Schistosomiasis is a major public health problem in 76 countries where it afflicts more than 200 million people [1]. Schistosomes exhibit a complicated life cycle including two free-living stages in water and parasitic stages in the intermediate snail host and definitive vertebrate host [2]. A large number of expressed mRNAs, genes and proteins have recently been identified or predicted in schistosomes from cDNA, genomic, and proteomic analyses [3–5]. However, in most cases, few functional details are available regarding these genes or proteins due to the lack of comprehensive molecular tools for schistosomes to elucidate their roles in vivo[6–8]. Some progress has been made toward the development and use of RNA interference methods, transfection methods, expression and integration of DNA constructs, and gene reporters [9–28]. There is a great need for further development and comparison of reporter genes and transfection protocols for different schistosome stages to provide researchers with a resource for choosing the most effective and appropriate tools for a particular experimental paradigm. In the present study, we evaluated the use of Gaussia luciferase as a reporter for use in schistosomes. In addition, we evaluated a variety of electroporation and biolistic transfection protocols to compare the effectiveness of these transfection protocols in different schistosome stages.
Gaussia luciferase is a new reporter luciferase from the marine copepod Gaussia princeps[29, 30]. This 185 amino acid (19.9 kDa) monomeric luciferase does not require any cofactors (e.g., ATP) and catalyzes the oxidation of the substrate coelenterazine in a reaction that emits light (480 nm) similar to Renilla luciferase. The reporter can be used in a Dual-Luciferase Assay with Photinus (Firefly) luciferase, enabling the use of a control luciferase reporter in experimental paradigms [32]. This new luciferase has several major advantages over previous luciferases (Firefly or Renilla): 1) human codon-optimized Gaussia Luciferase generates at least 100-fold higher bioluminescent signal intensity in mammalian cells, compared to Firefly and Renilla luciferases; 2) Gaussia luciferase possesses a natural secretory signal consisting of 16 amino acids, is secreted into the media, and thus expression can be evaluated without lysis of the cells or organisms; and 3) Gaussia luciferase coding region is 555 nt, the smallest luciferase reporter.
To evaluate Gaussia luciferase as a potential reporter for schistosomes, we transfected Firefly, Renilla, and Gaussia luciferase RNA into different schistosome stages and compared the level of luciferase activity observed in schistosome lysates. RNA reporters instead of DNA reporters were used to enable direct comparison of reporter activity and transfection efficiency independent of potential differences in promoter activity or RNA processing that could occur in different cells and stages. As illustrated in Figure 1, Gaussia luciferase led to much higher levels of luciferase activity than either the Firefly or Renilla luciferase reporter in all schistosome stages examined (some data not shown). We next analyzed the time course of Renilla and Gaussia luciferase activity in sporocysts (Figure 2A & B). Gaussia luciferase activity peaked rapidly within 3 hours, began to fall ~ 12 hours post-transfection, and was gone by 30 hrs. Renilla luciferase also peaked rapidly at 3 hrs, began to fall after ~ 12 hour post-transfection, but significant levels of luciferase remained after 48 hrs. Thus, Gaussia luciferase protein appears to have a significantly shorter half-life than Renilla. We estimate that Gaussia protein half-life in worms is ~ 9 hours. Similar data were observed in schistosomula (data not shown).
Figure 1. Expression of luciferase reporters in Schistosoma mansoni.

(A) Comparison of Firefly, Renilla and Gaussia luciferase activity in adults and miracidia following biolistic introduction of reporter RNAs. Firefly luciferase activity in miracidia (403 RLU/sec/mg) and adults (166 RLU/sec/mg) while much lower than other luciferases is reproducibly detected.
(B) Comparison of Firefly, Renilla and Gaussia luciferase activity in sporocyst lysates following introduction of reporter RNAs using either biolistics or electroporation. Firefly luciferase activity in sporocysts, while much lower than other luciferases, is reproducibly detected using either biolistics (24,286 RLU/sec/mg) or electroporation (16,734 RLU/sec/mg). (C) Comparison of Gaussia luciferase activity in schistosomula lysates following introduction of a reporter RNA using biolistics or electroporation. Biolistic and electroporation conditions used are provided in Table 1 and described in [10]. Luciferase activity in worm lysates in A–C was measured 3 hours following RNA introduction using the Promega Dual-Luciferase Assay as previously described [10] with the activities normalized to protein concentrations in worm lysates using the Pierce BCA protein assay kit and Compat-Able protein assay preparation reagent set (Pierce, Rockford, IL). Luciferase activity represents 1/8 of total luciferase activity in parasite lysates. Error bars show standard deviation of a minimum of two independent results. Experiments illustrated are representative and were repeated a minimum of three times with similar results using independently prepared parasites and RNA transcripts. Firefly and Renilla DNA templates and their corresponding RNA transcripts were prepared using the primers and methods previously described [10]. Gaussia luciferase templates for RNA transcription were prepared using the plasmid pGlu-Basic (Targeting Systems, Santee, CA) and the primers 5′-TAATACGACTCACTATAGGACAAGCTTGGTACCGAGC-3′ and 5′-T75GACAGTAAGAATTATTTCTAGATGC-3’. RNAs were prepared and purified as previously described [10]. Miracidia, sporocyts, schistosomula and adult worms were harvested and cultured as previously described [10, 12, 35].
Figure 2. Time course of Renilla and Gaussia luciferase activity.
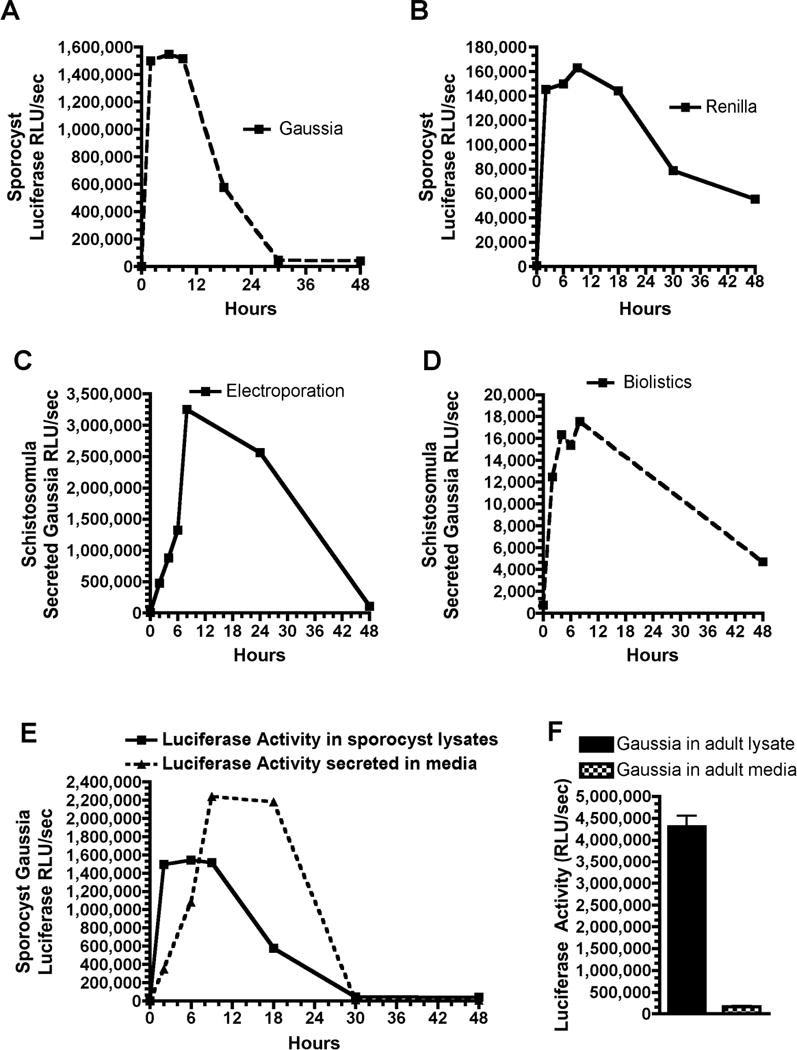
Time course of A) Gaussia and B) Renilla luciferase activity in sporocyts following electroporation of RNA reporters. Time course of secreted Gaussia luciferase activity following C) electroporation or D) biolistics of RNA reporter into schistosomula. E) Comparison of Gaussia luciferase activity over time in sporocyst lysates and secreted into the culture media following introduction of reporter RNA by electroporation. F) Comparison of Guassia luciferase activity in adult lysates and culture media following biolistic introduction of RNA reporter. Experiments were carried out as in Figure 1 except that 20 μl of culture media were directly assayed for luciferase secreted into the media. Luciferase activity represents 1/8 of the total Gaussia activity in worm lysates or culture media.
A unique attribute of Gaussia luciferase is the presence of a native secretory signal. To examine the secretion of Gaussia luciferase in schistosomes, we transfected a Gaussia RNA reporter into sporocycts and measured levels of luciferase activity in both parasite lysates and the culture media over time. As illustrated in Figure 2E, secretion of Gaussia luciferase increased in a linear fashion up to ~ 9 hr, was relatively stable in the media to ~22 hr, decayed relatively rapidly after 22 hr, and was gone by 30 hrs. Maximal Gaussia luciferase activity was ~ 1/3 greater in sporocyst media than in lysates. The time course of Gaussia secretion in schistosomula was similar to that observed in sporocysts (Figure 2C & D).
During the course of these studies we also compared levels of luciferase expression using available biolistic or electroporation RNA transfection methods in different schistosome stages (see Table 1). Notably, RNA electroporation of miracidia and adults under a variety of conditions was never successful. Whether this represents the presence of multiple membranes in miracidia that limit efficient transfection or degradation of the RNA at the schistosome surface remains to be determined. In contrast, biolistic introduction of nucleic acids into miracidia and adults is readily achieved (Figure 1A). However, we have not observed significant secretion of Gaussia luciferase following biolistic transfection of RNA into adults (Figure 2F). One possible interpretation of these results is that the prevailing biolistic conditions (1550 psi) might deliver the gold particles carrying the nucleic acid to a depth within the worms that cannot lead to secretion. However, we have observed gold particles in the tegument and GFP expression has been observed in the adult tegument using biolistics and an actin promoter [27]. Thus, it seems more likely that the adult tegumental syncitium might either degrade the Gaussia reporter protein during secretion or its structure limits secretion. Comparison of levels of luciferase expression observed in either sporocysts or schistosomula following RNA transfection using biolistics or electroporation demonstrated that electroporation leads to much higher levels of luciferase activity (Figure 1). Preliminary data suggest that this is also true for DNA transfection and that parameters in Table 1 can also be used for DNA transfection (data not shown). Overall, current methods for delivery and analysis of protein normalized luciferase reporter expression are most efficient in sporocysts and schistosomula. From these data, we suggest that additional development and analysis of vectors and expression elements for DNA transfection might be most readily achieved through electroporation of either sporocysts or schistosomula.
Table 1. S. mansoni RNA transfection conditions.
Biolistics were carried out using a Bio-Rad Biolistics PDS-1000/HE particle delivery system and electroporation using 4 mm cuvettes and a BTX ECM830 square wave electroporator as previously described [10, 13].
| Adults | Miracidia | Sporocyts | Schistosomula | |
|---|---|---|---|---|
| Number of Organisms | 50 | 800–2000 | 800–2000 | 100–500 |
| Post-Transfection Recovery Media | RPMI Media 1640 with 10% FCS and 200μg/ml penicillin and streptomycin | MEJMSE-J with 5 % FCS and 200μg/ml penicillin and streptomycin | MEJMSE-J with 5 % FCS and 200μg/ml penicillin and streptomycin | RPMI Media 1640 with 10% FCS and 200μg/ml penicillin and streptomycin |
| RNA amount | 1μg RNA per shot | 1μg RNA per shot | 1μg RNA per shot or 10 μg/ml in 200 μl of transfection buffer | 1μg per shot or 10 μg/ml in 200 μl of transfection buffer |
| Electroporation Conditions | —(a) | —(a) | 280V, 0.5ms, 1 pulse in MEJMSE-J | 125V, 20ms, (b) 1 pulse in RPMI Media 1640 |
| Biolistic Conditions | 1550 psi helium pressure, 15 inches Hg, 3 cm stage distance, 1.6 μm or 2.2 μm gold particles, double shots | 450 psi helium pressure, 15 inches Hg, 3 cm stage distance, 0.6 μm gold particles | 450 psi helium pressure, 15 inches Hg, 6 cm stage distance, 0.6 μm gold particles | 450 psi helium pressure, 15 inches Hg, 3 cm stage distance, 0.6 μm gold particles |
Although a variety of electroporation conditions were evaluated, conditions were not identified that enabled RNA transfection in adults and miracida of S. mansoni.
RNA transfection condidtions for schistosomula were described by Correnti et al. [12].
To date, fluorescent or luciferase reporters have been tested and used in schistosomes [10, 13, 16, 19, 21–24, 27, 31]. Each of these reporters has its advantages. Fluorescent reporters provide a non-invasive and extremely useful method for evaluating the localization of expression. Furthermore, a variety of differently colored fluorescent reporters are currently available. A potential limitation of these reporters is the level of expression observed. Analysis of several different fluorescent reporters we have examined (data not shown) indicates only a small percentage of the parasites exhibit expression requiring considerable time and effort to identify and to evaluate expression of the reporter in parasites. In addition, while fluorescence can be quantitated, its measurement is more complex than luciferase measurement in a luminometer. Luciferase reporters are not typically used as imaging reporters. However, they can be used as imaging reporters and Gaussia’s output compared to Renilla is ~ 200-fold better. An advantage of luciferase reporters is their high sensitivity and ease with which quantitative data can be obtained. As shown here, electroporation of a Gaussia reporter RNA into sporocysts and schistosomula leads to the expression of high levels of luciferase activity. Gaussia luciferase is not only more robust than other luciferases examined, but has the advantage of being secreted in several stages enabling its level of activity to be measured non-invasively in the media. This offers a variety of advantages for experimental paradigms, particularly if the amount of secretion is high. In our experiments, we have easily measured luciferase activity in 20 μl of the media which corresponds to ~200,000 relative light units/sec. The media can be concentrated to increase the sensitivity of the assay. As Gaussia is secreted from the parasites, this property might enable the identification of 1) snails infected with sporocysts expressing recombinant DNA, 2) parasites expressing Gaussia luciferase following limiting dilution in culture, or 3) possibly the expression of luciferase from schistosomes in vivo in the definitive host. For example, as DNA transfection and integration technologies are further developed, it may be possible to monitor expression of Gaussia DNA constructs driven by different promoters and secretory signals in adults and during schistosome development in mice through analysis of secreted luciferase in samples of blood drawn from infected hosts. Secretion of Gaussia luciferase might also offer a new tool for evaluation of transcriptional regulation associated with signaling pathways. Thus, the influence of host ligands on transcription of schistosome genes could be examined using Gaussia reporter DNAs driven by selected promoters in developing schistosomes in culture. Following DNA transfection, the role of various ligands in modulating transcription could be measured over time in culture media. Furthermore, the relatively short half-life of Gaussia protein might enable experimental approaches where the culture media is changed, the worms washed, and new ligands added to evaluate their role on transcriptional regulation of particular genes. Finally, Gaussia has recently been adapted for use in protein-fragment complementation assays (PCAs) to study the dynamics of protein-protein interactions in vivo and in vitro[32]. Using this strategy, a variety of potential applications might be devised to study protein interactions using PCA that could be implemented by schistosome researchers simply using RNA transfection [33].
In the current experiments, we have used a human codon-optimized form of the Gaussia coding region. It remains to be determined whether native Gaussia codons would lead to higher levels of Gaussia expression in schistosomes as human codon-optimized open reading frames may not be optimal for invertebrate expression. Substitution of a native, schistosome secretory signal might improve secretion and be necessary for adults. In addition, a polyclonal antibody against Gaussia luciferase is available commercially as an additional tool for Gaussia reporter studies (NanoLight Technology, Pinetop, AZ). Furthermore, a second secreted copepod luciferase (Metridia) was recently made commercially available (Ready-To-Glow™ Secreted Luciferase System, Clontech, Mountain View, CA). Gaussia can be used in co-transfection experiments with Firefly luciferase for transfection and data normalization within parasite lysates [34](data not shown). Finally, promoterless vectors and non-secreted forms of Gaussia are also commercially available (Targeting Systems, Santee, CA).
In summary, we have demonstrated that Gaussia luciferase is a robust and secreted luciferase reporter that may enable a variety of experimental approaches in schistosomes. The data illustrate that 1) Gaussia is the most robust luciferase reporter for all stages examined, 2) biolistics can be used to introduce RNA into miracidia and adults where electroporation is not effective, and 3) electroporation of luciferase reporters into sporocysts and schistosomula leads to the highest levels of luciferase activity. These data should facilitate additional studies and provide a framework for further development of DNA transfection technologies in schistosomes.
Acknowledgments
This work was supported by a grant from the Ellison Medical Foundation (ID-IA-0037-02) and a grant from the NIH (AI49558). Schistosome-infected hamsters and snails were kindly supplied by the NIAID Schistosomiasis Resource Center at the Biomedical Research Institute through NIAID contract N01-AI-30026.
References
- 1.Gryseels B, Polman K, Clerinx J, et al. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro-dos-Santos G, Verjovski-Almeida S, Leite LC. Schistosomiasis–a century searching for chemotherapeutic drugs. Parasitol Res. 2006;99:505–21. doi: 10.1007/s00436-006-0175-2. [DOI] [PubMed] [Google Scholar]
- 3.Hu W, Yan Q, Shen DK, et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat Genet. 2003;35:139–47. doi: 10.1038/ng1236. [DOI] [PubMed] [Google Scholar]
- 4.Verjovski-Almeida S, DeMarco R, Martins EA, et al. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat Genet. 2003;35:148–57. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RA, Ashton PD, Braschi S, et al. Oming in on schistosomes: prospects and limitations for post-genomics. Trends Parasitol. 2007;23:14–20. doi: 10.1016/j.pt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Brindley PJ, Pearce EJ. Genetic manipulation of schistosomes. Int J Parasitol. 2007;37:465–73. doi: 10.1016/j.ijpara.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Kalinna BH, Brindley PJ. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007 doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Geldhof P, Visser A, Clark D, et al. RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects. Parasitology. 2007:1–11. doi: 10.1017/S0031182006002071. [DOI] [PubMed] [Google Scholar]
- 9.Boyle JP, Wu XJ, Shoemaker CB, et al. Using RNA interference to manipulate endogenous gene expression in Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 2003;128:205–15. doi: 10.1016/s0166-6851(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 10.Cheng G, Cohen L, Ndegwa D, et al. The flatworm spliced leader 3′-terminal AUG as a translation initiator methionine. J Biol Chem. 2006;281:733–43. doi: 10.1074/jbc.M506963200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng GF, Lin JJ, Shi Y, et al. Dose-dependent inhibition of gynecophoral canal protein gene expression in vitro in the schistosome (Schistosoma japonicum) by RNA interference. Acta Biochim Biophys Sin (Shanghai) 2005;37:386–90. doi: 10.1111/j.1745-7270.2005.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correnti JM, Brindley PJ, Pearce EJ. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasitol. 2005;143:209–15. doi: 10.1016/j.molbiopara.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Davis RE, Parra A, LoVerde PT, et al. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci U S A. 1999;96:8687–92. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delcroix M, Sajid M, Caffrey CR, et al. A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem. 2006;281:39316–29. doi: 10.1074/jbc.M607128200. [DOI] [PubMed] [Google Scholar]
- 15.Dinguirard N, Yoshino TP. Potential role of a CD36-like class B scavenger receptor in the binding of modified low-density lipoprotein (acLDL) to the tegumental surface of Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 2006;146:219–30. doi: 10.1016/j.molbiopara.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Heyers O, Walduck AK, Brindley PJ, et al. Schistosoma mansoni miracidia transformed by particle bombardment infect Biomphalaria glabrata snails and develop into transgenic sporocysts. Exp Parasitol. 2003;105:174–8. doi: 10.1016/j.exppara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Kines KJ, Mann VH, Morales ME, et al. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Exp Parasitol. 2006;112:209–20. doi: 10.1016/j.exppara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Osman A, Niles EG, Verjovski-Almeida S, et al. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathog. 2006;2:e54. doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi A, Wippersteg V, Klinkert MQ, et al. Cloning of 5′ and 3′ flanking regions of the Schistosoma mansoni calcineurin A gene and their characterization in transiently transformed parasites. Mol Biochem Parasitol. 2003;130:133–8. doi: 10.1016/s0166-6851(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 20.Skelly PJ, Da’dara A, Harn DA. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33:363–9. doi: 10.1016/s0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 21.Wippersteg V, Kapp K, Kunz W, et al. Characterisation of the cysteine protease ER60 in transgenic Schistosoma mansoni larvae. Int J Parasitol. 2002;32:1219–24. doi: 10.1016/s0020-7519(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 22.Wippersteg V, Kapp K, Kunz W, et al. HSP70-controlled GFP expression in transiently transformed schistosomes. Mol Biochem Parasitol. 2002;120:141–50. doi: 10.1016/s0166-6851(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 23.Wippersteg V, Sajid M, Walshe D, et al. Biolistic transformation of Schistosoma mansoni with 5′ flanking regions of two peptidase genes promotes tissue-specific expression. Int J Parasitol. 2005;35:583–9. doi: 10.1016/j.ijpara.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Yuan XS, Shen JL, Wang XL, et al. Schistosoma japonicum: a method for transformation by electroporation. Exp Parasitol. 2005;111:244–9. doi: 10.1016/j.exppara.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Freitas TC, Jung E, Pearce EJ. TGF-beta Signaling Controls Embryo Development in the Parasitic Flatworm Schistosoma mansoni. PLoS Pathog. 2007;3:e52. doi: 10.1371/journal.ppat.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correnti JM, Jung E, Freitas TC, et al. Transfection of Schistosoma mansoni by electroporation and the description of a new promoter sequence for transgene expression. Int J Parasitol. 2007 doi: 10.1016/j.ijpara.2007.02.011. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Beckmann S, Wippersteg V, El-Bahay A, et al. Schistosoma mansoni: Germ-line transformation approaches and actin-promoter analysis. Expt Parasitol. 2007 doi: 10.1016/j.exppara.2007.04.007. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Morales ME, Mann VH, Kines KJ, et al. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. Faseb J. 2007;21:1–11. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- 29.Tannous BA, Kim DE, Fernandez JL, et al. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–43. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Verhaegent M, Christopoulos TK. Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal Chem. 2002;74:4378–85. doi: 10.1021/ac025742k. [DOI] [PubMed] [Google Scholar]
- 31.Wippersteg V, Ribeiro F, Liedtke S, et al. The uptake of Texas Red-BSA in the excretory system of schistosomes and its colocalisation with ER60 promoter-induced GFP in transiently transformed adult males. Int J Parasitol. 2003;33:1139–43. doi: 10.1016/s0020-7519(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 32.Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods. 2006;3:977–9. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- 33.Remy I, Michnick SW. Application of protein-fragment complementation assays in cell biology. Biotechniques. 2007;42:137–9. doi: 10.2144/000112396. passim. [DOI] [PubMed] [Google Scholar]
- 34.Cheng G, Cohen L, Mikhli C, et al. In vivo translation and stability of trans-spliced mRNAs in nematode embryos. Mol Biochem Parasitol. 2007 doi: 10.1016/j.molbiopara.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis FA. In: Schistosomiasis, in Current Protocols in Immunology. Coligan JA, et al., editors. John Wiley and Sons, Inc.; New York: 1998. pp. 19.1.1–1.28. [Google Scholar]


