portray the two assays being compared. Thus, the line of perfect agreement between the assays (shown with a dashed line) extends from the bottom left of the graph to the top right. “Outlier values” were specifically not excluded from any of the analyses because of the previously documented substantial disagreement between values obtained by different assays (28–30,33). The relationship between FT4 and TSH was investigated using log-transformed TSH values.
Results
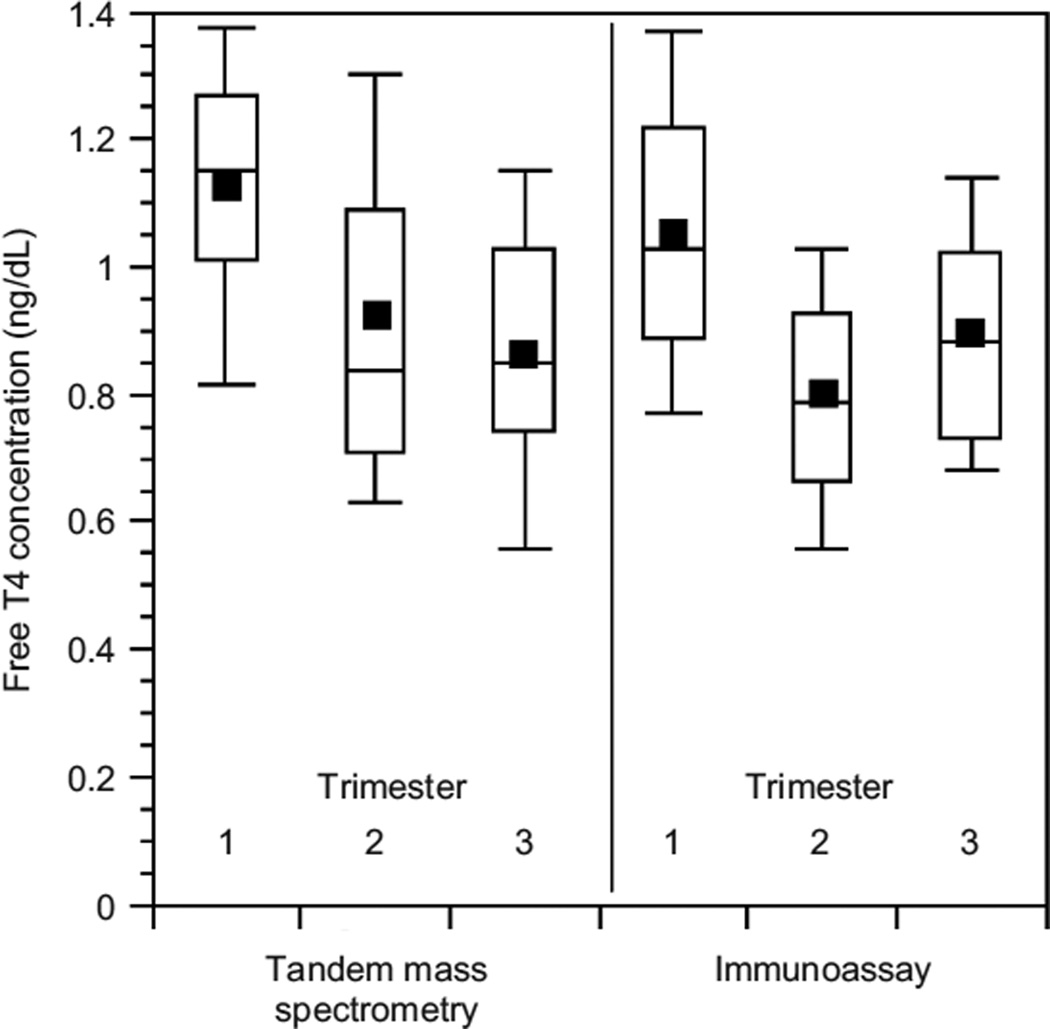
The demographic characteristics of the pregnant and non-pregnant subjects are shown in Table 1. All pregnant subjects were taking prenatal vitamins, were not taking other medications, and had a singleton gestation. Table 2 shows the mean value and standard error for all analytes in the nonpregnant state and for each of the trimesters of pregnancy. The mean, median, 10th, 25th, 75th, and 90th percentiles are shown for the FT4 concentrations measured by both LC/MS/MS and immunoassay in Figure 1. Formal FT4 reference intervals were not generated due to the small number of study participants, and the absence of data regarding thyroid autoimmunity.
Table 1.
The Demographic Characteristics of Study Subjects
| Characteristics | Pregnant | Nonpregnant |
|---|---|---|
| Number | 98 | 29 |
| Mean age (years) | 33 | 37 |
| Ethnic group/race | ||
| Caucasian (%) | 56 | 75 |
| African American (%) | 31 | 11 |
| Hispanic (%) | 2 | 0 |
| Asian (%) | 11 | 14 |
| Average weeks gestation | ||
| 1st trimester | 8.7 | |
| 2nd trimester | 17.8 | Not applicable |
| 3rd trimester | 28.7 |
Table 2.
Mean Values for all Analytes in Pregnant and NonPregnant Subjects*
| Nonpregnant | Pregnant |
|||
|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | ||
| Number of subjects | 29 | 59 | 35 | 26 |
| TT4 LC/MS/MS (µg/dL) | 7.12 ± 0.79 | 8.88 ± 2.67 | 10.67 ± 1.94a | 10.76 ± 2.00a |
| FT4 LC/MS/MS (ng/dL) | 0.93 ± 0.25 | 1.13 ± 0.23 | 0.92 ± 0.30a | 0.86 ± 0.21ab |
| FT4 IA ng/dL | 1.10 ± 0.25 | 1.05 ± 0.22 | 0.88 ± 0.17a | 0.89 ± 0.17a |
| TSH (MU/L) | 1.73 ± 1.13 | 1.13 ± 0.69 | 1.13 ± 0.54c | 1.04 ± 0.61d |
Values reported are mean ± standard error.
Significantly different from first trimester (p < 0.001).
Significantly different from second trimester (p < 0.001).
Not significantly different from first trimester.
Not significantly different from second trimester.
FT4, free thyroxine; IA, immunoassay; LC/MS/MS, isotope dilution liquid chromatography tandem mass spectrometry; TSH, thyrotropin; TT4, total thyroxine.
FIG. 1.
Box plot representation of the descriptive statistics for free thyroxine (FT4); measured by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) (the square depicts the mean, the line shows the median, the top and bottom of the box represent the 75% and 25% percentile respectively, and the whiskers show the 90th and 10th percentile).
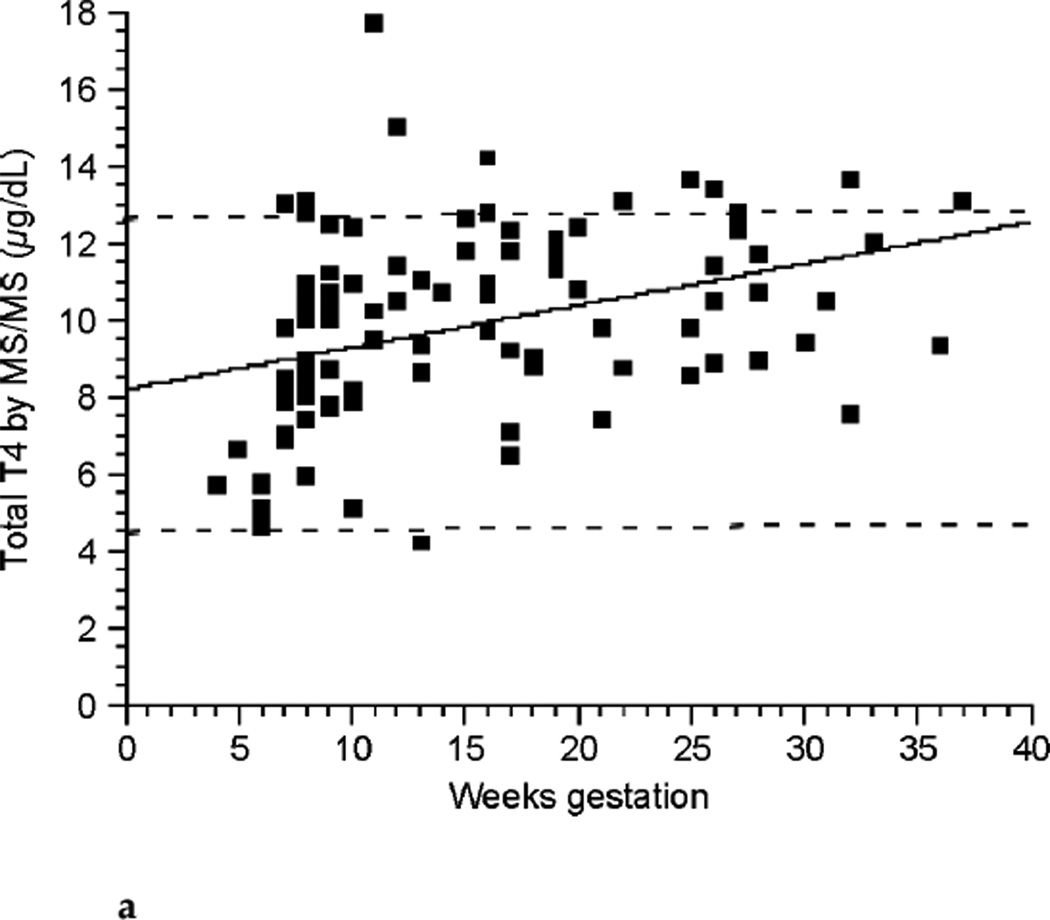
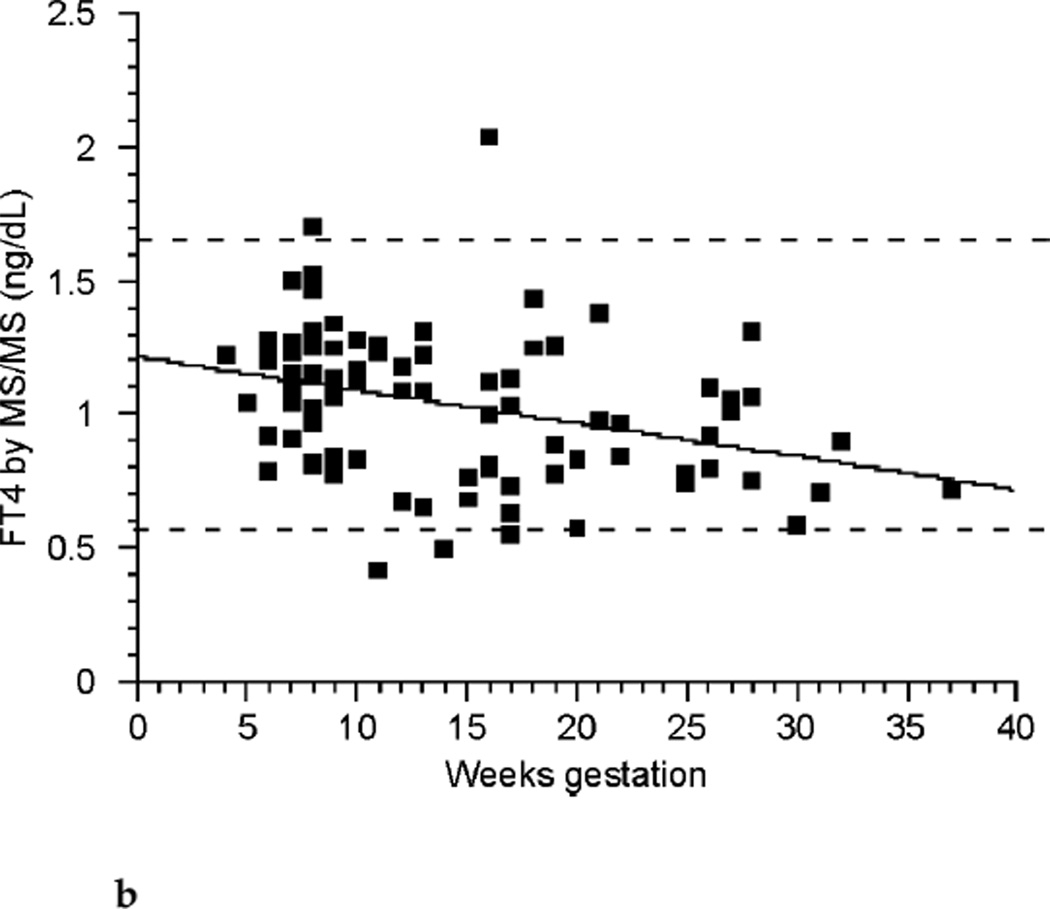
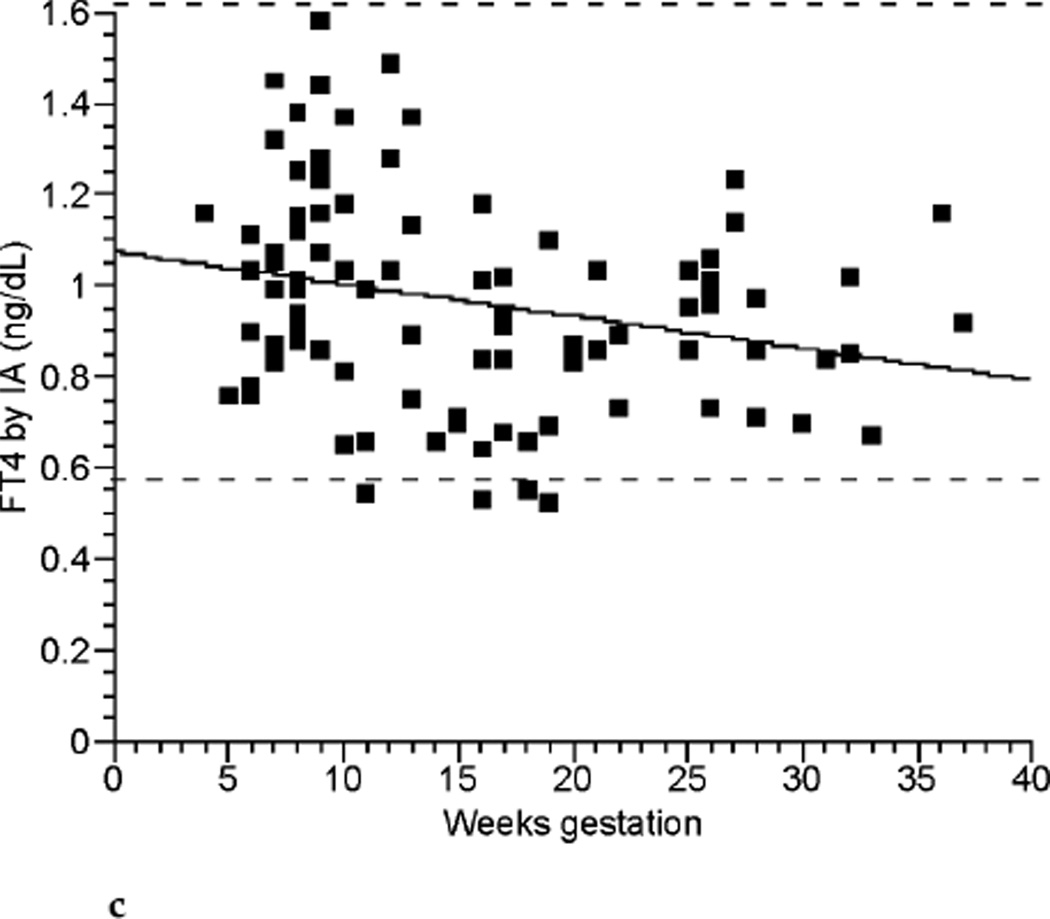
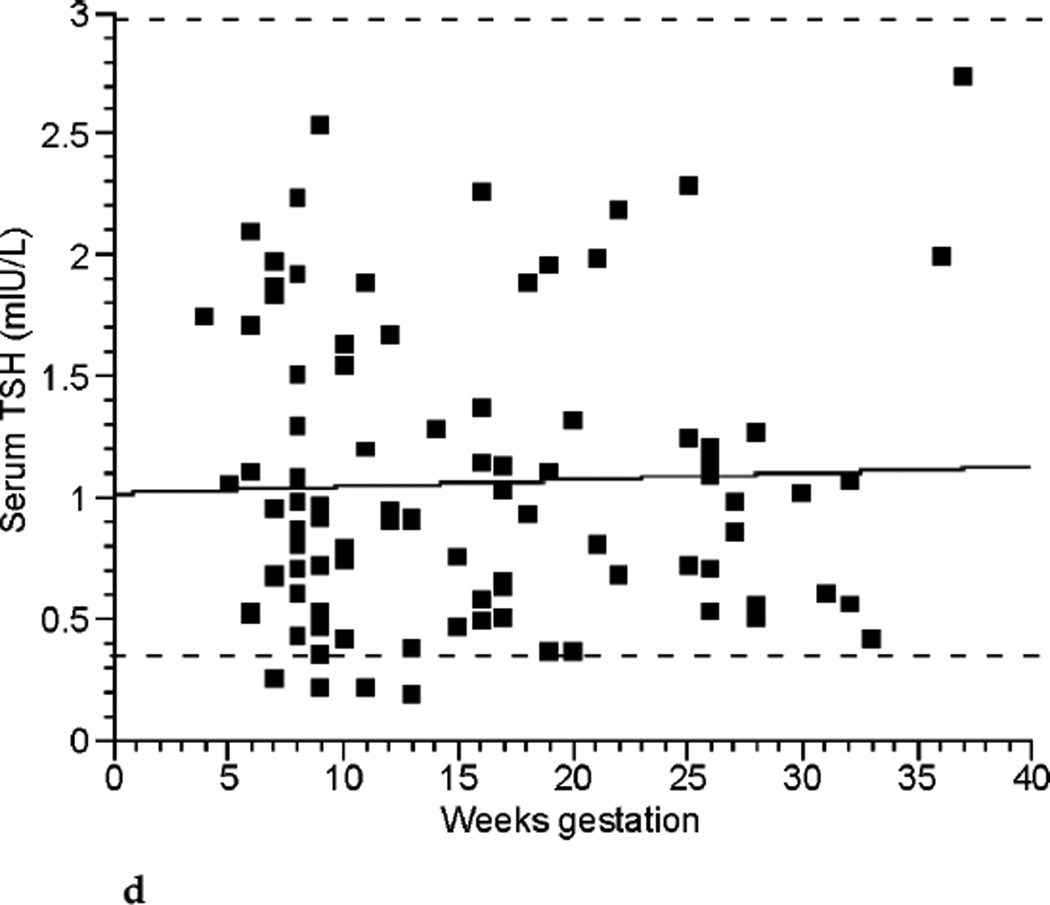
The relationships between TT4, FT4 by LC/MS/MS, FT4 by immunoassay, and TSH across the weeks of gestation are shown in Figure 2a, Figure 2b, Figure 2c, and Figure 2d respectively. TT4 levels increased as gestation progressed from the first to second trimester (p< 0.001). TT4 increased to approximately 120% of its first trimester value by the end of the second trimester, and to approximately 122% of its initial value by the end of the third trimester. FT4 decreased with each successive trimester when measured by LC/MS/MS. However, when meaen measured by immunoassay, FT4 decreased between the first and second trimester only (p <0.001). TSH remained statistically unchanged across trimesters.
FIG. 2a.
Total thyroxine (TT4) by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) (ng/dL) across gestational weeks of pregnancy (n ≤ 98).
FIG. 2b.
Free thyroxine (FT4) by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) (ng/dL) across gestational weeks of pregnancy (n ≤ 98).
FIG. 2c.
Free thyroxine (FT4) by immunoassay (IA) (ng/dL) across gestational weeks of pregnancy (n ≤ 98).
FIG. 2d.
Thyrotropin (TSH) (mUI/L) across gestational weeks of pregnancy (n ≤ 98).
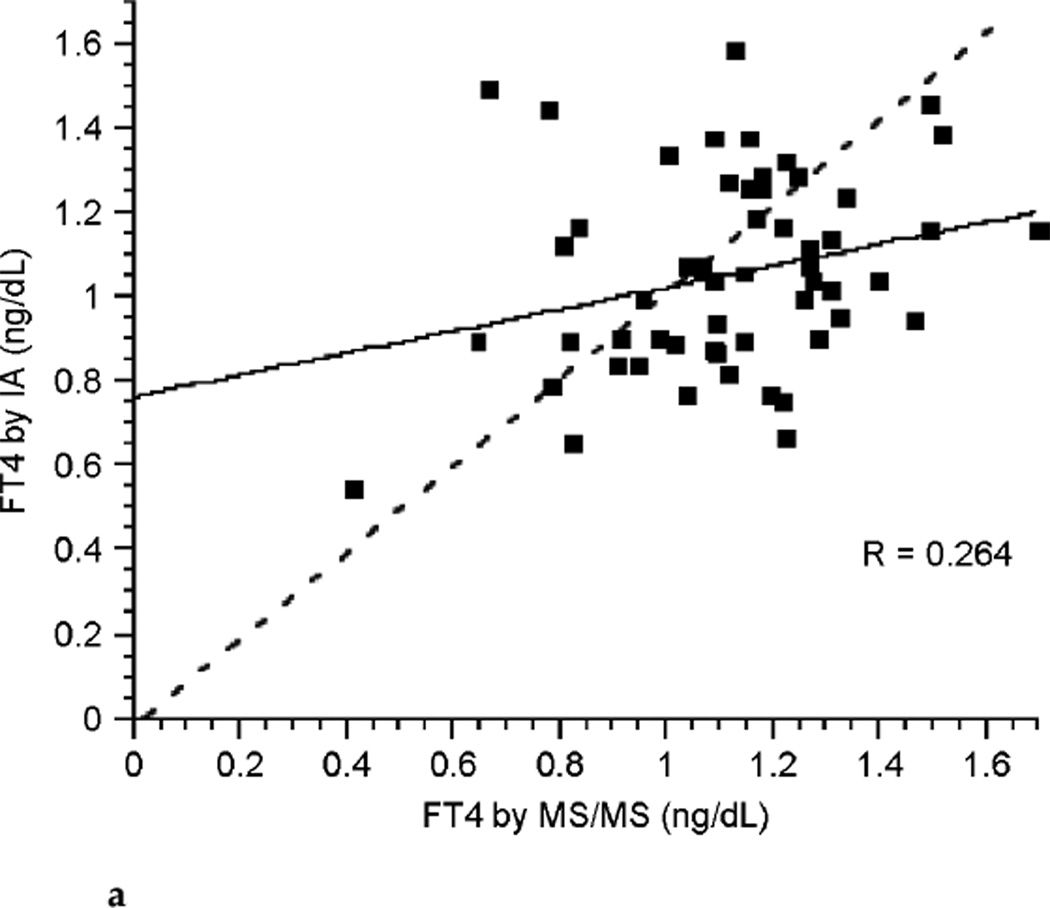
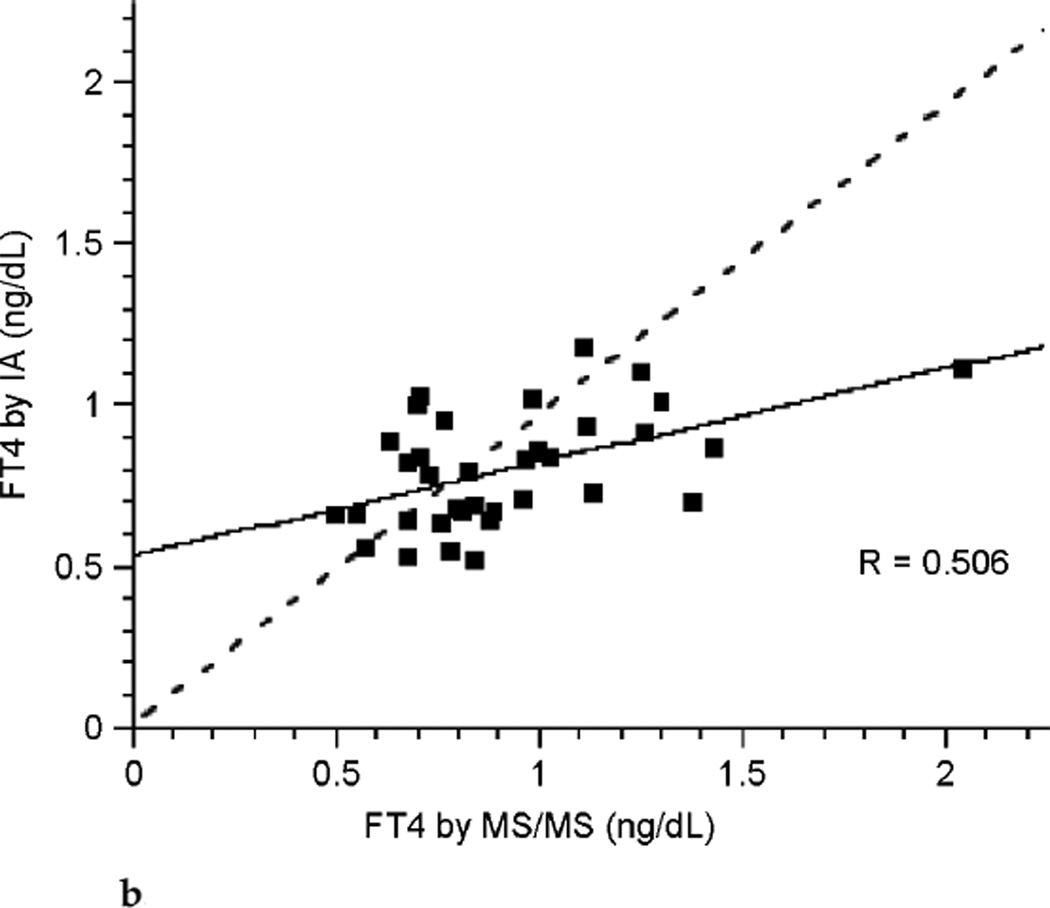
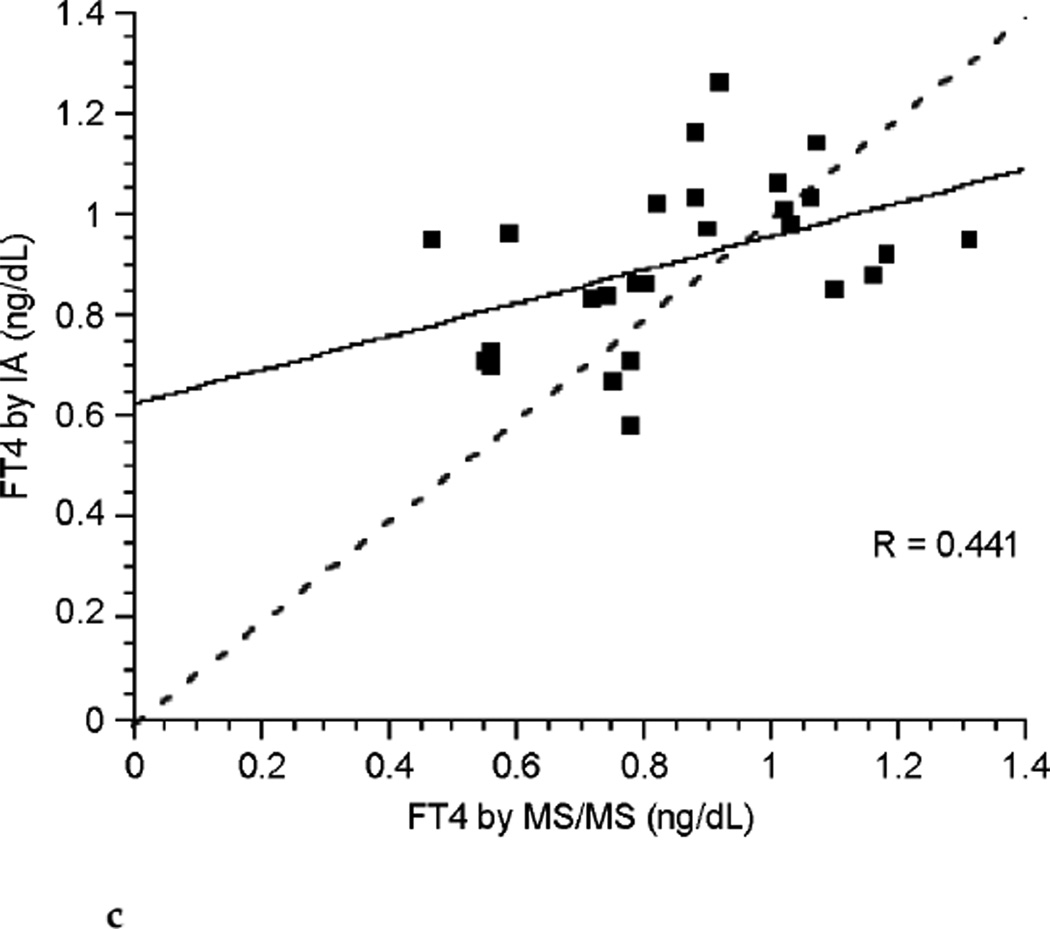
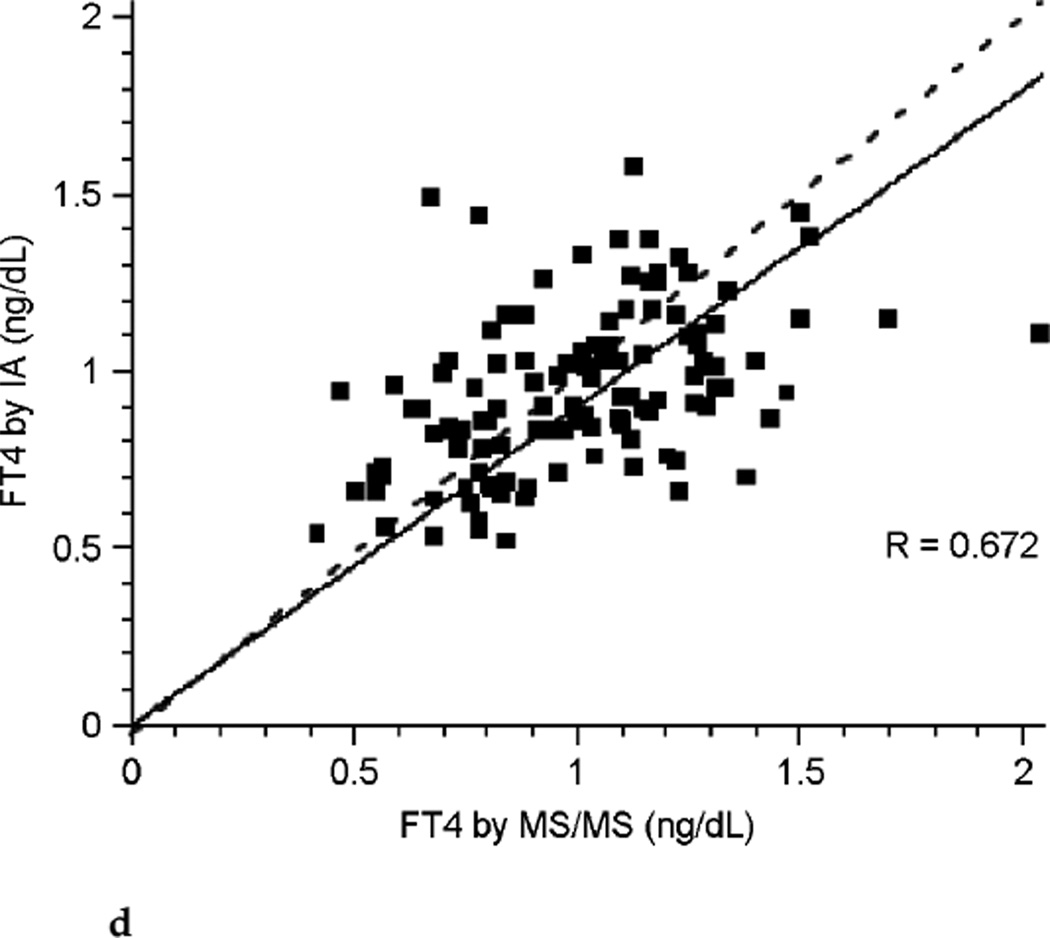
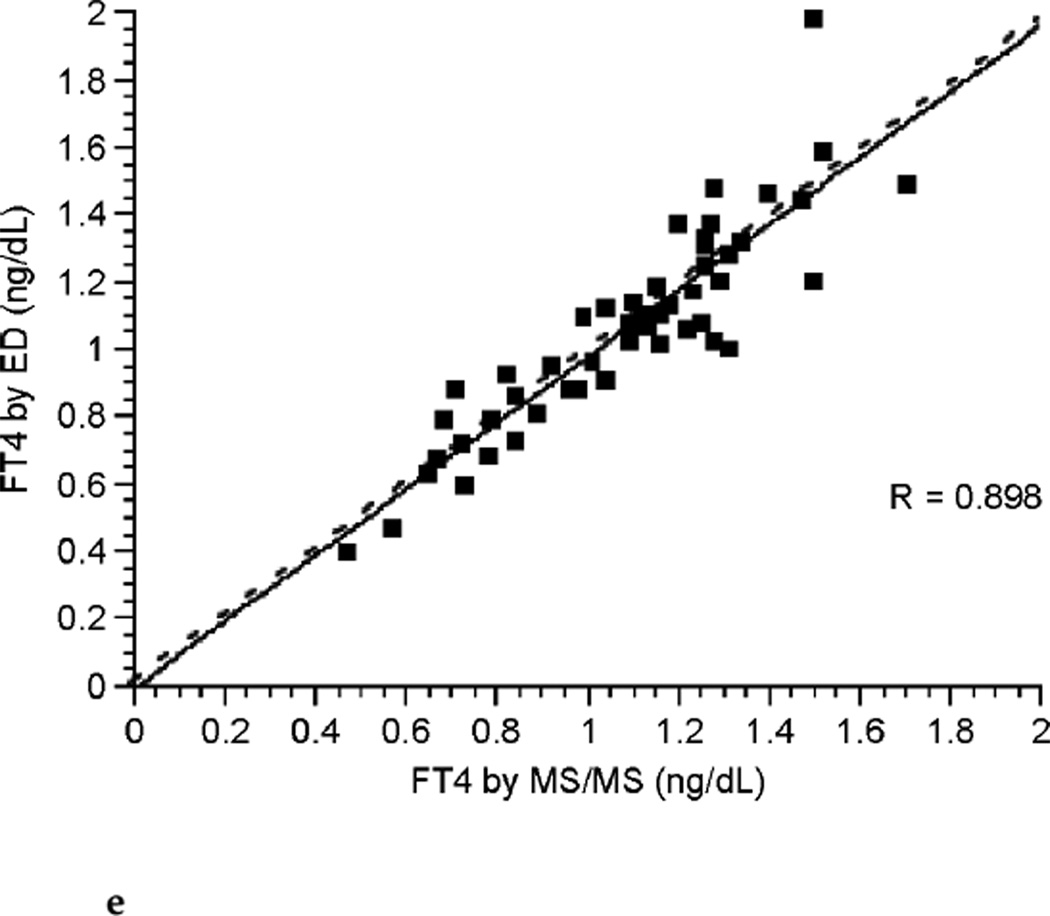
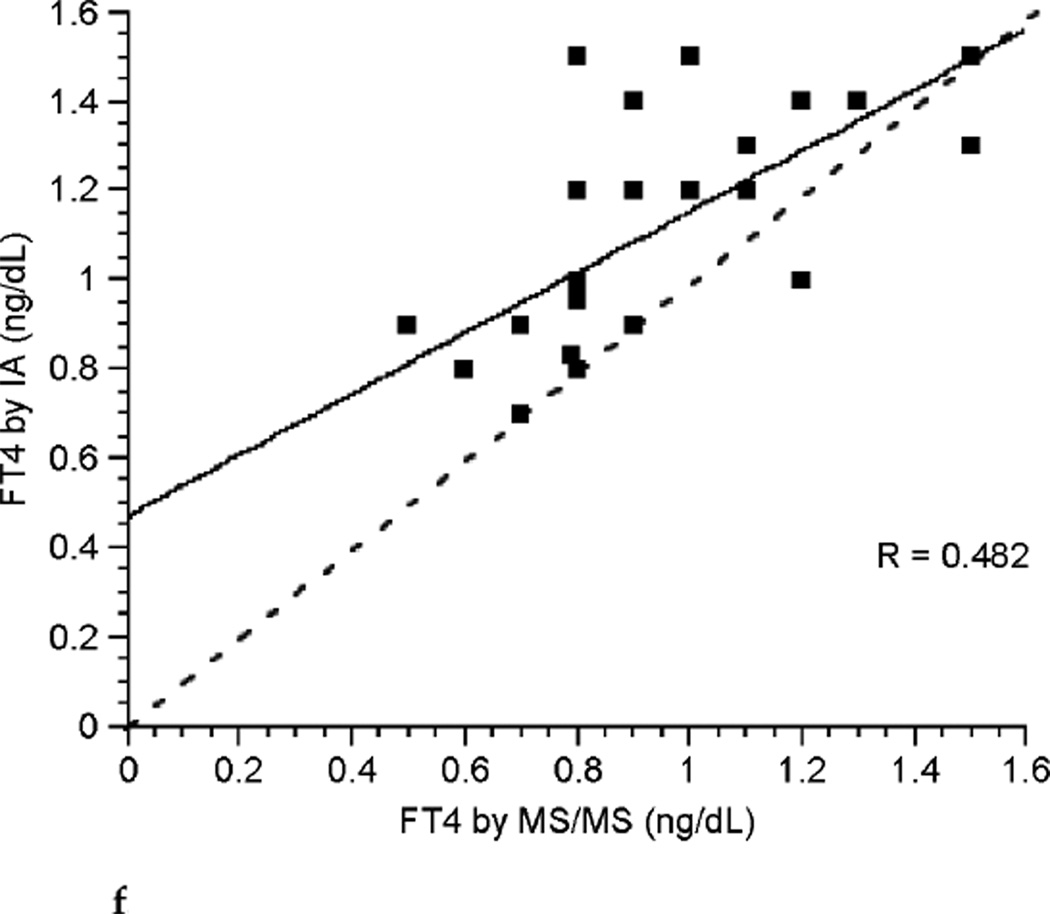
The correlation between FT4 measured by LC/MS/MS and immunoassay was poor during each of the three trimesters of pregnancy (see Fig. 3a, Fig. 3b, and Fig. 3c). Correlation coefficients were 0.265, 0.506, and 0.441 for each successive trimester respectively. Thus, agreement between these two assays was slightly better during the latter two-thirds of pregnancy. The correlation between assays was marginally improved for the group of pregnant patients as a whole with a correlation coefficient of 0.672 (see Fig. 3d), perhaps in part because of the larger sample size. In contrast, there was excellent agreement between the LC/MS/MS assay and equilibrium dialysis during pregnancy (see Fig. 3e). The correlation coefficient was 0.898. In nonpregnant patients the relationship between FT4 measured by LC/MS/MS and immunoassay was also poor (see Fig. 3f). The values for the LC/MS/MS value were both higher and lower than those obtained by immunoassay during pregnancy. However, LC/MS/MS readings were generally lower than immunoassay values in nonpregnant patients, with values for immunoassay mostly falling above the line presenting “perfect agreement” (see Fig. 3f).
FIG. 3a.
Correlation between free thyroxine (FT4) measured by immunoassay (IA) and isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS)during the first trimester of pregnancy (dashed line represents “perfect agreement”) (n ≤ 59).
FIG. 3b.
Correlation between free thyroxine (FT4) measured by immunoassay (IA) and isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) during the second trimester of pregnancy (dashed line represents “perfect agreement”) (n ≤ 35).
FIG. 3c.
Correlation between free thyroxine (FT4) measured by immunoassay (IA) and isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS)during the third trimester of pregnancy (dashed line represents “perfect agreement”) (n ≤ 26).
FIG. 3d.
Correlation between free thyroxine (FT4) measured by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) and immunoassay (IA) during all trimesters of pregnancy (dashed line represents “perfect agreement”) (n ≤ 98).
FIG. 3e.
Correlation between free thyroxine (FT4) measured by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) and equilibrium dialysis (ED) during all trimesters of pregnancy (dashed line represents “perfect agreement”) (n = 51).
FIG. 3f.
Correlation between free thyroxine (FT4) measured by isotope dilution liquid chromatography tandem mass spectrometry(LC/MS/MS)and immunoassay (IA) in nonpregnant patients (dashed line represents “perfect agreement”) (n = 28).
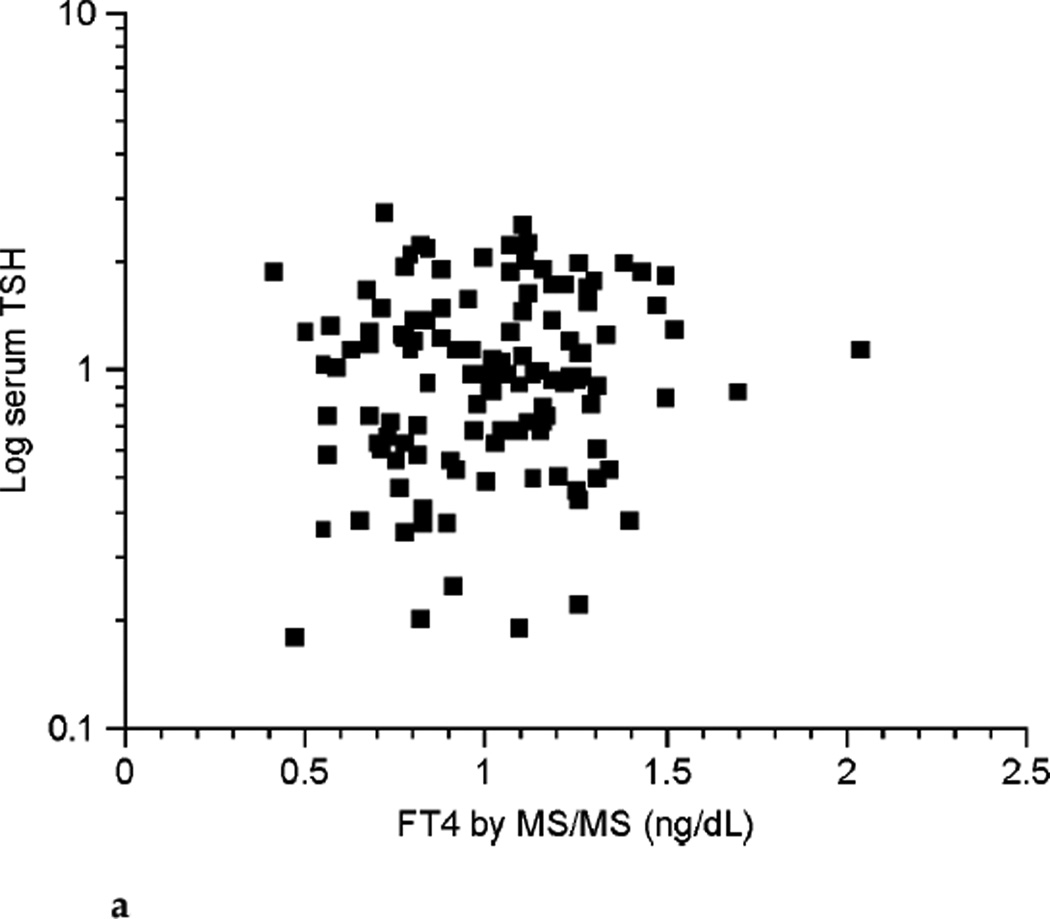
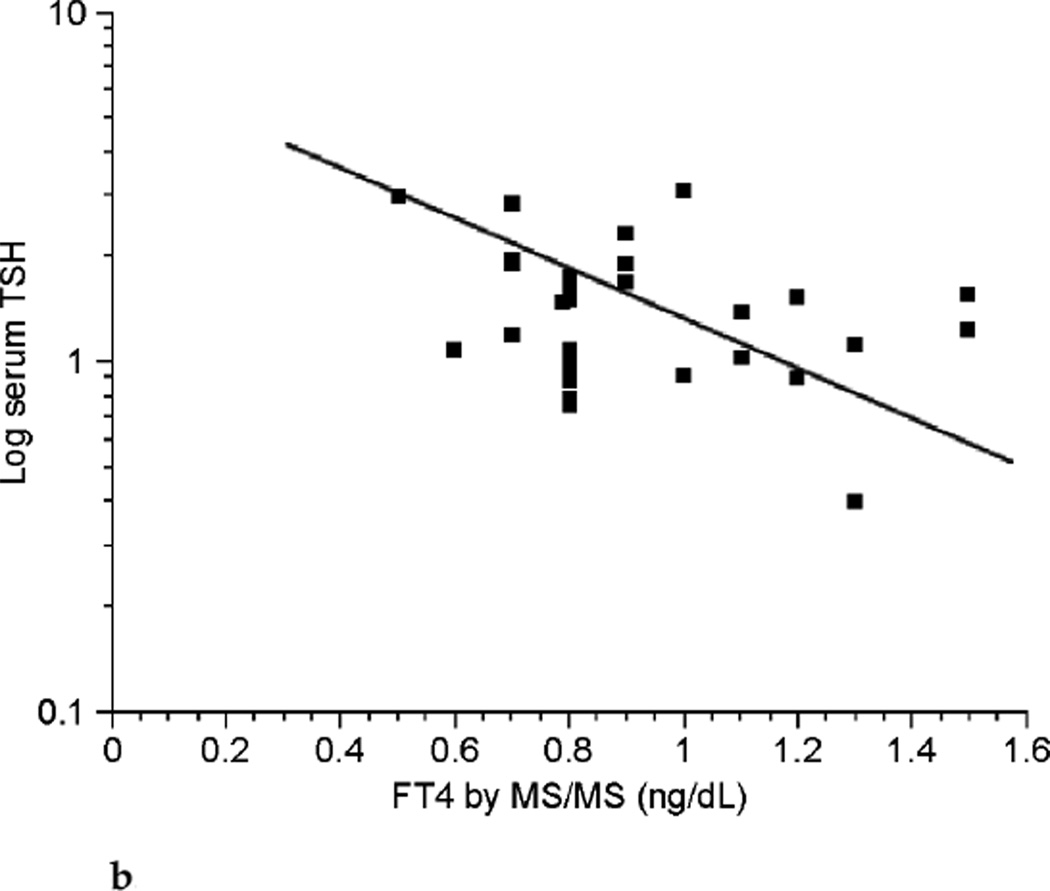
With the numbers of patients in this study no clear relationship could be shown between FT4 and log-transformed serum TSH in pregnancy (see Fig. 4a). However, despite the small number of patients there appeared to be an inverse relationship between the two parameters in the nonpregnant state (see Fig. 4b).
FIG. 4a.
Relationship between free thyroxine (FT4) by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) and log-transformed thyrotropin (TSH) values during pregnancy (n ≤ 98).
FIG. 4b.
Relationship between free thyroxine (FT4) by isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) and log-transformed thyrotropin (TSH) values in nonpregnant patients (n = 28).
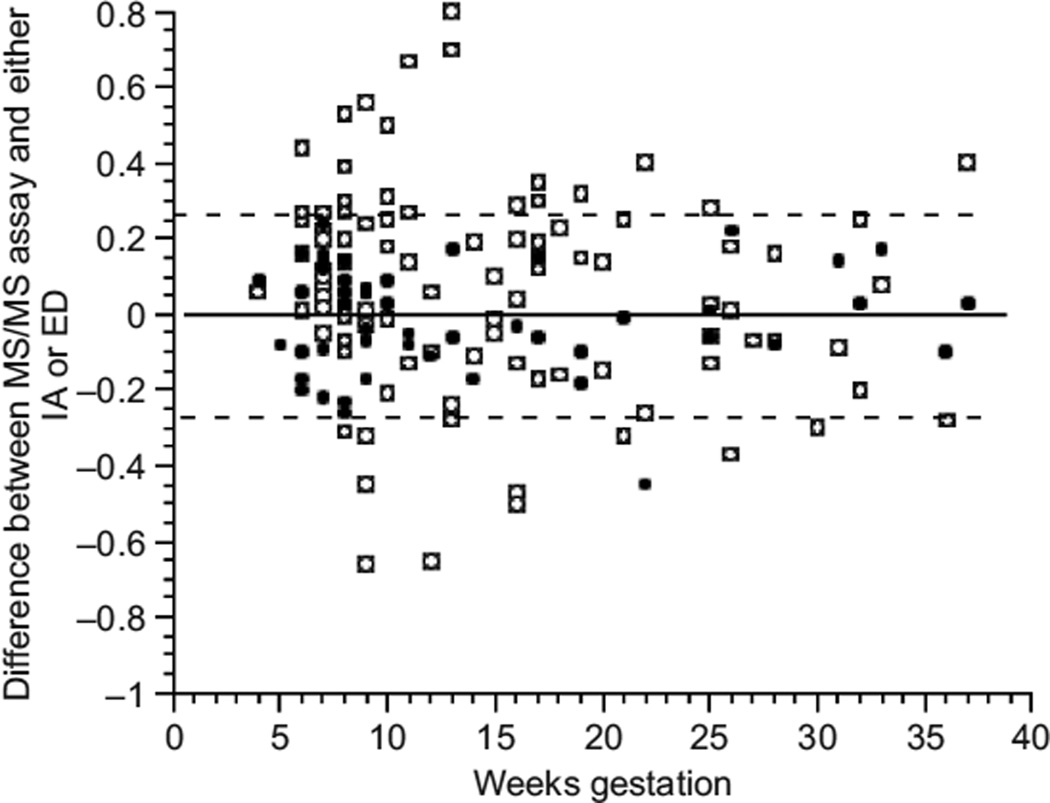
Figure 5 shows both the difference between the immunoassay and LC/MS/MS assays and the difference between the equilibrium dialysis and LC/MS/MS assays across the weeks of pregnancy. The disagreement between the immunoassay and LC/MS/MS was generally greater during the first trimester. However, the good agreement between equilibrium dialysis and LC/MS/MS persisted across the successive trimesters of pregnancy.
FIG. 5.
Difference between mass spectrometry (LC/MS/MS) and immunoassay (IA) values and between LC/MS/MS and equilibrium dialysis (ED) values across gestational weeks of pregnancy (open squares show the difference between isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) and IA; filled circles show the difference between LC/MS/MS and ED; dashed lines encompass all LC/MS/MS and ED differences except one outlier value).
Discussion
In agreement with many other studies, we observed that TT4 increased as pregnancy progressed. TT4 levels during pregnancy have been well studied both in countries with iodine sufficiency and iodine insufficiency and their values generally plateau during the second and third trimester at about 150% of their prepregnancy values (3,4,6,7,10,11,37– 39). The magnitude of the increase in this study was similar to that reported in previous studies. The nonpregnant and pregnant groups, however, were comprised of different individuals. Nevertheless, peak TT4 levels during pregnancy were still 150% of nonpregnancy levels.
Also, in agreement with other studies, we found that FT4 levels decreased as pregnancy progressed, while still remaining within the normal nonpregnant reference intervals for the commonly used clinical laboratories. The largest decline in FT4 concentration occurred as subjects progressed from the first to the second trimester. FT4 then did not change with progression into the third trimester when measured by immunoassay. However, regression models used to determine if FT4 differed by subject across trimester showed a further small decline in FT4 when this analyte was measured by LC/MS/MS.
However, in contrast to other studies, this study did not demonstrate a decreased serum TSH during the first trimester of pregnancy, or increasing TSH levels during the trimesters thereafter. Instead, we observed that TSH levels were relatively constant across pregnancy. We could find no obvious explanation for this unusual pattern. Certainly our sample size is relatively small. Our pregnant patient group was 31% African American. National Health and Nutrition Examination Survey (NHANES) III data suggested that African American females have lower serum TSH values than their white counterparts (14). Different references intervals for TSH levels have been suggested for different ethnic groups (13,15,16). However, our patient demographics would not be expected to mask the usual first trimester decline in serum TSH. It is possible, though not probable, that the first trimester blood draws were timed so that the usual TSH nadir was not captured. Blood draws were not scheduled at a specific time of day, so the known circadian rhythm in serum TSH could have affected our results (40,41). It is admittedly a weakness of our study that we did not check thyroid antibodies in our participants and also did not assess iodine status. All pregnant patients were supplemented with prenatal multivitamins, but information regarding the iodine content of the supplement was not collected.
With respect to measurements of FT4, the concentrations determined by LC/MS/MS do not agree with those determined by immunoassay. The disagreement is particularly marked during the first trimester of pregnancy. The concentrations were more in agreement in the third trimester and there was a little further improvement in the agreement in the second trimester. During pregnancy it appeared that the LC/MS/MS assay resulted in FT4 determinations that were both higher and lower than the immunoassay determinations. Thus, data points fell on both sides of the line depicting perfect agreement between the two assays (see Fig. 3a–3d). In contrast, a different pattern was seen in the non-pregnant patients, in whom LC/MS/MS determinations of FT4 concentrations were mostly lower than those resulting from immunoassay measurements (see Fig. 3f). It would thus seem that immunoassay-associated errors are different in the pregnant and nonpregnant state. During pregnancy both underreporting and overreporting of FT4 concentrations can occur, possibly due to a combination of protein binding-associated errors and heterophilic antibody–associated errors. It would be interesting to determine how these or other factors combine to produce the greatest disagreement between assays in the first trimester. In the nonpregnant patients, errors appeared to mostly be overreporting by immunoassay, possibly reflecting the greater specificity of LC/MS/MS technology.
When the discrepancy between LC/MS/MS and the other assays was visualized by plotting the difference between the two assays across the weeks of gestation (see Fig. 5), the greater disagreement between LC/MS/MS and immunoassay, with most disagreement occurring during the first trimester can be clearly appreciated. When LC/MS/MS per-MS performance was compared to that of the current gold standard equilibrium dialysis, there was excellent agreement seen in the pregnant patients. Not only was the disagreement between LC/MS/MS and equilibrium dialysis of a lesser magnitude, but also it was more constant across weeks of gestation. Thus, results obtained by LC/MS/MS measurement of FT4 appear to be equivalent to those obtained by equilibrium dialysis. We hypothesize that this closer agreement is achieved because LC/MS/MS methodology circumvents problems associated with protein binding and heterophilic antibodies. Presuming that equilibrium dialysis is an accurate assay for FT4, LC/MS/MS has a comparable accuracy based on its agreement with equilibrium dialysis.
The total time required for FT4 analysis in the method reported here was approximately 1.2 hours, which included a 1-hour centrifugation to obtain the ultrafiltrate. However, the second-generation method for FT4, which is currently in use in our bioanalytical laboratory, utilizes the more sensitive API-5000 tandem mass spectrometer. This requires only 150 µL of ultrafiltrate, which reduces the centrifugation time to 20 minutes, thus shortening the analysis time considerably. Thirty samples can be batched for simultaneous centrifugation. Measurement of FT4 by LC/MS/MS is, thus, less time consuming than measurement by equilibrium dialysis, and is comparable to the 15–30 minutes needed to perform an immunoassay. In addition to being much faster than equilibrium dialysis, LC/MS/MS is also less expensive once initial equipment costs have been covered. Mayo Clinic charges for measurement of FT4 by equilibrium dialysis are greater than $200, whereas Children’s National Medical Center charges $180 for the LC/MS/MS assay. Equipment costs of approximately $500,000 are paid off with approximately 3,500 assays. Furthermore, assays can be rapidly performed and a large volume of samples can be processed, resulting in an assay with high throughput. Measurement of FT4 and free tri-iodothymorine by LC/MS/MS is currently in routine clinical use at Children’s National Medical Center in Washington, D.C. It will be introduced shortly at Boston Children’s Medical Center. Tandem mass spectrometers are readily available in the market place and are already in use by many commercial laboratories for measuring analytes such as testosterone and 25-hydroxyvitamin D.
We believe that LC/MS/MS techniques will prove to be valuable in all clinical situations, but will be particularly invaluable in pregnancy, renal failure, autoimmune disease, and other specific disease states characterized by changes in protein binding or the presence of heterophilic antibodies. This assay is ideally suited for generating reliable, reproducible trimester-specific reference intervals for FT4. We concur with the proposals to document trimester-specific reference intervals for thyroid analytes based on large groups of women without evidence of thyroid disease (2,3,8,31,42). Furthermore, we propose that LC/MS/MS should be used for this purpose.
In conclusion, our data demonstrate the excellent agreement of LC/MS/MS with the gold standard equilibrium dialysis assay, but poor correlation with the notoriously inaccurate immunoassay. We believe that LC/MS/MS is the assay of choice for all clinical situations, because its superior performance provides more relevant FT4 values for use in clinical decision-making. Our current assay is eminently practical for clinical use because of its high throughput, ability to handle a large number of samples, and requirement for small sample size. LC/MS/MS has, in fact, already been shown to have better specificity for measurement of steroids (43) and vitamin D (44) and is at present the assay used by several commercial laboratories for measuring these analytes.
Acknowledgments
This work was supported in part by grant M01 RR 013297 from the National Center for Research Resources. Jacqueline Jonklaas is supported by National Center for Research Resources grant K23 RR16524. Offie Soldin is supported in part by NIH/NICHD grant 5U10HD047890-03 and by the Office of Research on Women’s Health grant 5U10HD047890-03 Obstetrics and Pharmacology Research Unit. Steven Soldin is partially supported by NIH GCRC grant number MO1-RR-020359 and by Applied Biosystems/Sciex.
References
- 1.Soldin OP. Thyroid function testing in pregnancy and thyroid disease: trimester-specific reference intervals. Ther Drug Monit. 2006;28:8–11. doi: 10.1097/01.ftd.0000194498.32398.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach-Huynh TG, Jonklaas J. Thyroid medications during pregnancy. Ther Drug Monit. 2006;28:431–441. doi: 10.1097/01.ftd.0000211834.41844.82. [DOI] [PubMed] [Google Scholar]
- 3.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–1090. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weeke J, Dybkjaer L, Granlie K, et al. A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol (Copenh) 1982;101:531–537. doi: 10.1530/acta.0.1010531. [DOI] [PubMed] [Google Scholar]
- 5.Soldin OP, Tractenberg RE, Soldin SJ. Differences between measurements of T4 and T3 in pregnant and nonpregnant women using isotope dilution tandem mass spectrometry and immunoassays: are there clinical implications? Clin Chim Acta. 2004;347:61–69. doi: 10.1016/j.cccn.2004.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 7.Liberman CS, Pino SC, Fang SL, Braverman LE, Emerson CH. Circulating iodide concentrations during and after pregnancy. J Clin Endocrinol Metab. 1998;83:3545–3549. doi: 10.1210/jcem.83.10.5163. [DOI] [PubMed] [Google Scholar]
- 8.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38:329–332. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 9.Demers LM, Spencer CA. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:19–56. doi: 10.1046/j.1365-2265.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 10.Berghout A, Endert E, Ross A, Hogerzeil HV, Smits NJ, Wiersinga WM. Thyroid function and thyroid size in normal pregnant women living in an iodine replete area. Clin Endocrinol (Oxf) 1994;41:375–379. doi: 10.1111/j.1365-2265.1994.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 11.Ballabio M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73:824–831. doi: 10.1210/jcem-73-4-824. [DOI] [PubMed] [Google Scholar]
- 12.Soldin OP, Hilakivi-Clarke L, Weiderpass E, Soldin SJ. Trimester-specific reference intervals for thyroxine and tri-iodothyronine in pregnancy in iodine-sufficient women using isotope dilution tandem mass spectrometry and immunoassays. Clin Chim Acta. 2004;349:181–189. doi: 10.1016/j.cccn.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price A, Obel O, Cresswell J, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. 2001;308:91–98. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 14.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination-Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 15.Price A, Davies R, Heller SR, Milford-Ward A, Weetman AP. Asian women are at increased risk of gestational thyrotoxicosis. J Clin Endocrinol Metab. 1996;81:1160–1163. doi: 10.1210/jcem.81.3.8772593. [DOI] [PubMed] [Google Scholar]
- 16.Walker JA, Illions EH, Huddleston JF, Smallridge RC. Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet Gynecol. 2005;106:1365–1371. doi: 10.1097/01.AOG.0000185475.61612.ea. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus JH, Premawardhana LD. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449–452. doi: 10.1136/jcp.2004.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soldin OP, Tractenberg RE, Pezzullo JC. Do thyroxine and thyroid-stimulating hormone levels reflect urinary iodine concentrations? Ther Drug Monit. 2005;27:178–185. doi: 10.1097/01.ftd.0000149954.20089.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 20.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 21.Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 22.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 23.Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA. Iodine status, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–355. doi: 10.1038/sj.ejcn.1600563. [DOI] [PubMed] [Google Scholar]
- 24.Price A, Griffiths H, Morris BW. A longitudinal study of thyroid function in pregnancy. Clin Chem. 1989;35:275–278. [PubMed] [Google Scholar]
- 25.Ball R, Freedman DB, Holmes JC, Midgley JE, Sheehan CP. Low-normal concentrations of free thyroxin in serum in late pregnancy: physiological fact, not technical artefact. Clin Chem. 1989;35:1891–1896. [PubMed] [Google Scholar]
- 26.Burrow GN. Thyroid function and hyperfunction during gestation. Endocr Rev. 1993;14:194–202. doi: 10.1210/edrv-14-2-194. [DOI] [PubMed] [Google Scholar]
- 27.Sapin R, D’Herbomez M, Schlienger JL. Free thyroxine measured with equilibrium dialysis and nine immunoassays decreases in late pregnancy. Clin Lab. 2004;50:581–584. [PubMed] [Google Scholar]
- 28.Roti E, Gardini E, Minelli R, Bianconi L, Flisi M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J Endocrinol Invest. 1991;14:1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
- 29.d’Herbomez M, Forzy G, Gasser F, Massart C, Beaudonnet A, Sapin R. Clinical evaluation of nine free thyroxine assays: persistent problems in particular populations. Clin Chem Lab Med. 2003;41:942–947. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Nelson JC, Weiss RM, Wilcox RB. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10:31–39. doi: 10.1089/thy.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 31.Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44–53. doi: 10.1089/thy.2005.15.44. [DOI] [PubMed] [Google Scholar]
- 32.Ekins R. Validity of analog free thyroxin immunoassays. Clin Chem. 1987;33:2137–2144. [PubMed] [Google Scholar]
- 33.Steele BW, Wang E, Klee GG, et al. Analytic bias of thyroid function tests: analysis of a College of American Pathologists fresh frozen serum pool by 3900 clinical laboratories. Arch Pathol Lab Med. 2005;129:310–317. doi: 10.5858/2005-129-310-ABOTFT. [DOI] [PubMed] [Google Scholar]
- 34.Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44:440–454. [PubMed] [Google Scholar]
- 35.Fiad TM, Duffy J, McKenna TJ. Multiple spuriously abnormal thyroid function indices due to heterophilic antibodies. Clin Endocrinol. 1994;41:391–395. doi: 10.1111/j.1365-2265.1994.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 36.Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358:113–118. doi: 10.1016/j.cccn.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitworth AS, Midgley JE, Wilkins TA. A comparison of free T4 and the ratio of total T4 to T4-binding globulin in serum through pregnancy. Clin Endocrinol (Oxf) 1982;17:307–313. doi: 10.1111/j.1365-2265.1982.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 38.Nissim M, Giorda G, Ballabio M, et al. Maternal thyroid function in early and late pregnancy. Horm Res. 1991;36:196–202. doi: 10.1159/000182160. [DOI] [PubMed] [Google Scholar]
- 39.Kung AW, Lao TT, Chau MT, Tam SC, Low LC. Goitrogenesis during pregnancy and neonatal hypothyroxinaemia in a borderline iodine sufficient area. Clin Endocrinol (Oxf ) 2000;53:725–731. doi: 10.1046/j.1365-2265.2000.01156.x. [DOI] [PubMed] [Google Scholar]
- 40.Scobbo RR, VonDohlen TW, Hassan M, Islam S. Serum TSH variability in normal individuals: the influence of time of sample collection. West Virginia Med J. 2004;100:138–142. [PubMed] [Google Scholar]
- 41.Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–139. [PubMed] [Google Scholar]
- 42.Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 43.Minutti CZ, Lacey JM, Magera MJ, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–3693. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- 44.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]