SUMMARY
Pesticides are important agricultural tools often used in combination to avoid resistance in target pest species, but there is growing concern that their widespread use contributes to the decline of pollinator populations. Pollinators perform sophisticated behaviours while foraging that require them to learn and remember floral traits associated with food, but we know relatively little about the way that combined exposure to multiple pesticides affects neural function and behaviour. The experiments reported here show that prolonged exposure to field-realistic concentrations of the neonicotinoid imidacloprid and the organophosphate acetylcholinesterase inhibitor coumaphos and their combination impairs olfactory learning and memory formation in the honeybee. Using a method for classical conditioning of proboscis extension, honeybees were trained in either a massed or spaced conditioning protocol to examine how these pesticides affected performance during learning and short- and long-term memory tasks. We found that bees exposed to imidacloprid, coumaphos, or a combination of these compounds, were less likely to express conditioned proboscis extension towards an odor associated with reward. Bees exposed to imidacloprid were less likely to form a long-term memory, whereas bees exposed to coumaphos were only less likely to respond during the short-term memory test after massed conditioning. Imidacloprid, coumaphos and a combination of the two compounds impaired the bees' ability to differentiate the conditioned odour from a novel odour during the memory test. Our results demonstrate that exposure to sublethal doses of combined cholinergic pesticides significantly impairs important behaviours involved in foraging, implying that pollinator population decline could be the result of a failure of neural function of bees exposed to pesticides in agricultural landscapes.
KEY WORDS: pesticide, Apis mellifera, long-term memory, pollinator decline, acetylcholine, imidacloprid
INTRODUCTION
In the last 20 years, pesticide use has shifted away from organophosphates and carbamates towards neonicotinoid compounds that are agonists of insect nicotinic acetylcholine receptors (nAChRs) (Buckingham et al., 1997; Elbert et al., 2008; Ihara et al., 2006). Unfortunately, because they are systemic insecticides that persist in plants throughout the growing season, they affect non-target organisms such as pollinators. For example, pollen and nectar that are collected and eaten by pollinators often contain these pesticides, even when the plant was only exposed to neonicotinoids as a seed treatment (Halm et al., 2006; Rortais et al., 2005). The extent to which neonicotinoids are implicated in pollinator population decline, however, is controversial (Maxim and van der Sluijs, 2010); some pollinators, such as honeybees, also experience stress from infestation with parasites and pathogens such as Varroa destructor and Nosema spp. (Dainat et al., 2012; Le Conte et al., 2010).
Neonicotinoids often affect non-target organisms through prolonged sub-lethal exposure (Halm et al., 2006) and may have even larger effects on survival when combined with exposure to other agrochemicals (Wu et al., 2011) or other forms of stress. Honeybees are likely to be exposed to additional potentially harmful chemicals during treatment for the mite V. destructor. For example, mite treatments are often themselves potent pesticides, like the organophosphate coumaphos (Mullin et al., 2010; Rosenkranz et al., 2010). This particular combination is of interest because of the potential for additive effects when the two compounds are administered simultaneously, as both neonicotinoids and coumaphos target cholinergic signalling. The targets of neonicotinoid pesticides, nAChRs, play an important role in honey bee learning and memory processes vital to successful foraging behaviour (Gauthier, 2010). Both acute and chronic administration of the neonicotinoid imidacloprid impairs olfactory learning and memory (Decourtye et al., 2004a; Decourtye et al., 2004b) probably as a result of a change in the way that neurons in the honeybee's mushroom bodies function (Gauthier, 2010). The organophosphate acetylcholinesterase (AChE) inhibitor coumaphos (commercially known as Checkmite) is used as a miticide in honeybee colonies but could potentially harm bees as well as their parasites (Hawthorne and Dively, 2011). The combination of two pesticides could be more toxic and have stronger effects on behaviour than exposure to a single compound because the same mechanisms are used to detoxify both, notably the p-glycoprotein xenobiotic efflux transporters and the cytochrome P450 monooxygenase enzymes (Johnson et al., 2009). Whether prolonged exposure to imidacloprid or other pesticides and their combinations has a stronger effect on learning and memory in bees or other pollinators is unknown (Biernaskie et al., 2009).
Efficient foraging by bees depends on their ability to rapidly learn, remember and communicate the identity and location of flowers offering nectar and pollen rewards (Biernaskie et al., 2009; Lihoreau et al., 2011). Substances such as cholinergic pesticides could have a profound influence on the bee's ability to forage successfully via their effects on learning and memory. A previous study of learning in bees demonstrated that bees subjected to spaced conditioning (intervals of 3 min or longer between trials) were more likely to form long-term olfactory memories than bees subjected to conditioning with shorter intervals (Menzel et al., 2001). In Drosophila, olfactory learning and memory acquired during spaced learning arise from different molecular mechanisms from those produced by massed conditioning (Isabel et al., 2004; Pagani et al., 2009). Whether cholinergic pesticides affect massed and spaced learning differently has not yet been tested.
Based on results from previous studies (Decourtye et al., 2004a; Decourtye et al., 2004b), we predicted that learning and memory would be impaired in honeybees subjected to prolonged exposure to sublethal doses of cholinergic pesticides and that the combination of substances that target cholinergic signalling would have a stronger effect than either substance alone. We used imidacloprid, a systemic neonicotinoid found in pollen and nectar, and the mite treatment coumaphos, an organophosphate AChE inhibitor that accumulates in hive wax and food stores treated with this compound (Mullin et al., 2010; Rortais et al., 2005). We identified a range of sub-lethal doses that were also relevant to field exposure levels (Decourtye et al., 2004b; Mullin et al., 2010; Rortais et al., 2005). Using a classical conditioning assay for olfactory learning (Bitterman et al., 1983), we specifically compared performance during both massed and spaced learning assays with the aim of testing how disruption of cholinergic signalling affected performance during acquisition and during short- to mid-term memory (STM) and early long-term memory (eLTM) recall tests (Menzel et al., 2001).
MATERIALS AND METHODS
Honeybees
Foraging adult worker honeybee colonies (Apis mellifera mellifera L.) were originally obtained from stock of the National Bee Unit (York, UK) and maintained at Newcastle University. Bees were collected in plastic vials at the colony entrance and placed on ice; when they stopped moving, they were immediately transferred to small plastic boxes where they were treated with pesticides as described below.
Pesticides
Imidacloprid and coumaphos were obtained in dry powder form (>99% purity, Sigma-Aldrich, St Louis, MO, USA). Solutions of imidacloprid, coumaphos and a combination of the two drugs were made to concentrations of 1 μmol l−1, 100 nmol l−1 and 10 nmol l−1. Imidacloprid was directly dissolved in 1 mol l−1 sucrose solution; however, coumaphos was first dissolved in DMSO to make a stock solution with a concentration of 10 mmol l−1 and then diluted with 1 mol l−1 sucrose. We used a concentration 0.001% DMSO after pilot studies indicated that concentrations less than 0.1% did not influence olfactory learning and memory. Fresh solutions were prepared weekly from frozen aliquots of the stock solutions.
Exposure to pesticides
Exposure to pesticides prior to the behavioural experiments was accomplished by adding pesticides to 1 mol l−1 sucrose solution and feeding it to adult workers ad libitum for 4 days prior to learning and memory experiments. Oral exposure was chosen to allow continuous, measurable exposure over 4 days; and although topical exposure to coumaphos may be more representative of its use as a mite treatment, both imidacloprid and coumaphos have been found in within-hive food stores, making oral administration a field-realistic exposure route (Mullin et al., 2010; Wu et al., 2011). After capture from the colony, cohorts of 20 honeybees were placed in plastic boxes (16.5×11×6.5cm) that had ventilation holes in the lid, and four holes in the sides to allow insertion of feeding tubes. Feeding tubes were made from 2 ml microfuge tubes with four, ~2 mm holes drilled along one side to allow the bees to insert their mouthparts into the feeding solution. The solution in each feeding tube was replenished daily. Control bees were fed 1 mol l−1 sucrose; pesticide treatment groups were fed 1 mol l−1 sucrose containing imidacloprid, coumaphos, or a combination of the two (see ‘Pesticides’ for information about concentrations). The bees were retained in the feeding boxes for 3 days prior to experimentation. On the 4th day, the entire cohort in each box was cold anaesthetized and each bee was transferred to a restraining harness as described previously (Wright et al., 2009). Each bee was allowed to recover for 1 h, fed 25 μl of the solution it had experienced for the previous 3 days, and left in a humidified plastic box at room temperature overnight. For the ‘reversal’ experiments, bees were fed the combined 100 nmoll−1 imidacloprid and 100 nmoll−1 coumaphos solution for 3 days, but for an additional 3 days afterwards, bees were fed 1 mol l−1 sucrose containing no pesticides, and were also fed 25 μl of uncontaminated sucrose solution after harnessing and training.
All treatments were administered to cohorts of 15–25 bees each week, and the surviving bees from all treatment groups were trained and tested in parallel. This process was repeated weekly until N≥25 conditioned bees was reached for each treatment group. We ran these experiments in parallel in order to distribute the variation caused by environmental conditions or other factors across all experimental conditions equally.
Determining consumption rates and sub-lethal dosage
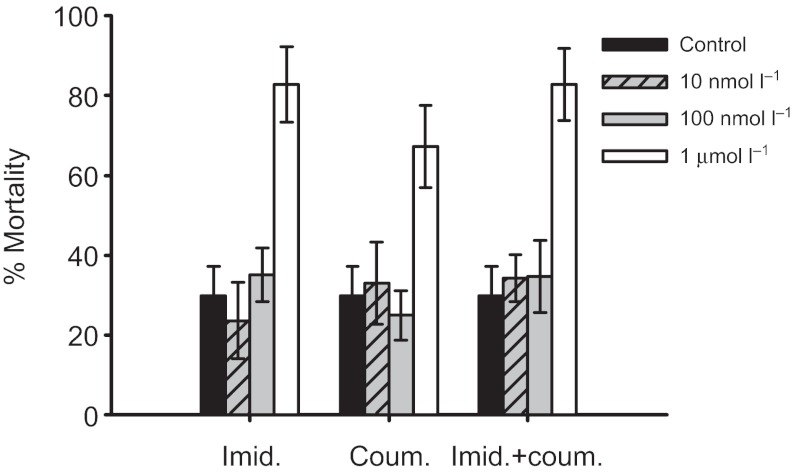
Preliminary experiments were performed to determine sub-lethal doses. The concentrations used were 10 nmol l−1, 100 nmol l−1 and 1 μmol l−1. Bees were kept as described above, and the number of bees surviving each day was recorded. Mortality rates were compared at the stage of the experiment where the bees had consumed pesticide solutions ad libitum for 3 days, and were to be harnessed ready for the learning and memory experiments (Fig. 1). On the basis of this, 100 and 10 nmol l−1 doses of all treatments were found to be sub-lethal, and so were used in the subsequent learning and memory experiments (see Results and Fig. 1 for details).
Fig. 1.
Survival data for bees fed different concentrations of pesticide treatments over a 3 day period prior to conditioning. (A) Imidacloprid, (B) coumaphos, (C) imidacloprid plus coumaphos (N≥3 replicates using cohorts of N≥15 bees for each treatment). For all treatments, the 1 μmol l−1 concentration caused greater mortality than the control. The 100 and 10 nmol l−1 concentrations did not increase mortality, so were selected for use in the subsequent learning and memory experiments.
During this phase of the experiment, food consumption was measured by weighing the feeding tubes before and after the bees had fed for 24 h, and average consumption per bee per day was calculated. There were no differences in daily food consumption between the control group and treatment groups fed 100 nmol l−1 concentrations of the pesticide solutions (Kruskal–Wallis, χ32=1.51, P=0.680). Mean consumption of sucrose syrup across all treatments was 143.95±3.55 mg per bee per day.
Based on the amount of sucrose syrup consumed (3 days consuming ~144 mg per bee per day, plus 27.5 mg on two subsequent days, totalling 487 mg over the whole experiment), we estimated that each bee fed the 10 nmol l−1 pesticide solution consumed ~1.3 ng of imidacloprid and/or 1.8 ng coumaphos over the 6 day experimental protocol. This amount of imidacloprid is within the range consumed by foraging bees feeding on imidacloprid-contaminated nectar (Rortais et al., 2005). Bees fed the 100 nmol l−1 pesticide solutions consumed imidacloprid at a concentration of 23.3 μg kg−1 of sucrose syrup, and coumaphos at a concentration of 33 μg kg−1. This imidacloprid concentration is within the range of previously published studies (Decourtye et al., 2004b). The coumaphos concentration we used was 6- to 60-fold lower than that found in a previous study that measured coumaphos within colony stores (180 p.p.b.) (Mullin et al., 2010).
Learning and memory experiments
Honeybees were trained using a procedure for olfactory conditioning of the proboscis extension reflex (Bitterman et al., 1983). The conditioned (CS) and unconditioned stimuli (US) were presented on a massed (30 s inter-trial interval) or a spaced schedule (10 min inter-trial interval) as described elsewhere (Menzel et al., 2001). The CS was an odour presented for 4 s duration, and the US was a reward of 0.2 μl of 1 mol l−1 sucrose solution. The odour stimulus arose from a 3 μl aliquot of 1-hexanol applied to a strip of filter paper placed within a glass tube and attached to the controlled air supply (the arena and training apparatus were as previously described) (Wright et al., 2008). Each subject received six conditioning trials. Bees that responded to the CS alone before training were excluded from conditioning. Bees that failed to respond to the odour during any of the six conditioning trials [even if they continued to exhibit a proboscis extension response (PER) in response to antennal stimulation] were defined as ‘non-responders’; these data were analysed separately. After conditioning, each bee was tested with the CS and a novel odour (2-octanone) at 10 min and 24 h. The order of presentation of the test odours was randomized across subjects, with a 3–5 min interval between each test. The 10 min test was performed to assess STM and the 24 h test was performed to test eLTM (Menzel et al., 2001). To measure memory, we compared the responses during the last acquisition trial with those during both recall tests within each treatment group: STM was assessed in terms of whether the response to the CS at the 10 min memory test was significantly less than that on the 6th training trial, and eLTM was assessed in terms of whether the response to the CS at the 24 h memory test was less than that at the STM test. Memory specificity was measured by comparing the response to the CS with the response to the novel odour, during the 10 min and 24 h memory tests. (We did not test beyond 24 h because most of the pesticide-treated bees died within 72 h of harnessing.)
Statistical analysis
Consumption and mortality data were analysed using a Kruskal–Wallis test. Comparisons between the proportion of ‘responders’ and ‘non-responders’ were analysed using logistic regression. Data for bees that responded during conditioning were analysed separately. The response of each subject to the odour stimulus during conditioning and testing was scored as a binary response (full proboscis extension or not) and analysed using binary logistic regression (lreg) (generalized linear model, GLM) in the statistics program SPSS. For logistic regression analysis of the acquisition data, the first training trial was excluded from the analysis to facilitate model fitting (all responses at this point were 0). Mean values for the probability of response, and standard errors of the means, are reported for each treatment, dose and odour presentation. Least squares post hoc tests (lsc) were performed for pair-wise comparisons.
The specificity of olfactory memory was tested in our experiments by presenting both a novel odour and the CS. To compare the relative response rate of our subjects, we calculated a ‘discrimination index’ (DI), represented as:
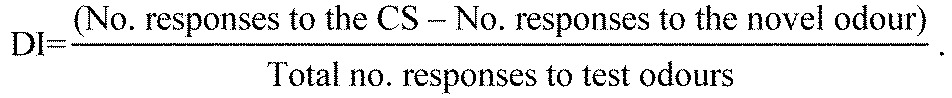 |
(1) |
RESULTS
Identification of sub-lethal doses of imidacloprid and coumaphos
Preliminary experiments were performed using three different concentrations of each pesticide to identify sub-lethal concentrations for use in the learning and memory experiments (Fig. 1). Unsurprisingly, all the compounds tested had some effect on mortality (lreg, imidacloprid, χ32=25.5, P<0.001; coumaphos, χ32=12.9, P=0.005; imidacloprid plus coumaphos, χ32=25.6, P<0.001). However, by comparing the different doses of each compound, it was found that only the 1 μmol l−1 concentration significantly increased mortality compared with the controls (imidacloprid, P<0.001; coumaphos, P=0.004; imidacloprid plus coumaphos, P<0.001). The 10 and 100 nmol l−1 concentrations of all treatments were found to cause no increase in mortality relative to the controls (imidacloprid: 10 nmol l−1 P=0.607, 100 nmol l−1 P=0.603; coumaphos: 10 nmol l−1 P=0.814, 100 nmol l−1 P=0.625; imidacloprid plus coumaphos: 10 nmol l−1 P=0.680, 100 nmol l−1 P=0.634).
Learning performance is impaired when bees are exposed to imidacloprid and coumaphos
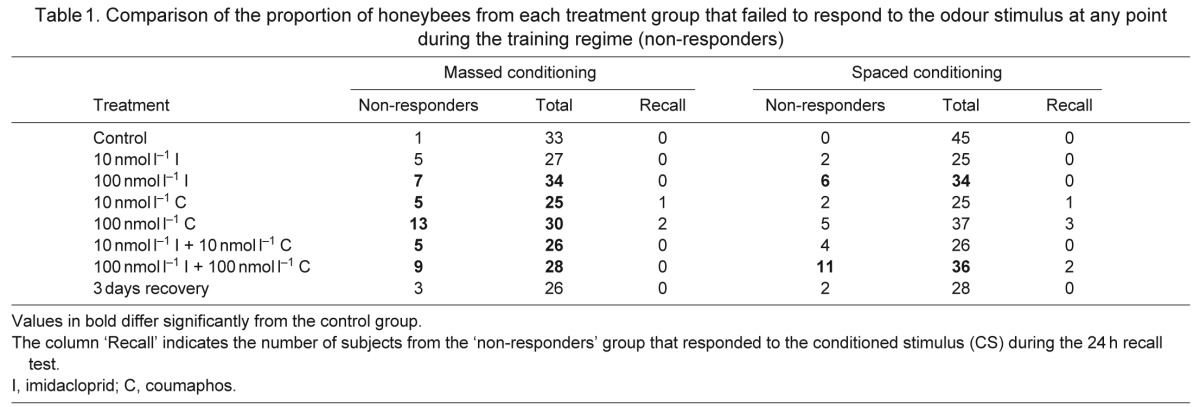
The proportion of non-responding bees in each group treated with pesticides was compared (Table 1). Pesticide exposure increased the proportion of non-responding bees in both the massed and spaced conditioning assays (Table 1; imidacloprid: lreg, χ22=6.10, P=0.047; coumaphos: lreg, χ22=7.66, P=0.022; imidacloprid plus coumaphos: lreg, χ32=12.7, P=0.005). For honeybees allowed to recover for 3 days after combined pesticide exposure, failure to respond during conditioning was not significantly different from the level exhibited by the control group during both types of conditioning assay (massed: lsc, P=0.220; spaced: lsc, P=0.639).
Table 1.
Comparison of the proportion of honeybees from each treatment group that failed to respond to the odour stimulus at any point during the training regime (non-responders)
Prolonged exposure to imidacloprid and coumaphos reduces the rate of olfactory learning
In the population of bees that exhibited olfactory learning, we found that 100 nmol l−1 doses of all compounds and their combinations affected the rate of olfactory learning in both massed and spaced conditioning. Each drug produced a slightly different effect on the acquisition curve in both learning assays (Fig. 2, Table 2). Exposure to imidacloprid influenced the rate of learning for bees trained with both massed (Fig. 2A; lreg, χ22=16.8, P<0.001) and spaced (Fig. 2D; lreg, χ22=19.8, P<0.001) conditioning protocols: the rate of acquisition was slower, as exhibited by the lower probability of responding during the first three trials, and the population reached a lower asymptote (trials 4–6). Imidacloprid had a stronger effect on spaced conditioning than on massed conditioning: both doses reduced acquisition during spaced conditioning (10 nmol l−1: lsc, P=0.004; 100 nmol l−1: lsc, P<0.001), whereas only the 100 nmol l−1 dose reduced the rate of learning during massed conditioning (lsc, P<0.001).
Fig. 2.
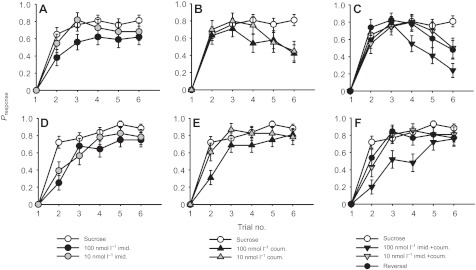
Rates of acquisition during olfactory conditioning (massed conditioning A–C, spaced conditioning D–F) are affected by pesticide treatment. Treatment with 100 nmol l−1 imidacloprid (A) and 10 and 100 nmol l−1 coumaphos (B) reduced the rate of acquisition (response probability Presponse). (C) Treatment with imidacloprid plus coumaphos had a similar effect on acquisition to that produced by 100 nmol l−1 coumaphos alone. Bees allowed to recover from the 100 nmol l−1 treatment did not differ from the control (sucrose). During spaced conditioning, 10 and 100 nmol l−1 imidacloprid (D) and 100 nmol l−1 coumaphos (E) reduced the rate of acquisition. (F) The 100 nmol l−1 dose of imidacloprid plus coumaphos strongly reduced the rate of acquisition. Bees allowed to recover from the 100 nmol l−1 combined imidacloprid plus coumaphos treatment (i.e. reversal) did not differ from the control. Sample sizes and pairwise comparison statistics for all treatments and doses are shown in Table 2. Note: the control group is the same for A–C and for D–F.
Table 2.
Sample sizes and post hoc pairwise comparison statistics for the acquisition data (Fig. 2)
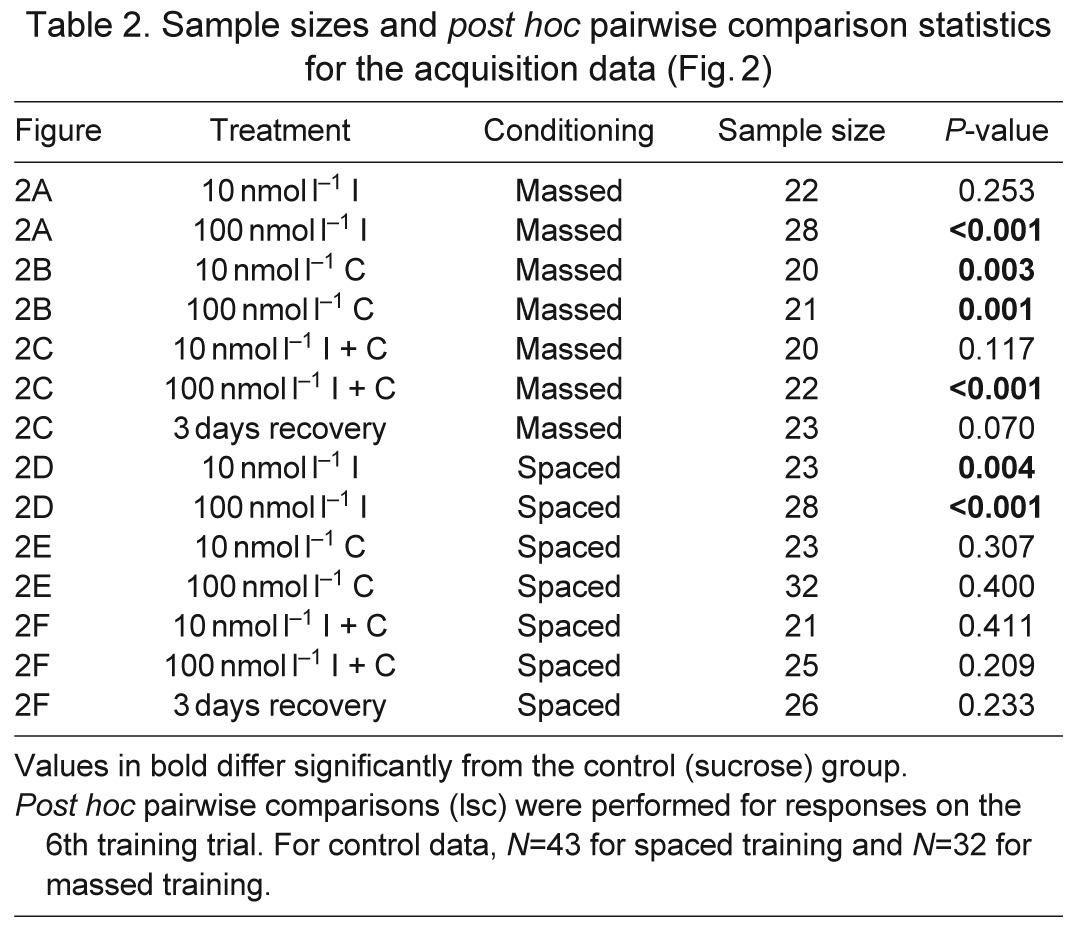
Coumaphos also impaired learning during both massed (Fig. 2B; lreg, χ22=11.3, P=0.003) and spaced conditioning (Fig. 2E; lreg, χ22=14.7, P=0.001), but the effects on massed learning were greater than those seen during spaced learning. During massed conditioning, the effect on acquisition was strikingly different to that produced by imidacloprid (Fig. 1A). Initially, coumaphos-treated bees responded as well as the control bees, but the number of animals responding to the CS began to decrease during the course of training, and by trial 6, significantly fewer animals responded to the CS (lsc, trial 6: 10 nmol l−1, P=0.003; 100 nmol l−1, P=0.001). This effect was not seen during the spaced conditioning protocol, for which the curves qualitatively resembled those produced by imidacloprid, where the rate of learning was slightly lower during the first three trials with the highest dose (lsc, P<0.001).
The effect of combined exposure to imidacloprid and coumaphos on the rate of acquisition during learning resembled both the strong effect of coumaphos on massed learning (Fig. 2C; lreg, χ32=18.3, P<0.001) and the impact of imidacloprid on the rate of acquisition during spaced conditioning at the highest doses (Fig. 2F; lreg, χ32=30.9, P<0.001). However, both the 10 and the 100 nmol l−1 treatment reduced the proportion of bees that responded on the 6th trial of massed learning (lsc, 10 nmol l−1, P=0.016; 100 nmol l−1, P<0.001) in the manner observed when bees were exposed to coumaphos alone. Bees that were fed the ‘reversal’ treatment did not perform differently from control animals during massed conditioning for most trials, but they also exhibited the decline on the last two trials shown by the bees subjected to the combined exposure. Their responses during spaced conditioning were not significantly different from the control (lsc, P=0.071).
Exposure to imidacloprid impairs memory formation
We measured how exposure to imidacloprid, coumaphos and their combination influenced short-/mid-term (STM) and eLTM memory by testing bees at 10 min and 24 h after conditioning (Fig. 3). The pesticides altered the way that bees responded during the STM and eLTM tests after both kinds of conditioning. Imidacloprid exposure impaired STM after massed but not spaced conditioning (Fig. 3A,D; massed: lreg, χ22=8.13, P=0.017; spaced: lreg, χ22=4.44, P=0.327). However, it reduced eLTM after conditioning in both assays (massed: lreg, χ22=6.54, P=0.038; spaced: lreg, χ22=11.5, P=0.003). Prolonged coumaphos exposure also reduced the average rate of response of the massed-conditioned bees on the 6th conditioning trial and during both of the recall tests (Fig. 3B,E; lreg, χ22=9.95, P=0.007).
Fig. 3.
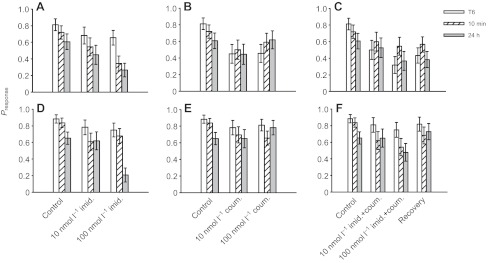
Pre-exposure to imidacloprid affects long-term memory (LTM) formation (massed conditioning A–C, spaced conditioning D–F). (A) After massed conditioning, bees exposed to 100 nmol l−1 imidacloprid had poor performance during the short-term memory (STM, 10 min) and LTM (24 h) tests. Exposure to coumaphos (B) and a mixture of imidacloprid and coumaphos (C) did not influence recall. (D) After spaced conditioning, imidacloprid affected recall at 24 h but not at 10 min. Exposure to coumaphos (E) and a mixture of imidacloprid and coumaphos (F) did not influence recall. Note: the control group is the same for A–C and for D–F. T6, 6th conditioning trial.
While the responses of the bees subjected to prolonged coumaphos exposure were lower than those of the control group, the average rate of response of these bees did not change from the 6th trial to the 10 min and 24 h tests (10 nmol l−1: lreg, χ22=0.137, P=0.934; 100 nmol l−1: lreg, χ22=1.14, P=0.565). This is especially apparent when the responses of the bees subjected to spaced conditioning were compared with those of the massed-conditioned bees: the responses of the spaced-conditioned bees during the recall test were unaffected by coumaphos exposure (lreg, χ22=2.12, P=0.344).
Exposure to the combination of imidacloprid and coumaphos caused effects most similar to those of coumaphos after massed conditioning. Response rates were lower at all time points (lreg, χ22=14.2, P=0.001) although no notable decrease in response rate equivalent to memory impairment was seen between the last acquisition trial and the memory tests (see Table 3). This effect was not reversed in bees that had been allowed to recover from combined pesticide exposure: response rates were still much lower than those of controls (lreg, χ12=18.1, P<0.001).
Table 3.
Sample sizes and post hoc pairwise comparison statistics for the recall test (Fig. 3)
After spaced conditioning, combined imidacloprid and coumaphos treatment also had an effect (lreg, χ22=9.18, P=0.010), and in this case a true memory impairment was observed, with response rates at the 10 min memory test being lower than those on the last acquisition trial (P=0.011). This effect was reversed in bees allowed to recover from the pesticide treatment, which did not respond differently to the controls (lreg, χ12=1.18, P=0.277).
Olfactory memory specificity is reduced after prolonged exposure to pesticides
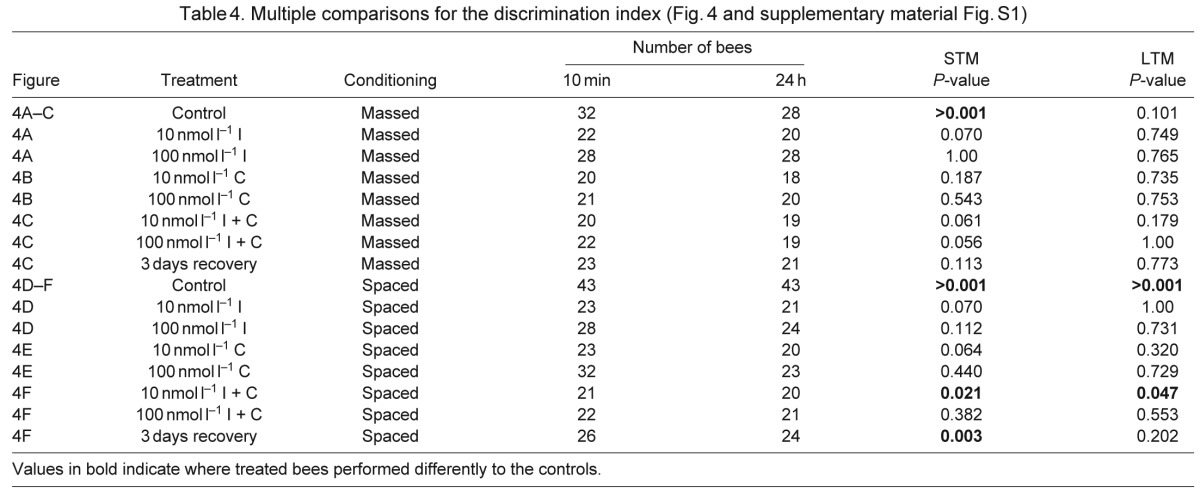
To compare the responses of the bees during the tests for STM and eLTM with those to the novel odour, we calculated a ‘discrimination index’ that reflected the proportion of bees from each treatment that responded to the CS in preference to the novel odour during each test (Fig. 4, Table 4; data for comparison of the CS and the novel odour are in supplementary material Fig. S1). All pesticide treatments affected the specificity of the responses during the recall test. It is notable that 100 nmol l−1 imidacloprid-treated bees were as likely to respond to the CS as to the novel odour at 24 h (the discrimination index in this case was less than 0, Fig. 4A,D). Treatment with 100 nmol l−1 coumaphos was also detrimental to the specificity of the test response; less than 10% of the bees preferentially responded to the CS (Fig. 4B,E). For the combined pesticide treatment at the 100 nmol l−1 concentration, the bees retained some specificity in the test response at 10 min after conditioning; however, when tested 24 h later, they failed to respond preferentially to the CS, even though their response rates to the test odours were still relatively high (Fig. 3C,F).
Fig. 4.
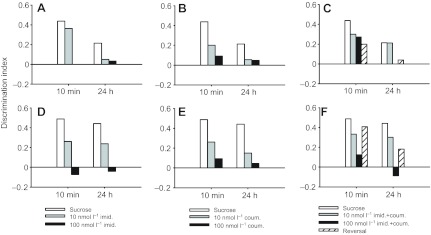
Pesticides affect odour discrimination during olfactory recall tests. A discrimination index was calculated to measure when bees responded to the conditioned stimulus (CS) at a greater rate than to a novel odour during the 10 min and 24 h recall tests. Values greater than zero reflect a preference for the CS over the novel odour; negative values reflect a preference for the novel odour. (A–C) Bees tested after massed conditioning with imidacloprid (A), coumaphos (B) or imidacloprid plus coumaphos (C). (D–F) Bees tested after spaced conditioning with imidacloprid (D), coumaphos (E) or imidacloprid plus coumaphos (F). Sample sizes and pairwise comparison statistics for all treatments and doses are shown in Table 4; recall response rates to the CS and the novel odour are reported in supplementary material Fig. S1.
Table 4.
Multiple comparisons for the discrimination index (Fig. 4 and supplementary material Fig. S1)
DISCUSSION
Combinations of sub-lethal doses of modern pesticides often produce additive or even synergistic effects on the mortality and behaviour of animals (Laetz et al., 2009). In our experiments, we combined a neonicotinoid pesticide, imidacloprid, with an AChE inhibitor, coumaphos, to simulate the situation where honeybees are exposed to pesticides in food and to miticides applied within the colony. We found that each of the cholinergic pesticides we examined had specific effects on learning and memory that were reflected in the responses of bees given the combination of pesticides, and that these effects on learning were additive. Combined pesticide exposure also strongly reduced the specificity of the response during the 24 h test. The influence of the pesticides on memory, however, was more complex and depended on pesticide exposure. Furthermore, bees allowed to recover for 3 days after pesticide exposure exhibited performance during conditioning that indicated they were still affected by exposure, but their responses during testing were not different from those of the control.
Because cholinergic signalling plays a key role in olfactory learning and memory, it is reasonable to assume that impairment in cholinergic signalling caused by prolonged exposure to nAChR agonists or AChE inhibitors should also lead to deficits in acquisition and, therefore, memory formation. In this study, disruption of cholinergic pathways by chronic exposure to imidacloprid or coumaphos affected performance during both massed and spaced learning. This may have been due to direct impairment of the neural circuits involved in olfaction or gustation, or to a disruption of the mechanisms of associative learning. Interestingly, in bees that could perform associative learning, prolonged exposure to imidacloprid produced different effects on learning and memory to those produced by coumaphos, although both compounds target cholinergic signalling pathways. However, it is possible that the partial agonist imidacloprid could in fact decrease cholinergic signalling by competing with the full agonist acetylcholine (ACh) for the receptor-binding sites (Déglise et al., 2002), whereas coumaphos will increase ACh signalling initially, via both nicotinic and muscarinic receptors, until either receptor desensitization of neuronal death occurs (Fukuto, 1990; Pohanka, 2011).
Although we observed a modest impairment in acquisition in coumaphos-treated bees during spaced conditioning, the main effect on acquisition was expressed as a decline in PER in the last three conditioning trials of massed conditioning. This precipitous decline during the last three trials was also observed in the honeybees that had been exposed to the combination of pesticides. These animals continued to respond to the US, but rather slowly, and their head and proboscis shook in a way that suggested that perhaps some additional effects on motor function may also be involved; although it should be noted that this was never seen with coumaphos-treated animals during spaced training. Organophosphate pesticides are known to affect motor function in many different animals including flies, fish and rodents: observed effects include tremors, unco-ordinated movement and transient paralysis (Miller and Kennedy, 1972; Moser, 1995; Patil and David, 2010). We have also observed altered motor behaviour in coumaphos-treated bees, including episodes of paralysis and decreased co-ordination (Williamson et al., 2013). Acute application of AChE inhibitors results in an acceleration of olfactory learning in honeybees, presumably because inactivation of the enzyme leads to a transient elevation of ACh during sensory stimulation (Guez et al., 2010). In our experiments, bees were continually exposed to low levels of an irreversible AChE inhibitor; this would result in an elevation of ACh in the synaptic cleft that would also lead to eventual desensitization of the over-stimulated cholinergic neurons rather than an increase in excitation (Chen, 2012; Hartmann et al., 2007; Palmer et al., 2013). The fact that bees simply did not respond during the last few trials of conditioning even though they were able to learn during the first few trials strongly suggests that the olfactory system and other circuits that rely on cholinergic signalling cannot cope with the high levels of ACh released during the rapid stimulation that occurs during massed conditioning. This would result in an inability of the neurons to detect and respond to the ACh produced by each episode of synaptic transmission resulting from olfactory stimulation, and hence lead to a failure in expression of the learned behaviour. This effect is observed in humans that have been poisoned with AChE inhibitors; an accumulation of Ach in the synaptic cleft leads to paralysis and death (Pope et al., 2005).
Imidacloprid impaired LTM after both massed and spaced conditioning, whereas coumaphos did not influence LTM. The response rates during our test periods for the control subjects were very high when compared with previous studies of massed and spaced conditioning in bees during memory recall tests (Menzel et al., 2001). This is probably due to the fact that the bees in our experiments were highly motivated as a result of the feeding regime. In the light of this, the fact that we observed a drop in the response after imidacloprid exposure strongly suggests that it influences LTM consolidation. In contrast, the responses of the coumphos-treated bees indicated that they did not forget that the odour was associated with a reward, but the response rate was equally high to the incorrect odour stimulus. Perhaps the most striking effect of prolonged imidacloprid and coumaphos exposure found in this study is the inability of treated bees to correctly select the conditioned odour rather than a novel odour during the memory tests. Both imidacloprid and coumaphos administered alone reduced the bees' ability to differentiate the olfactory stimuli during the tests, an effect that has previously been reported for coumaphos but not for imidacloprid (Weick and Thorn, 2002). The combination of the two compounds impaired olfactory discrimination after massed training, but only the higher dose impaired discrimination after spaced training. This effect was neither additive nor synergistic, which is in contrast to the effects seen on acquisition, where a small additive effect on learning impairment was observed. It is not clear whether the impairment of olfactory discrimination was caused by a true deficit in learning and memory consolidation (i.e. the bees did not learn the correct odour) or arose from a deficit in olfactory perception (i.e. the bees could not detect which odour was correct). Cholinergic signalling plays a key role in the antennal lobes, where odour information from the antennae is initially processed, in addition to its importance in the mushroom bodies, where olfactory information is integrated and learning and memory processes occur (Gauthier, 2010).
The contrasting effects of the two pesticides on memory and general responsiveness may be explained by the involvement of distinct sub-types of nAChRs in different aspects of the memory formation and retrieval process (Dacher et al., 2005; Gauthier et al., 1992). Previous studies using antagonists to block nAChR function have shown learning and memory impairments very similar to the ones we describe here (Gauthier et al., 2006; Lozano et al., 1996). Mecamylamine, a broad spectrum antagonist that blocks all nAChRs, impaired learning and responsiveness to the CS during and immediately after olfactory conditioning, but did not affect LTM (Lozano et al., 1996). This is reminiscent of the effects we report for coumaphos, which by raising ACh levels throughout the brain will also affect all nAChRs. In another study, ɑ-bungarotoxin, a specific antagonist of a particular nAChR subtype, also impaired learning, but had much more dramatic effects on LTM (Gauthier et al., 2006). This effect is very similar to our observed effects of imidacloprid, and it is known that imidacloprid acts on ɑ-bungarotoxin-sensitive receptors (Déglise et al., 2002; Jepson et al., 2006).
Our data clearly show that bees have difficulty performing simple learning and memory tasks when they have experience prolonged exposure to combinations of pesticides as adult foragers. Foraging for food is a demanding task that requires the bees not only to learn but also to optimize their foraging strategies by accurately learning and remembering which flowers offer the best rewards (Biernaskie et al., 2009; Lihoreau et al., 2011). Comparisons of laboratory learning tests and foraging in the field suggest that learning ability is a good predictor of foraging ability at the colony level (Raine and Chittka, 2008). Our data, in combination with other studies that have revealed foraging and communication impairments in bees exposed to imidacloprid or other neonicotinoid pesticides (Eiri and Nieh, 2012; Henry et al., 2012; Whitehorn et al., 2012), imply that commonly used pesticides are a strong candidate for the observed decline in pollinator populations, and that the simultaneous exposure to multiple pesticides additively amplifies this effect on important behaviours.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Malcolm Thompson for beekeeping and the National Bee Unit at York (UK) for donating bee colonies.
LIST OF SYMBOLS AND ABBREVIATIONS
- ACh
acetylcholine
- AChE
acetylcholinesterase
- CS
conditioned stimulus
- LTM
long-term memory
- nAChR
nicotinic acetycholine receptor
- PER
proboscis extension response
- STM
short-term memory
- US
unconditioned stimulus
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/216/10/1799/DC1
COMPETING INTERESTS
No competing interests declared.
FUNDING
This work was funded in part by a UK government Insect Pollinators Initiative (BBSRC, NERC, Wellcome Trust, DEFRA, and the Scottish Government) [grant no. BB/I000143/1 to G.A.W.]. Deposited in PMC for release after 6 months.
REFERENCES
- Biernaskie J. M., Walker S. C., Gegear R. J. (2009). Bumblebees learn to forage like Bayesians. Am. Nat. 174, 413-423 [DOI] [PubMed] [Google Scholar]
- Bitterman M. E., Menzel R., Fietz A., Schäfer S. (1983). Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107-119 [PubMed] [Google Scholar]
- Buckingham S. D., Lapied B., Corronc H., Sattelle F. (1997). Imidacloprid actions on insect neuronal acetylcholine receptors. J. Exp. Biol. 200, 2685-2692 [DOI] [PubMed] [Google Scholar]
- Chen Y. (2012). Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology 33, 391-400 [DOI] [PubMed] [Google Scholar]
- Dacher M., Lagarrigue A., Gauthier M. (2005). Antennal tactile learning in the honeybee: effect of nicotinic antagonists on memory dynamics. Neuroscience 130, 37-50 [DOI] [PubMed] [Google Scholar]
- Dainat B., Evans J. D., Chen Y. P., Gauthier L., Neumann P. (2012). Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 78, 981-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourtye A., Armengaud C., Renou M., Devillers J., Cluzeau S., Gauthier M., Pham-Delegue M. H. (2004a). Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 78, 83-92 [Google Scholar]
- Decourtye A., Devillers J., Cluzeau S., Charreton M., Pham-Delègue M. H. (2004b). Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 57, 410-419 [DOI] [PubMed] [Google Scholar]
- Déglise P., Grünewald B., Gauthier M. (2002). The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 321, 13-16 [DOI] [PubMed] [Google Scholar]
- Eiri D. M., Nieh J. C. (2012). A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J. Exp. Biol. 215, 2022-2029 [DOI] [PubMed] [Google Scholar]
- Elbert A., Haas M., Springer B., Thielert W., Nauen R. (2008). Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 64, 1099-1105 [DOI] [PubMed] [Google Scholar]
- Fukuto T. R. (1990). Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 87, 245-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. (2010). State of the art on insect nicotinic acetylcholine receptor function in learning and memory. Adv. Exp. Med. Biol. 683, 97-115 [DOI] [PubMed] [Google Scholar]
- Gauthier M., Belzunces L. P., Zaoujal A., Colin M. E., Richard D. (1992). Modulatory effect of learning and memory on honey-bee brain acetylcholinesterase activity. Comp. Biochem. Physiol. 103C, 91-95 [Google Scholar]
- Gauthier M., Dacher M., Thany S. H., Niggebrügge C., Déglise P., Kljucevic P., Armengaud C., Grünewald B. (2006). Involvement of alpha-bungarotoxin-sensitive nicotinic receptors in long-term memory formation in the honeybee (Apis mellifera). Neurobiol. Learn. Mem. 86, 164-174 [DOI] [PubMed] [Google Scholar]
- Guez D., Zhu H., Zhang S. W., Srinivasan M. V. (2010). Enhanced cholinergic transmission promotes recall in honeybees. J. Insect Physiol. 56, 1341-1348 [DOI] [PubMed] [Google Scholar]
- Halm M. P., Rortais A., Arnold G., Taséi J. N., Rault S. (2006). New risk assessment approach for systemic insecticides: the case of honey bees and imidacloprid (Gaucho). Environ. Sci. Technol. 40, 2448-2454 [DOI] [PubMed] [Google Scholar]
- Hartmann J., Kiewert C., Duysen E. G., Lockridge O., Greig N. H., Klein J. (2007). Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 100, 1421-1429 [DOI] [PubMed] [Google Scholar]
- Hawthorne D. J., Dively G. P. (2011). Killing them with kindness? In-hive medications may inhibit xenobiotic efflux transporters and endanger honey bees. PLoS ONE 6, e26796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M., Béguin M., Requier F., Rollin O., Odoux J. F., Aupinel P., Aptel J., Tchamitchian S., Decourtye A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science 336, 348-350 [DOI] [PubMed] [Google Scholar]
- Ihara M., Brown L. A., Ishida C., Okuda H., Sattelle D. B., Matsuda K. (2006). Actions of imidacloprid, clothianidin and related neonicotinoids on nicotinic acetylcholine receptors of American cockroach neurons and their relationships with insecticidal potency. J. Pestic. Sci. 31, 35-40 [Google Scholar]
- Isabel G., Pascual A., Preat T. (2004). Exclusive consolidated memory phases in Drosophila. Science 304, 1024-1027 [DOI] [PubMed] [Google Scholar]
- Jepson J. E., Brown L. A., Sattelle D. B. (2006). The actions of the neonicotinoid imidacloprid on cholinergic neurons of Drosophila melanogaster. Invert. Neurosci. 6, 33-40 [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Pollock H. S., Berenbaum M. R. (2009). Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 102, 474-479 [DOI] [PubMed] [Google Scholar]
- Laetz C. A., Baldwin D. H., Collier T. K., Hebert V., Stark J. D., Scholz N. L. (2009). The synergistic toxicity of pesticide mixtures: implications for risk assessment and the conservation of endangered Pacific salmon. Environ. Health Perspect. 117, 348-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Conte Y., Ellis M., Ritter W. (2010). Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie (Celle) 41, 353-363 [Google Scholar]
- Lihoreau M., Chittka L., Raine N. E. (2011). Trade-off between travel distance and prioritization of high-reward sites in traplining bumblebees. Funct. Ecol. 25, 1284-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano V. C., Bonnard E., Gauthier M., Richard D. (1996). Mecamylamine-induced impairment of acquisition and retrieval of olfactory conditioning in the honeybee. Behav. Brain Res. 81, 215-222 [DOI] [PubMed] [Google Scholar]
- Maxim L., van der Sluijs J. P. (2010). Expert explanations of honeybee losses in areas of extensive agriculture in France: Gaucho (R) compared with other supposed causal factors. Environ. Res. Lett. 5, 014006 [Google Scholar]
- Menzel R., Manz G., Menzel R., Greggers U. (2001). Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn. Mem. 8, 198-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Kennedy J. M. (1972). Flight motor activity of houseflies as affected by temperature and insecticides. Pestic. Biochem. Physiol. 2, 206-222 [Google Scholar]
- Moser V. C. (1995). Comparisons of the acute effects of cholinesterase inhibitors using a neurobehavioral screening battery in rats. Neurotoxicol. Teratol. 17, 617-625 [DOI] [PubMed] [Google Scholar]
- Mullin C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., Vanengelsdorp D., Pettis J. S. (2010). High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5, e9754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M. R., Oishi K., Gelb B. D., Zhong Y. (2009). The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139, 186-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M. J., Moffat C., Saranzewa N., Harvey J., Wright G. A., Connolly C. N. (2013). Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nature Commun. 4, 1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V. K., David M. (2010). Behavioral and morphological endpoints: as an early response to sublethal malathion intoxication in the freshwater fish, Labeo rohita. Drug Chem. Toxicol. 33, 160-165 [DOI] [PubMed] [Google Scholar]
- Pohanka M. (2011). Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 155, 219-223 [DOI] [PubMed] [Google Scholar]
- Pope C., Karanth S., Liu J. (2005). Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 19, 433-446 [DOI] [PubMed] [Google Scholar]
- Raine N. E., Chittka L. (2008). The correlation of learning speed and natural foraging success in bumble-bees. Proc. Biol. Sci. 275, 803-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rortais A., Arnold G., Halm M. P., Touffet-Briens F. (2005). Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie (Celle) 36, 71-83 [Google Scholar]
- Rosenkranz P., Aumeier P., Ziegelmann B. (2010). Biology and control of Varroa destructor. J. Invertebr. Pathol. 103 Suppl. 1, S96-S119 [DOI] [PubMed] [Google Scholar]
- Weick J., Thorn R. S. (2002). Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 95, 227-236 [DOI] [PubMed] [Google Scholar]
- Whitehorn P. R., O'Connor S., Wackers F. L., Goulson D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351-352 [DOI] [PubMed] [Google Scholar]
- Williamson S. M., Baker D. D., Wright G. A. (2013). Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee, Apis mellifera. Invert. Neurosci. doi 10.1007/s10158-012-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. A., Choudhary A. F., Bentley M. A. (2009). Reward quality influences the development of learned olfactory biases in the honeybee. Proc. R. Soc. B 276, 2597-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Anelli C. M., Sheppard W. S. (2011). Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 6, e14720 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.