Abstract
Introduction
Cardiopulmonary bypass (CPB) produces inflammation and oxidative stress which contribute to post-operative complications following cardiac surgery. F2-Isoprostanes (F2-IsoPs) are products of lipid oxidative injury and represent the most accurate markers of oxidative stress. CPB is associated with elevated IsoPs in adults undergoing cardiac surgery. The relationship between F2-IsoPs and peri-operative end-organ function in infants with single ventricle physiology, however, has not been well studied.
Materials and Methods
Twenty infants (age 3m – 12m) with univentricular physiology undergoing elective Stage II palliation (bidirectional cavopulmonary anastomosis) were prospectively enrolled. Blood samples were collected prior to surgical incision (T0), 30 minutes after initiation of CPB (T1), immediately after separation from CPB (T2), and 24 hours post-operatively (T3). Plasma F2-IsoP levels were measured at each time point and correlated with indices of pulmonary function and other relevant clinical variables.
Results
Plasma F2-IsoPs increased significantly during surgery, with highest levels seen immediately after separation from CPB (p < 0.001). After separation from CPB, increased F2-IsoP was associated with lower arterial pH, higher PaCO2, and decreased lung compliance (ρ = −0.564, 0.633, −0.783; p = 0.012, 0.004, <0.001 respectively). Post-CPB F2-IsoPs did not correlate with duration of CPB, arterial lactate, or immediate post-operative outcomes.
Discussion
CPB produces oxidative stress in infants with single ventricle physiology as quantified by elevated F2-IsoP levels. Increased F2-IsoP levels correlated with impaired ventilation in the post-operative period. The extent to which F2-IsoPs and other bioactive products of lipid oxidative injury might predict and/or contribute to organ specific stress warrants further investigation.
Keywords: Isoprostanes, oxidative stress, cardiopulmonary bypass, congenital heart disease
INTRODUCTION
Cardiopulmonary bypass (CPB) produces inflammation and oxidative stress which contribute to post-operative complications following cardiac surgery [28, 29]. Atrial fibrillation [4, 24], pulmonary dysfunction [2, 22], acute renal injury [13], and neurocognitive dysfunction [8, 25] all represent important end-organ injuries thought to be related to peri-operative oxidative stress. Post-operative pulmonary dysfunction in infants with single ventricle physiology is particularly problematic due to their unique physiology which requires low pulmonary resistance for passive pulmonary blood flow. The relationship between the extent of CPB-induced oxidative injury and the severity of post-operative pulmonary dysfunction in this population, however, has not been clearly demonstrated.
Isoprostanes (IsoPs) are prostaglandin-like molecules formed in vivo by free radical mediated oxidation of arachidonic acid which can be measured in all biologic fluids, including plasma, urine, and cerebrospinal fluid [15, 18, 19]. The F2-IsoPs (named for their F-type prostane ring) are a particularly stable isoform which provide the most accurate measure of oxidative stress in vivo [12]. Cardiac surgery results in elevated F2-IsoP levels in adults [27], and studies comparing operations with and without CPB have found significantly higher plasma F2-IsoP levels in patients exposed to CPB [6], suggesting this form of extracorporeal circulation is a unique contributor to oxidative stress.
Plasma levels of inflammatory cytokines, complement fragments, and other biomarkers of oxidative stress are known to be elevated in children and infants following cardiac surgery [5, 7, 16], but their association with end-organ dysfunction is less clear. Furthermore, there is no published data on the time course and extent of F2-IsoP elevation during cardiac surgery in pediatric patients, much less regarding the relationship of peri-operative F2-IsoP levels with post-operative pulmonary function in this population. The purpose of this investigation was to determine the time course of peri-operative oxidative injury, as measured by plasma F2-IsoP levels, and to test the hypothesis that oxidative injury correlates with post-operative pulmonary dysfunction in a population of children with single ventricle physiology undergoing Stage II bidirectional cavopulmonary anastomosis (BCPA).
METHODS
Patient selection
Infants with single ventricle physiology undergoing elective Stage II palliation (BCPA) between the ages of 3 and 12 months at Vanderbilt Children’s Hospital were enrolled prospectively. Patients were excluded if they had a history of premature birth (<36 weeks gestational age) or known genetic syndromes. Of 34 infants who presented for Stage II palliation between January, 2009 and March, 2010, 24 were eligible. Of these, 20 patients were enrolled, and 19 completed the study. The study protocol was approved by the Vanderbilt University Institutional Review Board, and written informed consent was obtained from the parents of all infants at the time of enrollment.
Anesthesia and cardiopulmonary bypass
Patients received pre-operative sedation with oral midazolam (0.5–0.7 mg/kg). General endotracheal anesthesia was induced with a mixture of inhaled sevoflurane, nitrous oxide, and oxygen, and maintained with isoflurane, fentanyl, and pancuronium. During mechanical ventilation, isoflurane was equilibrated to a 0.2 – 1% end-tidal concentration, and oxygen concentration was titrated to a goal systemic oxygen saturation of 75 – 90%. Continuous monitoring included standard 5-lead electrocardiography, pulse oximetry, end-expiratory CO2, invasive arterial blood pressure, central venous pressure, rectal and nasal temperatures, and cerebral oximetry using near-infrared spectrophotometry (NIRS) (Somanetics, INVOS; Troy, MI). Methylprednisolone (7 mg/kg) was administered intravenously to all patients prior to the initiation of CPB.
All operations were performed on CPB with bicaval venous cannulation. The extracorporeal circuit included a roller pump (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI), tubing set, oxygenator with hard-shell venous reservoir, hemoconcentrator, and continuous in-line monitoring (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI). The circuit was primed with a mixture of albumin, packed red blood cells, fresh frozen plasma and plasmalyte-A. After pre-bypass ultrafiltration of this mixture, mannitol, sodium bicarbonate, calcium chloride, and heparin were added to the washed circuit prime in doses adjusted for body surface area. Perfusion flow rates during normothermia were 100–175 ml/kg/min, and pump flow was gradually reduced during cooling per standard protocol. Moderate hypothermia (34–35°C) was achieved for cases not requiring aortic cross clamping (n = 14), while lower core temperatures (31–32°C) were achieved during aortic cross clamping (n = 5). In 5 cases requiring aortic cross-clamp, standard cold-blood cardioplegia solution (containing sodium bicarbonate, lidocaine, heparin, and potassium chloride) was used for myocardial protection. Modified ultrafiltration was performed in all patients after termination of bypass with a pediatric hemoconcentrator (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI).
Unfractionated heparin (400 U/kg) was administered intravenously and an activated clotting time (ACT) of > 400 seconds (International Technidyne Corporation, Edison, NJ) was verified prior to the initiation of CPB. ACT was monitored every 30 minutes and was maintained > 400 seconds during CPB. Heparin was neutralized with protamine in doses adjusted for the amount of circulating heparin and the patient’s ACT at the end of CPB.
Blood sampling
Blood samples (5mL) were collected after anesthesia induction and before initiation of surgery (baseline, T0), 30 minutes after the initiation of CPB (T1), after separation from bypass and protamine administration (T2), and 24 hours after separation from CPB (T3). Blood was drawn from an indwelling arterial line (or from an indwelling central venous line in cases where an arterial line was not available), transferred into tubes containing potassium EDTA, and placed immediately on ice. Samples were centrifuged at 3200g for 15 minutes within 1 hour of collection, and the supernatant plasma was distributed in 0.5 – 1 mL aliquots which were then stored at −70 °C for later analysis.
Isoprostane analysis
Plasma sample preparation
One ml of plasma was added to 1.0 ng of [2H4]-15-F2t-IsoP ([2H4]-8-iso-PGF2a; Cayman Chemical, Ann Arbor, MI) internal standard. The solution was then processed by methods previously described such that derivatized F2-IsoP compounds were isolated, dried, and re-dissolved for GC/MS analysis [17, 21].
Mass Spectrometric analysis of F2-IsoP
GC/NICI-MS was carried out on an Agilent 5973 Inert Mass Selective Detector that is coupled with an Agilent 6890n Network GC system (Agilent Labs, Torrance, CA) by methods previously described. Levels of endogenous F2-IsoPs in a biological sample are calculated from the ratio of intensities of the ions m/z 569 (major ion generated by the F2-IsoP derivative) to m/z 573 (corresponding ion generated by the internal standard). The precision of this assay in biological fluids is ±6% and the accuracy is 94% [17, 21].
Clinical data collection
Clinical parameters including vital signs, cerebral oximetry, ventilatory parameters (including FiO2, tidal volume [TV], peak inspiratory pressure [PIP], and positive end expiratory pressure [PEEP]), and laboratory values including arterial blood gas analysis and hematrocrit were recorded at the time of each blood sample collection.
Lung compliance was determined using the following equation:
Information regarding duration of CPB, temperature, inotropic support, ventilator time, length of ICU stay, length of hospital stay, and postoperative complications was collected from the patient medical record. Pre-operative data including Stage I operation, baseline hemodynamics (pulmonary artery pressures and pulmonary vascular resistance), and outpatient medications were also collected from the medical record and recorded.
Statistical Analysis
Data are presented as mean ± standard deviation (SD) unless otherwise noted. F2-IsoP levels were compared over time using repeated measures ANOVA, and pair wise comparisons were made using the Tukey’s Honestly Significant Difference (HSD) test in order to adjust for inflated type I error in the setting of multiple comparisons. Associations between F2-IsoP levels and continuous clinical parameters were analyzed using Spearman’s rank correlation. Tests were two-tailed and a p-value of <0.05 was considered significant. Statistical analysis was performed using SPSS software v.19.0 (SPSS Inc., Chicago, IL), R software 2.10.1 (R-project.org), and GraphPad Prism software v.5.0 (GraphPad software Inc., San Diego, CA).
RESULTS
Patient characteristics
Of 19 patients who underwent BCPA, 11 (58%) had hypoplastic left heart syndrome, 2 (11%) had another form of single right ventricle, and 6 (31%) had a single left ventricle. Eighteen patients (95%) underwent at least one concomitant operation, most often pulmonary artery augmentation at the site of Blalock-Taussig (BT) shunt takedown and BCPA construction (n = 12, 63%). Two patients (11%) had bilateral BCPA. No patients were left with antegrade pulmonary blood flow. Average CPB time was 89.1 ± 42.1 minutes. Five patients (26%) required aortic cross-clamping, with mean duration of 17.4 ± 18.6 minutes (Table 1).
Table 1.
Patient characteristics
| Variable | Data (n = 19) |
|---|---|
| Age (mo) | 4.7 ± 2.2 |
| Gender – n (%) | |
| Male | 15 (79) |
| Female | 4 (21) |
| Race – n (%) | |
| Caucasian | 16 (84) |
| African American | 1 (5) |
| Hispanic | 2 (11) |
| Weight (kg) | 6.1 ± 1.0 |
| Primary Diagnosis –n (%) | |
| HLHS | 11 (58) |
| Other single RV | 2 (11) |
| Other single LV | 6 (31) |
| Concomitant operations – n (%) | |
| PA augmentation | 12 (63) |
| Atrial septectomy | 3 (16) |
| Removal RV-PA connection | 3 (16) |
| DKS | 1 (5) |
| Bilateral BCPA | 2 (11) |
| Baseline SpO2 (%) | 82.1 ± 7.4 |
| Baseline Hgb (g/dL) | 13.2 ± 2.0 |
| CPB time (min) | 89.1 ± 42.1 |
| X-clamp time (min) – *n = 5 | 17.4 ± 18.6 |
n, Number of patients; HLHS, hypoplastic left heart syndrome; RV, right ventricle; LV, left ventricle; PA, pulmonary artery; DKS, Damus-Kaye Stansel procedure; BCPA, bidirectional cavopulmonary anastomosis; SpO2, oxygen saturation by pulse oximetry; Hgb, hemoglobin; CPB, cardiopulmonary bypass; X-clamp, aortic cross-clamp
Clinical outcomes
Median peri-operative length of stay was 11 days (range 4–54 days). Median intubation time was 1 day (range 1–25 days), and median duration of intensive care unit (ICU) admission was 4 days (range 1–32 days). Four patients (21%) had persistent pleural effusions requiring chest tube placement for more than 5 days, with a median duration of chest tube drainage of 3 days (range 2–21 days).
Six patients (32%) required reoperation, including re-exploration, delayed chest closure, or wound debridement. Four patients (21%) with persistently elevated cavopulmonary pressures required inhaled nitric oxide (iNO) therapy in the immediate post-operative period, but only 1 of these was discharged on long-term pulmonary vasodilator therapy. Intubation time for these 4 patients was slightly longer than the rest of the cohort (median 2.5 days, range 2–7 days). Other post-operative complications included infection in 4 (21%), new thrombosis in 5 (26%), and arrhythmia in 1 (5%).
F2-Isoprostane Levels
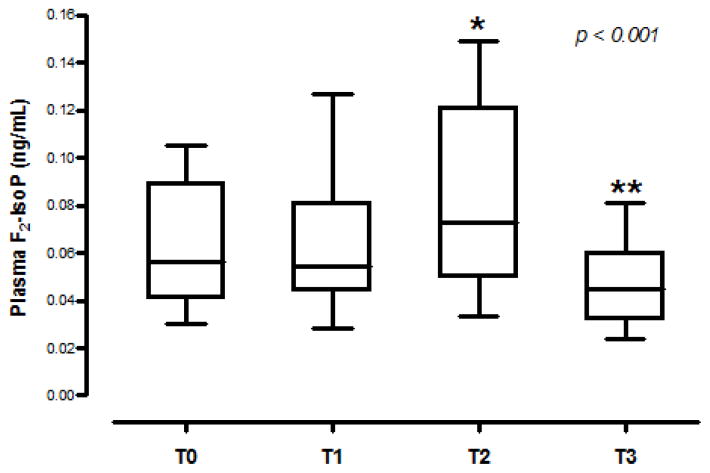
Plasma F2-IsoP changed significantly over time, with the highest levels seen immediately after separation from bypass (p < 0.001). There was a significant increase from baseline (T0, 0.062 ± 0.024 ng/mL) to after separation from bypass (T2, 0.084 ± 0.037 ng/mL) (95% CI for the change = 0.01 to 0.04, p = 0.001), with return to baseline levels or lower by 24 hours post-operatively (T3, 0.047 ± 0.016 ng/mL) (Figure 1). A positive correlation was observed between baseline F2-IsoP level (T0) and post-CPB F2- IsoP level (T2) (ρ = 0.678, p=0.001).
Fig 1. F2-Isoprostane levels increase after CPB.
Box-and-whisker plot demonstrating F2- Isoprostane (IsoP) levels at each time point: T0 (Baseline), T1 (30 minutes on CPB), T2 (immediately post-bypass), and T3 (24h post-bypass). The middle line represents the median value; the central box represents the values between the upper and lower quartiles; the vertical lines extend to 1.5 interquartile ranges from both ends of the box, excluding outliers (n = 19). (*) indicates p < 0.05 comparing T0 and T2, (**) indicates p < 0.05 comparing T2 and T3.
F2-Isoprostane correlations with pulmonary function and clinical variables
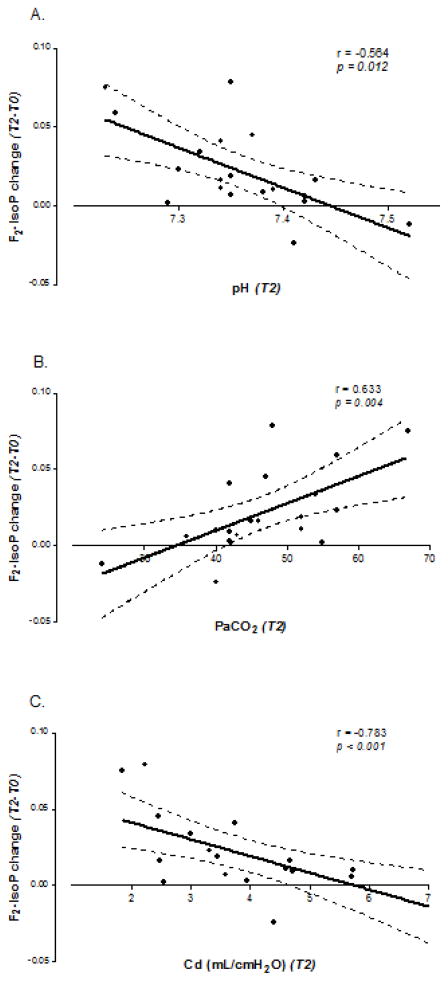
The change in plasma F2-IsoP levels from baseline (T0) to after separation from bypass (T2) was highly correlated with lower pH, higher PaCO2, and decreased lung compliance (ρ = −0.564, 0.633, −0.783; p = 0.012, 0.004, <0.001 respectively) (Figure 2a, 2b, 2c). Increased F2-IsoP levels did not correlate with duration of CPB, blood transfusion requirement, highest PaO2 documented on bypass, lactate, cerebral oxygenation, or transpulmonary gradient. Furthermore, F2-IsoP levels did not correlate with short-term post-operative outcomes such as ventilatory support requirement, duration of chest tube drainage, or length of hospital stay (Table 2).
Fig 2. Increase in F2-Isoprostane levels correlates with markers of impaired respiratory mechanics.
Correlation between F2-Isoprostane (F2-IsoP) change from baseline (T0) to post-bypass (T2) and pH (a), PaCO2 (b), and dynamic lung compliance (Cd) (c). Solid lines indicate linear regression fit; dotted lines indicate 95% confidence intervals for the mean.
Table 2.
Spearman rank correlations between clinical variables and F2-Isoprostane change from baseline (T0) to post-bypass (T2)
| Variable | Correlation coefficient (ρ) | p-value |
|---|---|---|
|
| ||
| Intra-operative variables | ||
| CPB time | 0.070 | 0.776 |
| Blood product transfusion requirement | −0.332 | 0.165 |
| Peak PaO2 on CPB | 0.217 | 0.373 |
|
| ||
| Indicators of peri-operative hemodynamic status | ||
| Highest peri-operative lactate | −0.263 | 0.277 |
| Lowest peri-operative cerebral oxygen saturation (NIRS) | 0.429 | 0.397 |
| Transpulmonary gradient (T2) | −0.120 | 0.683 |
|
| ||
| Indicators of peri-operative respiratory mechanics | ||
| pH (T2) | −0.564 | 0.012* |
| PaCO2 (T2) | 0.633 | 0.004* |
| Lung compliance (Cd) (T2) | −0.783 | <0.001* |
|
| ||
| Post-operative outcomes | ||
| ICU days | 0.046 | 0.855 |
| Chest tube days | 0.364 | 0.138 |
| Total hospital days | 0.203 | 0.420 |
CPB, cardiopulmonary bypass; NIRS, near infrared spectrophotometry; ICU, intensive care unit
DISCUSSION
Our results show that plasma F2-IsoP levels are elevated in children with univentricular physiology undergoing Stage II surgical palliation (BCPA) on CPB. The temporal profile of oxidative stress surrounding CPB demonstrated in this study is similar to that observed in adults, although the highest levels in adults were during CPB [27] whereas our patients demonstrated the greatest elevation immediately following separation from bypass. This difference may be due to higher priming blood volumes required for infant CPB, resulting in dilutional changes in concentration and therefore relatively lower measured F2-IsoPs while on CPB. We also observed an association between increased circulating F2-IsoP levels and decreased pulmonary compliance in the immediate post-operative period. Because all patients received pressure-controlled ventilation after CPB, decreased pulmonary compliance resulted in lower pH and higher PaCO2, which were also correlated with an increase in F2-IsoP levels.
Pulmonary dysfunction is a known complication of cardiac surgery and CPB related to ischemia-reperfusion injury, blood transfusion, hypothermia, endothelial dysfunction, activation of inflammatory mediators, increased vascular permeability, and pulmonary edema [22, 26]. Despite improvements in peri-operative management, including modified ultrafiltration to reduce total body water and pulmonary edema following CPB, pulmonary complications remain a major cause of morbidity following cardiac surgery [3, 10, 14].
Oxidative stress is known to contribute to lung injury after cardiac surgery, as well as in other pulmonary diseases in which F2-IsoP levels are known to be elevated [2, 11, 20, 23]. While F2-IsoPs are biologically inactive, other forms of isoprostanes produced in response to oxidative stress, specifically those with E-type prostane rings, exert biologic effects in nearly all cell types within the lung, including airway smooth muscle, vascular smooth muscle and endothelium, and alveolar epithelial cells [11]. Because the biologically active isoprostane isoforms are relatively unstable, they are more difficult to analyze than the F2-IsoP isoform. Elevated F2-IsoPs, therefore, may represent not only a marker of overall oxidative stress following cardiac surgery, but may also indicate ongoing cellular injury and oxidative stress due to the concomitant accumulation of biologically active isoprostanes. As our results suggest, this ongoing damage is evident within the lung tissue of infants undergoing CPB.
The extent and causes of post-operative pulmonary dysfunction are particularly relevant to patients with BCPA physiology. Decreased pulmonary compliance can lead to impaired pulmonary blood flow, cyanosis, and decreased cardiac output. Diastolic dysfunction and impaired ventricular filling – often seen in the immediate post-operative period as a result of CPB-induced myocardial ischemia-reperfusion injury, inflammation, and edema – can further worsen pulmonary blood flow in this physiology which relies on passive pulmonary blood flow and low filling pressures. Furthermore, as pulmonary and cerebral circulations are now coupled in series, pulmonary vascular dysfunction can contribute to cerebral venous congestion and impaired cerebral perfusion. Because CO2 has opposing effects on the pulmonary and cerebral circulation (with vasoconstricting effects in the lung and vasodilating effects in the brain), ventilatory management strategies after BCPA have been studied closely. Pulmonary vasodilators, such as inhaled nitric oxide, have been shown to improve pulmonary blood flow and cardiac output in patients struggling with cyanosis following BCPA [1]. Conversely, elevated PaCO2 – a potent pulmonary vasoconstrictor – causes cerebral vasodilation and improved cerebral blood flow which, in BCPA physiology, also translates to improved pulmonary blood flow and cardiac output during the post-operative period [9]. For this reason, many institutions use controlled hypoventilation as a strategy for maintaining slightly higher PaCO2 following BCPA. In our study, higher PaCO2 after separation from bypass was associated with increased F2-IsoP production, suggesting more overall oxidative injury and raising questions about how ventilatory manipulations may impact peri-operative oxidative stress and ongoing lung injury.
One notable observation in this work is that there was no clear correlation between the extent of F2-IsoP elevation and intra-operative variables expected to contribute to oxidative stress such as duration of CPB, blood transfusion requirement, and highest PaO2 on bypass. Furthermore, increased F2-IsoPs were not associated with commonly used clinical monitoring parameters including arterial blood lactate, cerebral oxygenation, or transpulmonary gradient. Similarly, peri-operative F2-IsoP levels did not significantly impact short term clinical outcomes, including ventilatory support requirement, duration of chest tube drainage, or length of hospital stay. Additional intra-operative procedures, including aortic cross-clamp, also had no impact on F2-IsoP levels or short-term clinical outcomes.
Study Limitations
Studies in pediatric cardiac surgery are complicated by the heterogeneity of cardiac lesions and the variety of operations these children undergo, which limits statistical power and clinical applicability. Despite attempts to minimize this problem by studying a relatively homogeneous group of patients, even this select group included a variety of diagnoses and surgical techniques which certainly affected the power of our analysis. In addition, small sample size limited our ability to perform more in-depth statistical analysis, including multivariable linear regression and/or subgroup comparisons to identify predictors of increased F2-IsoP production. Finally, we recognize that children with single ventricle physiology represent a very complex group of patients whose F2-IsoP production may differ significantly from other children requiring surgical intervention on CPB. Future work should explore F2-IsoP trends in a larger cohort of pediatric patients, ideally comparing peri-operative F2-IsoP production in children with cyanotic lesions to those with non-cyanotic lesions. Additional focus on organ specific assessment of lipid oxidation and long term outcomes should also be considered.
Conclusions
CPB produces oxidative stress in infants with single ventricle physiology as quantified by elevated F2-IsoP levels. In this group, increased F2-IsoP levels correlated with decreased pulmonary compliance and markers of impaired ventilation in the immediate post-operative period. The extent to which F2-IsoPs and other bioactive products of lipid oxidative injury might contribute to peri-operative organ specific stress warrants further investigation. The utility of F2-IsoPs as a predictor of long term outcomes requires ongoing study.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Prince Kannankeril, C. Michael Stein, L. Jackson Roberts III, Sean Davies, Gregg Stanwood, Louis Muglia, and Judy Aschner, as well as Tom Klein, M.S. for their advice in project development as well as their editorial and technical assistance.
FUNDING SOURCES
Supported by NIH Grants NS050396 (B.M.), GM07569–32 (E.A.), 1 UL1 RR024975 from NCRR/NIH (Vanderbilt Institute for Clinical and Translational Research), and P30HD15052 (Vanderbilt Kennedy Center).
Abbreviations
- CPB
cardiopulmonary bypass
- F2-IsoPs
F2-Isoprostanes
- BCPA
bidirectional cavopulmonary anastomosis
- ACT
activated clotting time,
Footnotes
This paper is submitted with the full knowledge and authorization of all listed authors.
References
- 1.Agarwal HS, Churchwell KB, Doyle TP, Christian KG, Drinkwater DC, Jr, Byrne DW, Taylor MB. Inhaled nitric oxide use in bidirectional Glenn anastomosis for elevated Glenn pressures. Ann Thorac Surg. 2006;81:1429–1434. doi: 10.1016/j.athoracsur.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Angdin M, Settergren G, Starkopf J, Zilmer M, Zilmer K, Vaage J. Protective effect of antioxidants on pulmonary endothelial function after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:314–320. doi: 10.1016/s1053-0770(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 3.Apostolakis EE, Koletsis EN, Baikoussis NG, Siminelakis SN, Papadopoulos GS. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg. 5:1. doi: 10.1186/1749-8090-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: a meta-analysis. Eur J Cardiothorac Surg. 2009;35:521–527. doi: 10.1016/j.ejcts.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Caputo M, Mokhtari A, Rogers CA, Panayiotou N, Chen Q, Ghorbel MT, Angelini GD, Parry AJ. The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2009;138:206–214. doi: 10.1016/j.jtcvs.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalca V, Sisillo E, Veglia F, Tremoli E, Cighetti G, Salvi L, Sola A, Mussoni L, Biglioli P, Folco G, Sala A, Parolari A. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. Ann Thorac Surg. 2006;81:562–567. doi: 10.1016/j.athoracsur.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, Schmid ER, Schmitt B. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38:1323–1332. doi: 10.1016/j.freeradbiomed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Clancy RR. Neuroprotection in infant heart surgery. Clin Perinatol. 2008;35:809–821. viii. doi: 10.1016/j.clp.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Hoskote A, Li J, Hickey C, Erickson S, Van Arsdell G, Stephens D, Holtby H, Bohn D, Adatia I. The effects of carbon dioxide on oxygenation and systemic, cerebral, and pulmonary vascular hemodynamics after the bidirectional superior cavopulmonary anastomosis. J Am Coll Cardiol. 2004;44:1501–1509. doi: 10.1016/j.jacc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Yao T, Wang W, Zhu D, Zhang W, Chen H, Fu W. Continuous ultrafiltration attenuates the pulmonary injury that follows open heart surgery with cardiopulmonary bypass. Ann Thorac Surg. 2003;76:136–140. doi: 10.1016/s0003-4975(03)00264-9. [DOI] [PubMed] [Google Scholar]
- 11.Janssen LJ. Isoprostanes and lung vascular pathology. Am J Respir Cell Mol Biol. 2008;39:383–389. doi: 10.1165/rcmb.2008-0109TR. [DOI] [PubMed] [Google Scholar]
- 12.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C, Lamy A, Legare JF, Mazer CD, McCluskey SA, Rubens FD, Sawchuk C, Beattie WS. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 14.Keenan HT, Thiagarajan R, Stephens KE, Williams G, Ramamoorthy C, Lupinetti FM. Pulmonary function after modified venovenous ultrafiltration in infants: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2000;119:501–505. doi: 10.1016/s0022-5223(00)70129-2. discussion 506–507. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Stern A, Roberts LJ, Morrow JD. The isoprostanes: novel prostaglandin-like products of the free radical-catalyzed peroxidation of arachidonic acid. J Biomed Sci. 1999;6:226–235. doi: 10.1007/BF02253564. [DOI] [PubMed] [Google Scholar]
- 16.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–413. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 17.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 18.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow JD, Chen Y, Brame CJ, Yang J, Sanchez SC, Xu J, Zackert WE, Awad JA, Roberts LJ. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab Rev. 1999;31:117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med. 2002;166:S25–30. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 22.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121:1269–1277. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 23.Ovechkin AV, Lominadze D, Sedoris KC, Robinson TW, Tyagi SC, Roberts AM. Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem. 2007;113:1–12. doi: 10.1080/13813450601118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramlawi B, Otu H, Mieno S, Boodhwani M, Sodha NR, Clements RT, Bianchi C, Sellke FW. Oxidative stress and atrial fibrillation after cardiac surgery: a case-control study. Ann Thorac Surg. 2007;84:1166–1172. doi: 10.1016/j.athoracsur.2007.04.126. discussion 1172–1163. [DOI] [PubMed] [Google Scholar]
- 25.Ramlawi B, Rudolph JL, Mieno S, Khabbaz K, Sodha NR, Boodhwani M, Levkoff SE, Marcantonio ER, Sellke FW. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006;244:593–601. doi: 10.1097/01.sla.0000239087.00826.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stayer SA, Diaz LK, East DL, Gouvion JN, Vencill TL, McKenzie ED, Fraser CD, Andropoulos DB. Changes in respiratory mechanics among infants undergoing heart surgery. Anesth Analg. 2004;98:49–55. doi: 10.1213/01.ANE.0000096005.25218.74. table of contents. [DOI] [PubMed] [Google Scholar]
- 27.Ulus AT, Aksoyek A, Ozkan M, Katircioglu SF, Basu S. Cardiopulmonary bypass as a cause of free radical-induced oxidative stress and enhanced blood-borne isoprostanes in humans. Free Radic Biol Med. 2003;34:911–917. doi: 10.1016/s0891-5849(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 28.Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Warren OJ, Watret AL, de Wit KL, Alexiou C, Vincent C, Darzi AW, Athanasiou T. The inflammatory response to cardiopulmonary bypass: part 2--anti-inflammatory therapeutic strategies. J Cardiothorac Vasc Anesth. 2009;23:384–393. doi: 10.1053/j.jvca.2008.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.