Abstract
The mammalian superior colliculus (SC) and its nonmammalian homolog, the optic tectum, constitute a major node in processing sensory information, incorporating cognitive factors, and issuing motor commands. The resulting action—to orient toward or away from a stimulus—can be accomplished as an integrated movement across oculomotor, cephalomotor, and skeletomotor effectors. The SC also participates in preserving fixation during intersaccadic intervals. This review highlights the repertoire of movements attributed to SC function and analyzes the significance of results obtained from causality-based experiments (microstimulation and inactivation). The mechanisms potentially used to decode the population activity in the SC into an appropriate movement command are also discussed.
Keywords: saccade, fixation, head, gaze shift, reach, vibrissae, pinnae, sonar, vector averaging, vector summation
Introduction
Visualize a pitcher throwing a fastball to the batter, who swings the bat and makes solid contact with the baseball. It is driven sharply and directly back at the pitcher, who in turn reacts to snatch the ball just before it hits him. To record the out and also to save himself from injury, the pitcher must rely on visual cues about the location and trajectory of the ball after it is hit, auditory information such as the timing and intensity of the contact between the bat and ball, as well as proprioceptive signals and/or internal representations of body positions after releasing the ball. Neural signals representing these events must be integrated and transformed into a coordinated movement of the eyes, head, hands, and body to make the catch. Such a simple and seemingly reflexive orienting behavior is produced by a rather complicated set of neural processes that successfully accomplish the sensory to motor transformation.
The superior colliculus (SC) is a major node for mediating sensorimotor transformations (Hall & Moschovakis 2004, Sparks & Mays 1990, Stein & Meredith 1993). Residing on the roof of the brain stem, this subcortical structure contains seven alternating fibrous and cellular laminae. Neurons in the superficial layers (stratum zonale, stratum griseum superficiale, and stratum opticum) are responsive nearly exclusively to visual stimuli appearing at specific locations in the contralateral hemifield. A subset of cells in the intermediate (stratum griseum intermedium, and stratum album intermedium) and deeper (stratum griseum profundum, and stratum album profundum) layers, collectively referred to as the deep layers, expresses sensitivity to sensory stimuli from several modalities (e.g., vision, audition, somatosensation), and another overlapping group of neurons discharges a vigorous premotor burst during the orienting movement. This review is intended to provide a critical assessment on various topics related to the motor functions of the SC. The repertoire of movements attributed to the SC and its nonmammalian homolog, the optic tectum (OT), is considered first, followed by an evaluation of collicular mechanisms for saccade generation. We specifically address the dynamic features of ensemble activity that permit a balance between fixation and redirection of the visual axis and the computational rules that best explain how population activity in the SC is decoded to produce saccades.
Repertoire of Movements Produced by the Superior Colliculus
Eye Movements
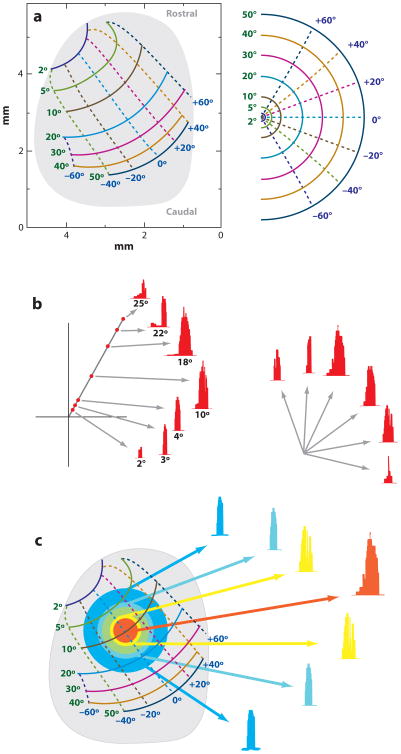
When the head is restrained from moving, neurons in the deep layers of the SC discharge a burst prior to contraversive saccades. The elevated activity exists for only a range of saccade vectors that define each cell's movement field (Straschill & Hoffmann 1970, Wurtz & Goldberg 1972). The response is maximal for an optimum amplitude and direction, also known as the center or “hot spot” of the movement field, and graded for increasing and decreasing vectors (Figure 1b). Neurons in the SC are organized according to their movement field centers (Figure 1a) (Sparks et al. 1976). Cells located rostrally within the SC discharge vigorously for small-amplitude saccades, whereas units found at more caudal locations burst optimally for large-size movements. Neurons within the medial and lateral regions are most active during saccades with upward and downward components, respectively. Micro-stimulation of the SC produces saccade vectors that conform to this organization (McHaffie & Stein 1982, Robinson 1972, Stanford et al. 1996, Straschill & Rieger 1973). The topography of saccade amplitude onto the SC is logarithmic: A disproportionately large amount of SC tissue is allocated for small saccades, corresponding to movements to a parafoveal space, whereas a relatively compressed region is attributed for larger amplitude saccades to peripheral locations. In contrast, the saccade direction map along the mediolateral extent of the SC is fairly linear (Ottes et al. 1986).
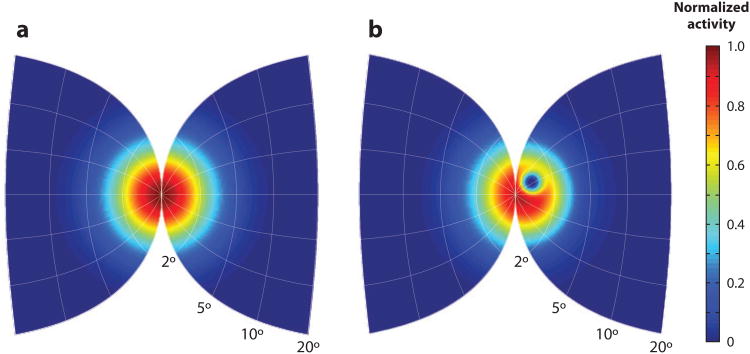
Figure 1.
Fundamental properties of the superior colliculus (SC) for the generation of saccades. (a) A schematic of the topographic organization of contralateral saccade vectors (left) is encoded in retinotopic coordinates. Isoradial and isodirectional bands are shown as solid and dashed lines, respectively. The radial and directions bands are identified in green and blue numbers, respectively. Each band is represented in a different color. The mapping of these bands in the contralateral hemifield is shown in the right panel. A disproportionately large amount of SC space is used to produce small amplitude saccades relative to the caudal SC areas that produce larger vectors. (b) Neurons in the deep SC layers discharge for a range of saccade amplitudes and directions. Its location on the SC map dictates the optimal vector for which the cell emits its maximal burst. Burst profiles are shown for different amplitude saccades in the optimal direction (left) and for several optimal amplitude saccades in various directions (right). Adapted from Sparks & Gandhi (2003). (c) Population response for the generation of a saccade can be envisioned as a mound of activity across a large portion of the deep SC layers. The amplitude and direction of the executed saccade typically matches with the vector encoded at the locus of maximal activity. Neurons that are active but are located away from the center exhibit a suboptimal burst. Adapted from Sparks & Gandhi (2003).
Because each SC neuron bursts for a range of saccades, it follows that a population of cells, generally envisioned as a Gaussian mound, emits spikes for every movement (Figure 1c). In general, ∼28% of the neurons in the deep layers (Munoz & Wurtz 1995b) discharge a premotor burst prior to the generation of a saccade. The size of the active population and the total number of spikes produced in the premotor burst are invariant across all saccades (Anderson et al. 1998, Van Opstal & Goossens 2008), but the mound is centered at the site corresponding to the executed saccade vector. To produce a wide range of saccade amplitudes from a relatively constant output, SC projections to the brainstem burst generator have to be weighted. Consistent with this hypothesis, the number of terminal boutons deployed onto the horizontal component of the burst generator increases monotonically across the rostral-caudal extent of the SC (Moschovakis et al. 1998).
The terminology used to categorize saccade-related neurons in the SC has evolved as investigators probed for specific functionality. It is important to note that these neurons are distinctly different from visual neurons, which reside in the superficial layers of the SC. Visual neurons respond only to sensory (visual) stimulation, and although saccade-related neurons can have sensory responses, they are primarily involved with motor function. The current nomenclature for saccade-related neurons is based primarily on studies performed by Munoz and colleagues (Munoz & Guitton 1991; Munoz & Wurtz 1993a, 1995a). Three major classes exist within the SC:
Saccade-related burst neurons emit a high-frequency volley of spikes prior to producing the high-speed eye movement.
Buildup neurons discharge a low-level or prelude response that accumulates gradually during the sensorimotor integration period before transitioning into a high-frequency burst to produce the saccade. This low-frequency discharge has been attributed to processes such as motor preparation, target selection, attention, and working memory (see Sparks 1999 for a critical commentary).
Fixation neurons in the rostral SC discharge at a tonic rate during visual fixation and pause activity during most, but not all, saccades.
Although used for classification purposes, detailed analyses show that discharge features of SC neurons actually span the continuum across these categories, and in some cases, the labels can offer inaccurate insights into a neuron's function. For example, the so-called fixation neurons actually discharge for a range of saccade amplitudes that include both micro- (Hafed et al. 2009) and small-amplitude macrosaccades (Gandhi & Keller 1997, Krauzlis et al. 1997, Munoz & Wurtz 1993a).
Along with providing saccade vector coordinates, population activity in the SC has also been noted to contribute to the kinematics of a saccade. The dual-coding hypothesis (Sparks & Mays 1990) states that the firing rate of the high-frequency burst contributes to the speed of the movement, whereas the locus of population activity on the SC map indicates the desired saccade vector. Indeed, amplitude-matched visually guided saccades are faster than memory-guided eye movements (Gnadt et al. 1991, Smit et al. 1987), and the accompanying premotor discharge is more vigorous when the visual target remains illuminated (Edelman & Goldberg 2003). Stimulation-evoked saccades also exhibit a similar relationship with stimulation parameters. Their velocity waveforms scale, up to a saturation limit, with stimulation frequency (Stanford et al. 1996) and intensity (Van Opstal et al. 1990). The amplitude is site specific provided that the stimulation duration is long enough to complete the movement. Prolonged stimulation produces a “staircase” of saccades interrupted with brief intersaccadic intervals (Breznen et al. 1996, Missal et al. 1996, Robinson 1972, Stryker & Schiller 1975).
Eye movements other than saccades have also been associated with SC function (for a review, see Gandhi & Sparks 2004). Briefly, a subset of neurons that discharge during saccades alter their response characteristics during combined saccade-vergence movements (Walton & Mays 2003). Stimulation of the SC can either perturb coordinated saccade-vergence movements (Chaturvedi & van Gisbergen 1999), and for some sites in the rostral SC, microstimulation can even induce vergence eye movements (Chaturvedi & Van Gisbergen 2000) and lens accommodation (Sawa & Ohtsuka 1994). Activity of neurons in the rostral SC is also correlated with smooth-pursuit eye movements (Krauzlis 2003; Krauzlis et al. 1997, 2000), although microstimulation of the region does not produce such movements (Basso et al. 2000). A recent study also demonstrated that microstimulation of the barn-owl OT evokes pupil dilation (Netser et al. 2010).
Eye and Head Movements
When the head is free to move, large-amplitude changes in the line of sight cannot be produced by a saccadic eye movement alone. Such gaze shifts are generally executed as a coordinated movement of the eyes and head (Figure 2a) (also see Freedman 2008 for a review). Typically, the onset of the gaze shift is initiated by a saccadic eye-in-head movement, and the head movement lags behind. The offsets of the gaze shift and ocular saccade often coincide, although some discrepancies have been reported. The time of peak head velocity appears synchronized to the end of the gaze shift (Chen & Tehovnik 2007), and the head movement continues for 100–200 ms after gaze shift has terminated, during which the eyes counter-rotate in the orbits to stabilize gaze. By varying the initial positions of the eyes in orbits (and restraining movements of the torso and lower extremities), the same amplitude gaze shift can be produced by different combinations of eye and head movements.
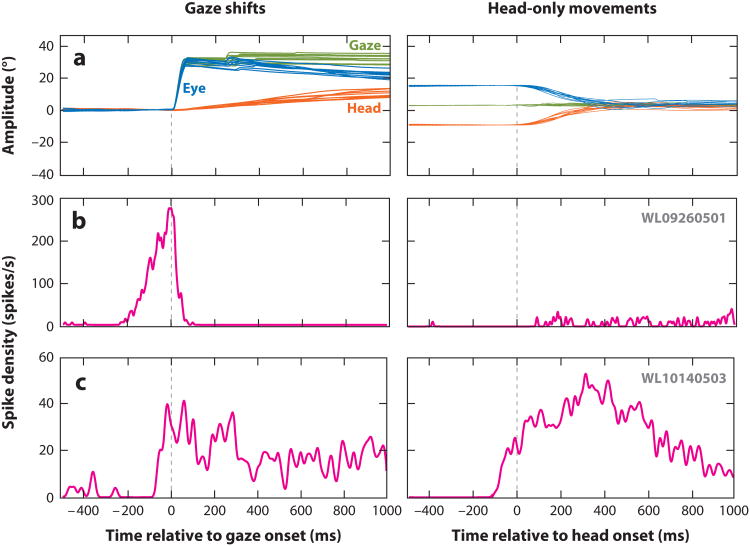
Figure 2.
Activity of superior colliculus (SC) neurons during coordinated eye-head movements (gaze shifts) and head-only movements. (a) Several examples of gaze shifts (left) and head-only movements (right) are plotted as a function of time. For gaze shifts (left), the change in line of sight (equivalently, gaze or eye-in-space) (green traces) is produced initially by rapidly moving the eyes within the orbits (eye-in-head) (blue traces). The head movement (orange traces) typically lags gaze onset, but it can continue for several hundred milliseconds after the termination of the gaze shift, during which the eyes counter-rotate in the orbits. During head-only movements (right), gaze remains stable as the eyes counter-rotate in the orbits. (b) Average spike density waveform of a SC neuron that resembles a classical gaze-related burst neuron. This cell produced a high-frequency burst for optimal size gaze shifts (left), while its activity was negligible for all head-only movements (right). (c) Average spike density waveform of another SC neuron that responds during head-only movements (right). It also discharges for gaze shifts (left), but the duration of activity outlasts the duration of the gaze shift and is better correlated with head duration. Note that the firing rate is too low to be a high-frequency burst even when optimal-amplitude head-only movements and gaze shifts were produced. The traces illustrated in the top row are not the specific movements generated during the neural recordings shown in the bottom two rows. Adapted from Walton et al. (2007), with permission.
The subset of SC neurons that discharge a high-frequency premotor burst before head-restrained saccades also exhibit similar neural activity prior to head-unrestrained gaze shifts. The high-frequency component is optimal for a desired change in gaze, not its individual eye or head component (Freedman & Sparks 1997). Suprathreshold microstimulation of the SC in cats and monkeys produces coordinated eye-head movements with characteristics comparable to visually guided gaze shifts (Freedman et al. 1996; Guillaume & Pélisson 2001, 2006; Harris 1980; Klier et al. 2001; Paré & Guitton 1994; Roucoux et al. 1980). Data suggest that the SC encodes gaze displacement in retinal coordinates (Klier et al. 2001). For a given stimulation site, roughly the same amplitude gaze shift is elicited across variations in the initial eye-in-head position. The amplitudes of the saccadic eye component and the head movement vary inversely as a function of initial eye-in-head position. Thus, as the eyes are initially deviated increasingly in the direction opposite to that of the stimulation-evoked movement, the amplitude of the saccadic eye component increases and that of the accompanying head movement decreases. In species with a negligible oculomotor range, such as the owl and the bat, the change in gaze is produced nearly entirely by the head (du Lac & Knudsen 1990, Valentine et al. 2002).
Changing the frequency of stimulation proportionally modifies the speed of both eye and head components and, therefore, also the speed of the gaze shift (Freedman et al. 1996). Interestingly, prolonged stimulation continues to drive the head movement, albeit at a slower speed, even after the gaze shift comes to an end (du Lac & Knudsen 1990, Freedman et al. 1996). The head-movement amplitude in such cases violates its lawful relationship with gaze amplitude and instead correlates better with stimulation duration. These results suggest that the place code component of the dual-coding hypothesis appears valid only for the line of sight (gaze).
The processing of SC activity by neural elements controlling the neck musculature is not dependent on the generation of a gaze shift. Electromyography (EMG) of neck muscles reveals a transient sensory response linked to the onset of a visual stimulus in the ipsilateral hemifield (Corneil et al. 2004), but perhaps most effectively during reflexive movement tasks (see Pruszynski et al. 2010); the SC could be the primary source of this short-latency response. Low-frequency stimulation of the SC also evokes low-level EMG in the deep neck muscles contralaterally, even when the head is restrained (Corneil et al. 2002a, Roucoux et al. 1980). The EMG response is generally smaller, but not negligible, when stimulation is applied within the rostral SC, and it increases for stimulation delivered at more caudal sites. For any SC site, neck muscle EMG also increases during the period leading to saccade onset, reflecting a correlation with movement preparation (Corneil et al. 2007, Rezvani & Corneil 2008). When the head is unrestrained, similar stimulation parameters can evoke head-only movements (the eyes counter-rotate in the orbits) that precede the onset of a gaze shift (Corneil et al. 2002b, Pélisson et al. 2001). When a gaze shift does follow, the EMG response increases significantly prior to the higher velocity head movement associated with the gaze shift. These results are consistent with the hypothesis that the SC output is processed by two separate pathways in the brain stem. The oculomotor pathway produces the saccadic eye component of the gaze shift and the head pathway innervates the neck muscles. A key distinction between the two is that the eye pathway is potently inhibited by the pontine omnipause neurons (OPNs). These neurons prevent the premature execution of eyemovements until the SC output reaches a threshold, which is usually associated with the high-frequency burst. An absent or significantly weaker gating mechanism on the head pathway permits the generation of head-only movements that can precede gaze shifts (Corneil et al. 2002b, Gandhi & Sparks 2007, Grantyn et al. 2010, Guitton et al. 1990).
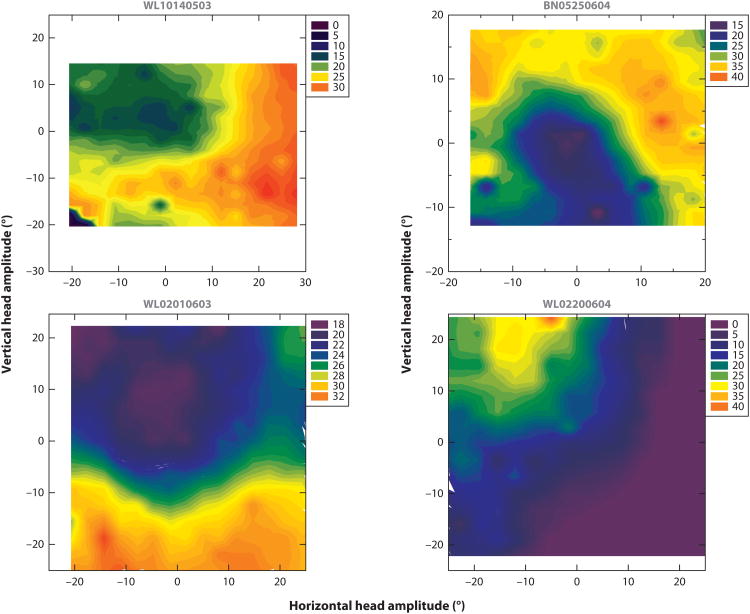
We can generate head movements without changing our line of sight, such as when nodding. Does the SC contribute to the generation of such head-only movements? Or is the relationship between SC output and neck-muscle response constrained only to head movements associated with gaze shifts? Many SC neurons in the deeper layers indeed exhibit various types of modulations when nonhuman primates generate active head-only movements in a controlled, experimental setting (Figure 2a) (Walton et al. 2007). Some neurons increase their firing rates (Figure 2c), whereas others show modest suppression. The maximal firing rates of these neurons are an order of magnitude lower than the high-frequency bursts observed for head-restrained saccades and head-unrestrained gaze shifts (Figure 2b). In general, there are substantial differences between SC neurons that respond during head-only movements and those that are active prior to gaze shifts. For example, no reliable relationship is observed between firing rate and head movement parameters such as velocity, amplitude, or position. High-frequency-burst neurons respond prior to the generation of a limited range of gaze vectors; in contrast, no such circumscribed response fields are noted for head-only movements (Figure 3). Although head-movement related neurons are found throughout the deeper layers of the SC, they lack a topographical organization based on head amplitude or direction. Thus, although SC neurons exhibit responsivity to head-only movements, they appear to be different from those generating head movements that accompany gaze shifts.
Figure 3.
Movement field characterization for head-only movements of four superior colliculus (SC) neurons. Average firing rate during the head-only movement (legend key) is plotted as a function of its horizontal and vertical components. The filled contour plots were constructed from individual trial data points. Adapted from Walton et al. (2007), with permission.
Pinnae and Whisker Movements
In addition to saccadic movements of the eyes and head (if it is unrestrained), stimulation of the SC can evoke movements of the whiskers and pinnae, more commonly in species that routinely use these mobile sensors for interactions with the environment (Cowie & Robinson 1994, Hemelt & Keller 2008, McHaffie & Stein 1982, Stein & Clamann 1981, Valentine et al. 2002). In the echolocating bat, for example, contralateral pinnae movements can be evoked with low-threshold currents (<25 μA) and with latencies (∼20 ms) as short as those for saccades in nonhuman primates (Valentine et al. 2002). Stimulation of rostral sites evokes forward movements of both ears, as if orienting to a target that is straight ahead. Large-amplitude, backward movement of both ears is evoked from posterior sights, conforming to the notion of orienting to a stimulus that is behind the animal. Medial and lateral sites evoke pinnae movements with upward and downward directions, respectively. With the exception of stimulation of rostral sites, the con-tralateral pinna moves first. Recruitment of the ipsilateral ear as well as the complexity of pinnae movement is often a function of stimulation parameters at posterior sites, suggesting that the entire response is not determined solely by the locus of stimulation. The topography of pinnae movements is similar in cats (Stein & Clamann 1981), although descriptions of SC stimulation-evoked pinnae movements are scarce in other animals. It is generally reported that stimulation of ventral regions in monkeys (Cowie & Robinson 1994) and caudal sites in rodents (McHaffie & Stein 1982) are most effective at producing ear movements.
Hemelt & Keller (2008) recently performed a thorough investigation of SC control of vibrissae movements in rats. Stimulation of effective sites evoked a sustained protraction of the whisker pad that lasted for the duration of the stimulation. A frequency of 333 Hz appeared optimal for producing large-amplitude movements, which scaled with current intensity above threshold (∼25 μA). Thus, the protraction magnitude was not site specific, unlike for saccades. The deeper layers of the rodent SC exhibits a dorsoventral topography for the laterality of vibrissae movements: Protractions of the contralateral and ipsilateral whisker pads were evoked from dorsal and ventral aspects, respectively. Bilateral movements were evoked from intermediate regions, but current spread to both dorsal and ventral regions could account for the observation.
What is the role of the SC in producing vibrissae movements? Kinematic properties of sustained vibrissae protraction (amplitude) evoked from the SC are distinct from the rhythmic whisking behavior (frequency) associated with the motor cortex (Cramer & Keller 2006). Hence, the SC may regulate the amplitude and positional control of whisking (Hemelt & Keller 2008). Interestingly, the putative tecto-facial neurons in the region, from which stimulation-evoked vibrissae movements are likely produced, do not respond to trigeminal inputs, precluding them from directly mediating the short-latency reflex loop connecting the trigeminal and facial neurons (Kleinfeld et al. 1999, Hemelt & Keller 2008).
Eye-Head-Body Movements
A combined eye-head movement may not be sufficient to produce very large changes in gaze, such as when looking behind. Coordination across multiple body segments, including the body and feet, is required (e.g., Hollands et al. 2004, McCluskey & Cullen 2007). In such cases, the gaze shift is not necessarily completed in a single movement. Instead, multiple, smaller-amplitude movements with brief intervals of steady gaze are used to fixate the desired location (Anastasopoulos et al. 2009; but also see Degani et al. 2010). To the best of our knowledge, descriptions of extracellular recordings from SC neurons during controlled eye-head-body movements do not exist in literature. A microstimulation approach, however, has been applied and has implicated the SC in controlling body movements. Microstimulation can induce whole-body movements in freely moving cats (Hess et al. 1946, Schaefer 1970, Syka & Radil-Weiss 1971), whole-body and circling behavior in rodents (Dean et al. 1986, Tehovnik & Yeomans 1986), whole-body turns in frogs (Ewert 1984), body and tail movements in goldfish (Herrero et al. 1998), head and body movements in snakes (Dacey & Ulinski 1986), and swimming in lampreys (Saitoh et al. 2007). When permitted by the oculomotor range, stimulation also produces an eye movement, and like the cases described with pinnae, vibrissae, and head movements, the extent of the accompanying body movement varies with stimulation parameters. For example, the frequency of contraversive circling increases monotonically with the frequency and current of stimulation delivered to the caudal SC in rodents (Tehovnik 1989). Tail movements are evoked by stimulation of increasingly posterior sites in the goldfish OT, and both the amplitude and complexity of the eye-body-tail movement increases with stimulation parameters (Herrero et al. 1998). Stimulation duration also plays a critical role in determining the movement evoked by the lamprey OT. A site that evokes just eye movements with short-duration stimulation can also produce complex, swimming patterns with substantially prolonged stimulation duration (Saitoh et al. 2007). These prolonged stimulation results are reminiscent of the complex actions evoked by long-duration stimulation of the precentral cortex in monkeys (Graziano et al. 2002).
The predominant motor function of the SC is to shift gaze toward a stimulus located in the contralateral hemifield. Neural commands for such orienting or approach movements are relayed through the crossed tecto-(reticulo-) spinal tract, also referred to as the predorsal bundle. However, the SC also participates in the generation of ipsiversive eye-head-body movements that resemble aversive actions generated to escape from predators or avoid harmful situations. Commands for movements away from a stimulus are processed by the ipsilateral tecto-(reticulo-)spinal projection that originates laterally and ventrally in the SC (Sparks 1986). Indeed, stimulation of ventral SC sites in the rat protracts the ipsilateral whisker pad (Hemelt & Keller 2008). Small punctate lesions laterally in the frog OT impairs kinematics of escape behavior but keeps prey capture behavior intact (King & Comer 1996). Stimulation of the caudal extent of the rodent SC produces ipsiversive head and body movements, including circling (Sahibzada et al. 1986). Stimulation of posterior sites in the goldfish can induce ipsiversive tail movements that reflect an escapelike swimming response (Herrero et al. 1998). Furthermore, properties of the escape response (number of tail beats, velocity, etc.) depend on stimulation parameters, suggesting that the SC activity both triggers and modulates the kinematics of the escape or avoidance response.
Reach Movements
Interacting with the environment can also require coordinating gaze shifts with arm movements, for example, when extending the arm to catch a ball. Neurons in the deeper layers of the SC and the underlying reticular formation are active prior to such reach movements (Werner 1993). Many show a single or biphasic burst of activity that correlates with simultaneously recorded EMG activity of shoulder, arm, and trunk muscles in nonhuman primates. The strongest correlations are obtained between activity in reticular formation neurons and EMG of proximal limb muscles (Stuphorn et al. 1999, Werner et al. 1997a). The posterior deltoid shoulder muscle in humans can also exhibit a brief EMG response linked to visual target onset (only when a reflexive manual response is required), and it has been speculated that the transient burst could be relayed through the SC (Pruszynski et al. 2010). The majority of SC “reach” neurons are intermingled with but distinct from visuomotor and motor neurons that burst for saccadic eye movements (Stuphorn et al. 2000, Werner et al. 1997b). A subset of reach neurons is modulated by the axis of visual fixation. Reach-related activity in another group of neurons was independent of gaze, and these neurons were found in the deepest portion of the SC and the underlying reticular formation. As observed for head movements (see above), reach-related neurons do not exhibit a topographical organization normally associated with saccade-related responses in the SC.
Results from microstimulation studies have been interpreted in support of a functional role of the SC in arm-movement control. Qualitatively assessed observation of proximal shoulder movement was reported after stimulation of deep SC layers (Cowie & Robinson 1994), but current spread to the underlying reticular formation could have evoked the movement. In addition, the movement could have been a generalized twitch or shrug of the contralateral shoulder, and not necessarily a reach action. Stimulation of the SC during forelimb movements by cats does, however, perturb the ongoing trajectory (Courjon et al. 2004).
Collicular response also exhibits modulation during other aspects of visually guided movements. For example, when reaching for an object, the visual axis often shifts to the target position and maintains a prolonged fixation until the hand makes contact (Neggers & Bekkering 2000, 2001). The extended fixation is reflected in enhanced activity in neurons in the rostral pole of the SC, potentially revealing a neural substrate of “gaze anchoring” during coordinated eye-hand movements (Reyes-Puerta et al. 2010). Another group of neurons in the SC responds specifically when the hand comes in con-tact with an object (Nagy et al. 2006), and the magnitude of activity increases with the intensity of the push. As these neurons are located in the intermediate and deep layers, it is likely that the activity reflects a premotor signal, although a sustained somatosensory component cannot be ruled out. On average, the neural responses are comparable for both ipsilateral and contralateral arms. As with reach movements, no topographical organization was detected.
Sonar Vocalization
Much like primates inspect the environment by shifting gaze between objects of interest, echolocating bats navigate in darkness by emitting sonar vocalization and processing returning echoes. Thus, sonar vocalization along with head and pinnae movements constitute the major effectors bats use to detect targets, and studies indicate that the SC participates in the movement of all three (Schuller & Radtke-Schuller 1990, Sinha & Moss 2007, Valentine et al. 2002). Microstimulation of the SC evokes sonar vocalizations whose time-frequency characteristics resemble the calls the same bats produced for echolocation for tracking targets (Valentine et al. 2002). The threshold for evoking a vocalization is typically less than 10 μA. The response latency is normally greater than 100 ms, and pinnae and/or head movements typically precede the onset of vocalization. The number of sonar pulses elicited increases with stimulation parameters, particularly current intensity and duration.
Extracellular activity recorded during sonar calls generated by bats during target tracking reveals two bouts of increased activity (Sinha & Moss 2007). The short-lead event is tightly coupled to and leads vocal onset by less than 5 ms, and the long-lead event is more variable and precedes the call by 20–30 ms; the early activity is short lived, as it returns to baseline between the two epochs. Sinha & Moss (2007) propose that the short-lead event triggers or times the vocalization, which in turn gates the neural response to the aural output but preserves sensitivity to its echo. The long-lead event, in contrast, could represent premotor activity, as its interval is correlated with call duration during target tracking.
Differential Control of Extraocular and Nonextraocular Muscles
Assessments based on neural activity offer correlative support for a functional role of the SC in the control of extraocular and skeletomotor actions. Microstimulation-based results suggest causality but indicate that the SC output alone is sufficient. In contrast, inactivation experiments appear best suited to address whether SC signals are necessary to produce gaze shifts coordinated across multiple effectors. Injections of only hundreds of nanoliters of either lidocaine or muscimol produce profound effects on head-restrained saccades encoded by the inactivated region (Aizawa & Wurtz 1998; Hikosaka & Wurtz 1983, 1985, 1986; Lee et al. 1988; Quaia et al. 1998). Such saccades generally display longer reaction times, attenuated velocity profiles, and prolonged durations. The direct connectivity between the SC and the saccade burst generator in the brainstem (reviewed by Moschovakis et al. 1996) can readily account for the observed deficits.
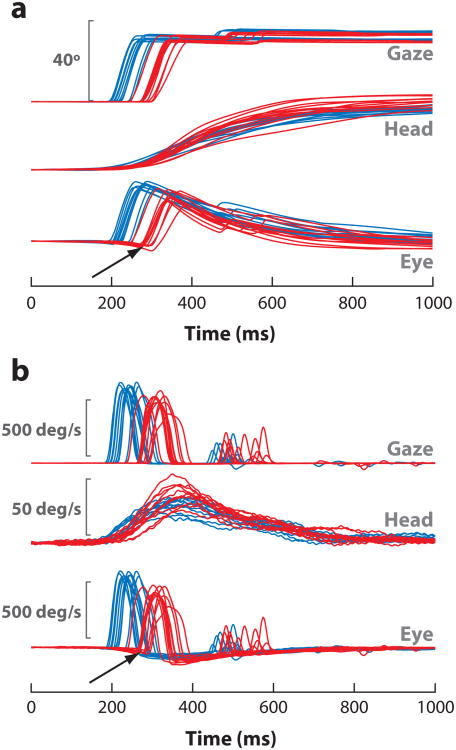
Gaze shifts generated with the head free to move are also expected to be compromised given the contributions of the saccadic eye component. The more interesting issue then is whether the head component of the combined movement is also attenuated after SC inactivation. Intuitively, a pronounced effect is expected because a weaker SC command is attempting to move a significant inertial load. Reversible inactivation with lidocaine does compromise the latency, velocity, and duration of head-unrestrained gaze shifts, but the effect is manifested nearly entirely by the saccadic ocular component (Figure 4) (Walton et al. 2008). The initiation of the head movement is delayed, but the change is modest compared with the increase in reaction time of the gaze shift. All other head-movement features remain unattenuated; somewhat counter-intuitively, the peak head velocity increases slightly.
Figure 4.
Effects of reversible inactivation with lidocaine on head-unrestrained gaze shifts. (a) Position and (b) velocity waveforms of several ∼40° gaze shifts are plotted before (blue) and after (red) a microinjection in the caudal superior colliculus. The onsets of both gaze and head components are delayed, but the head movement initiates sooner. Thus, the eyes counter-rotate in the orbits before gaze onset (arrows). Peak gaze velocity is also reduced after the inactivation. In contrast, the peak head velocity sometimes increases a modest amount. Adapted from Walton et al. (2008), with permission.
A negligible or absent effect of small-scale inactivation of the mammalian SC seems to generalize to all skeletomotor actions. For example, neither whisker movements in rodents (A. Keller, personal communication) nor reach movements in primates (K.P. Hoffmann, personal communication) appears compromised after localized SC inactivation. The active avoidance response typically triggered by a fearful conditioned response also remains intact after a lesion in the rodent SC contralateral to the conditioned stimulus (Cohen & Castro-Alamancos 2007). Both latency and kinematics remain unaltered during active head-only movements after inactivation of the monkey SC (Walton et al. 2008).
One possible explanation for the differential effect on extraocular and skeletomotor effectors is grounded in the distinct encoding mechanisms for gaze and other muscles. Motor commands for redirecting the line of sight appear to be mediated by a place code, whereas putative signals for skeletomotor actions have a weak, if any, topographical organization. Thus, it is possible that a chemical injection of hundreds of nanoliters of lidocaine or muscimol is not sufficient to dampen the SC drive to nonextraocular muscles. Lidocaine injections roughly an order of magnitude larger in volume also fail to produce deficits on head movements in monkeys (Walton et al. 2008), although increasingly larger inactivations/lesions have yielded deficits in head movements in cats (Isa & Sasaki 2002, Lomber et al. 2001). A drawback of very large inactivations, however, is that the deficits could be evoked by other factors. Consider the example in which head movements and gaze shifts are severely compromised after kainic acid lesions in the SC and the underlying reticular formation in cat (Isa & Sasaki 2002). It is difficult to discount the possibility that the observed effect is largely due to inactivation of the underlying mesencephalic reticular formation, which is known to produce severe deficits in head movements, even with small volume injections (Klier et al. 2002, Waitzman et al. 2000). Also, unilateral cryogenic inactivation of the superficial and deeper layers produces profound deficits on the generation of gaze shifts, including head movements (Lomber et al. 2001). These deficits, however, are absent after bilateral cooling (Lomber & Payne 1996), suggesting that the lack of overt orienting behavior may occur from deficits in nonmotoric function such as spatial hemineglect.
Another interpretation of the inactivation results is that skeletomotor function is also controlled by extracollicular pathway(s) that can fully compensate for an absent or weakened SC signal. One parallel input likely originates in the cortex, which has access to the spinal cord through cortico-spinal and cortico-reticulo-spinal pathways. For head movements, the supplementary eye fields (Chen & Walton 2005, Martinez-Trujillo et al. 2003) and premotor cortical areas may be the main sources of cortical input. Stimulation of the frontal eye fields also induces neck muscle activity (Elsley et al. 2007, Guitton & Mandl 1978) and coordinated eye-head movements (Chen 2006, Knight & Fuchs 2007, Tu & Keating 2000), but a significant component of its output is thought to connect through the SC (Hanes & Wurtz 2001, Komatsu & Suzuki 1985, Stanton et al. 1988). The putative cortical and SC pathways likely merge in the mesencephalic and pontine reticular formations. The convergence, however, is most likely a nonlinear combination of two (or more) streams; if they did combine linearly, inactivation of one pathway is predicted to produce a partial deficit in head movements. The parallel pathways probably express redundant motor commands, and the efficacy of each individual channel can be gated or modulated by cognitive, mechanical (length-tension property), and proprioceptive contributions. Thus, in the case where one pathway is weakened, the intact drive could enhance its throughput to control the compromised effectors. Perhaps simultaneous lesions of more than one pathway may unmask an impaired output. Recall the classic finding that saccades can be executed after a lesion of either the SC or the frontal eye fields, but not after inactivation of both structures (Schiller et al. 1979). A comparable observation also exists for sensory processes that mediate the active avoidance response in rodents (Cohen & Castro-Alamancos 2007): A lesion of either the SC or the somatosensory thalamus has no effect on the active-avoidance response triggered by a conditioned stimulus applied contralaterally, but simultaneous inactivation of both structures abolishes the behavior. If the cortical component is indeed an important parallel pathway, then deficits in skeletomotor actions should be prevalent after tectal lesions in species that lack a neocortex. Indeed, lesions of the OT in the barn owl and frog do appear to compromise a large range of contraversive head (Knudsen et al. 1993, Wagner 1993) and body movements (King & Comer 1996, Kostyk & Grobstein 1987), respectively.
Fixation Control by Rostral Superior Colliculus
Robinson's (1972) demonstration of a topographical organization of saccades evoked by microstimulation of the primate SC established the long-standing perspective that the deeper layers of the SC consist of a uniform saccade zone. Conforming to this idea, the rostral pole of the SC is predicted to encode very-small-amplitude eye movements. Nevertheless, the saccade-zone hypothesis was questioned with the discovery of so-called fixation neurons in the rostral pole of the SC. These cells discharge at a tonic rate during fixation and pause during most saccades (Munoz & Guitton 1989, 1991; Munoz & Wurtz 1993a; Peck 1989). Inactivation of the rostral SC reduces the latency of large-amplitude saccades and disrupts the animal's ability to maintain visual fixation during delayed or memory-guided tasks (Munoz & Wurtz 1993b). Conversely, stimulation of this region increases the latency of large-amplitude saccades (Munoz & Wurtz 1993b). Such data led to the view that the rostral pole of the SC serves as a “fixation zone” that stabilizes gaze. The theory implements the idea of a “see-saw”-like lateral interaction network in the SC, in which the rostral portion functions as an inhibitory system that facilitates visual fixation by suppressing activity in the caudal SC and thereby preventing saccade generation (Meredith & Ramoa 1998, Munoz & Istvan 1998). Fixation itself was proposed to be mediated through direct projections from the rostral SC to the OPNs (Büttner-Ennever et al. 1999, Gandhi & Keller 1997, Paré & Guitton 1994). However, several studies have disputed the proposed fixation hypothesis. First, physiological data revealed differences between the discharge properties of OPNs and fixation-related neurons in the rostral SC. For example, the end of pause in the rostral SC neurons lags the resumption of activity in the OPNs as well as saccade offset (Everling et al. 1998). Second, large-amplitude saccades perturbed by stimulation in the rostral SC can be better interpreted as colliding saccades instead of interrupted saccades associated with stimulation of the OPNs (Gandhi & Keller 1999). Finally, neural recordings have revealed that fixation-related neurons within the rostral SC actually discharge a burst for small contraversive saccades (Gandhi & Keller 1997, Krauzlis et al. 1997, Munoz & Wurtz 1993a), and this activity pattern is comparable to the bursts generated for larger saccades by neurons in the caudal SC. An enhanced neural response can be observed for even the involuntary microsaccades (typically <12 min arc) that occur while fixating (Hafed et al. 2009, Rolfs 2009, Steinman et al. 1973). Furthermore, the burst exhibits selectivity for a specific amplitude and direction and is continuous with the topography of the saccade zone. Thus, SC function has reverted back to the classic view in which it comprises a continuous representation of all saccade amplitudes and directions, with the rostral SC representing some of the smallest eye movements.
It then becomes important to ask, (how) can a continuous saccade vector map that encodes for microsaccades and small-amplitude movements in the rostral SC preserve fixation? One potential mechanism utilizes the observation that many rostral SC neurons discharge a burst for a range of saccades in both ipsiversive and contraversive directions (Hafed et al. 2009, Munoz & Wurtz 1993a), functionally linking the two visual fields to represent central locations. Hafed et al. (2009) proposed a model in which fixation is maintained by balancing the premotor activity across the rostral regions of the two colliculi. The locus of activity in their model is extracted from a stochastic process with a mean of zero amplitude. Microsaccades are triggered when the balance of activity sufficiently shifts the locus of activity from zero. Their model also simulates the effects of inactivation of the rostral SC. When a subset of model neurons is silenced, the spatial distribution of active neurons on the intact side is reduced to maintain the balance of motor activity and thus preserve fixation (Figure 5). In addition, the reduction of activity also results in less overall variability, causing a lower probability for stochastic fluctuations in the locus of activity and therefore producing fewer microsaccades.
Figure 5.
Schematic of distribution of activity in deep superior colliculus (SC) layers during fixation. (a) Population activity across the two colliculi is balanced during fixation of a visual target. (b) An inactivation of a small region in the rostral SC (blue circle inside right SC) induces a compensatory shift in activity in the intact side (left SC). Ensemble activity on the lesion side is also redistributed due to inter- and intracollicular interactions. The net activity across the two SC, however, remains balanced to preserve continued fixation on or near the foveal target. Illustration generated based on results by Hafed et al. (2009).
There is a concrete, conceptual difference between a mechanism based on the balance of ipsiversive and contraversive movement commands (Hafed et al. 2009) and a scheme based on gaze stabilization (Munoz & Guitton 1989; Munoz & Wurtz 1993a, 1993b). Nevertheless, a commonality can exist between the two as demonstrated by the observation that the timing of microsaccades relative to stimulus onset also influences saccade reaction times (Hafed & Krauzlis 2010, Rolfs et al. 2006). Peripheral visual stimuli presented during fixation but in register with microsaccades were more effective in attenuating the visual bursts in the caudal SC than when shown in absence of microsaccades (Hafed & Krauzlis 2010). Correspondingly, the reaction times of saccades directed to the visual target were greater if the stimulus was presented around the time of a microsaccade. These observations suggest that as the balance of activity shifts more toward one colliculus to trigger a microsaccade, the resulting greater activity in the rostral SC acts through the lateral interaction network to suppress visual and premotor build up in the caudal SC. Therefore, the basic mechanisms of a gaze-stabilization theory could exist within principles based on balancing motor activity across the SC.
Mechanisms for Decoding Superior Colliculus Activity
When immersed within a visual environment with many potential stimuli, the oculomotor system must first select an object for a saccade goal. The oculomotor system has served as a useful tool to probe mechanisms of target selection (Schall 1995, Schall & Thompson 1999), and the SC, among other structures, plays a major role in this process (Kim & Basso 2008, McPeek & Keller 2002, Shen & Paré 2007). The typical laboratory paradigm involves presentation of multiple visual stimuli, in which a specific feature, such as color, distinguishes the singleton (saccade target) from distractors. Each visual stimulus excites an ensemble of SC neurons in the deeper layers, and the result of the competing populations governs the observed eye movement. Given that the saccade usually ends on one of the visual stimuli, a computational mechanism that computes the vector average across all the active populations is a poor predictor of the observed data. Accurate performance is better associated with the degree of separation between target and distractor activity distributions (Kim & Basso 2008). A winner-takes-all scheme, in which the saccade is driven to the stimulus represented with the highest ensemble activity better utilizes the separation between activity distributions and therefore performs better than an averaging mechanism. A Bayesian model, maximum a posteriori estimate (Kim & Basso 2010), performs slightly better than either an averaging or a winner-takes-all mechanism, suggesting that a read-out mechanism for target and/or motor selection in the SC may be based on a probability framework.
Although these competitive mechanisms can contribute to selecting the neural population that will ultimately guide the eye movement, they do not indicate how the spatially coded contribution of each neuron within the selected (or winner) population of SC neurons is integrated and decoded to produce an eye movement. Two controversial models have dominated the oculomotor field in hypothesizing the proper mechanism for deciphering SC activity: vector averaging and vector summation. Early saccade models utilized static-ensemble-coding schemes, in which SC motor activity specifies only the metric coordinates of the saccade displacement. Dynamical properties such as trajectory and kinematics are assumed to be reflected by the operation of a feedback mechanism downstream of the SC, such as in the pons (Jürgens et al. 1981, Robinson 1975) or cerebellum (Lefèvre et al. 1998, Quaia et al. 1999). The vector-averaging model (Figure 6a) hypothesized that an active population in the SC is computed by taking the weighted average of the vector contribution of each neuron (e.g., Lee et al. 1988, Walton et al. 2005). The saccade goal S⃗ is computed by
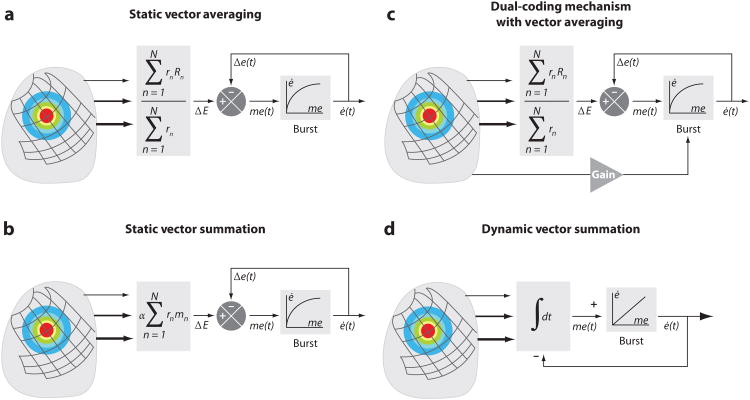
Figure 6.
Frameworks of contemporary models for decoding superior colliculus (SC) activity to generate saccadic eye movements. (a) Static averaging decoding model that defines desired saccade metrics by using a vector averaging computation (rn is the mean firing rate of cell n and R⃗n is the optimal vector encoded by that cell). (b) Static summation decoding model uses vector summation to define saccade metrics (m⃗n is the vector contribution of cell n and α is a fixed scaling constant). For both static averaging and summation models, the trajectory and kinematics are controlled downstream by nonlinear local feedback. (c) Dual-coding hypothesis model shares some of the framework of the static vector-averaging model. In addition, the firing rate of the SC across time can modulate the gain of the burst generator. In this manner, SC output now contributes to both metrics and kinematics. (d) Dynamic summation model integrates across time the spikes from an active population. The accumulating activity specifies the intended movement trajectory. Each spike from an SC cell adds a fixed, site-specific “mini” vector contribution to the movement command. In contrast to the other frameworks, the movement is controlled downstream by linear feedback. The projections from the SC are weighted (thickness of lines and size of arrows) according to its origin site along the rostral-caudal dimension. Model parameters: ΔE, desired eye displacement; Δe(t), current eye displacement; me(t), dynamic motor error; ė(t), current eye velocity;∫ dt, temporal integration; “burst,” brainstem burst generator. Adapted from Goossens & Van Opstal (2006).
where rn is the mean firing rate of cell n in the motor map and R⃗n is the optimal vector encoded by that neuron. In this format, the level of activity has no direct relation to either saccade trajectory or its kinematics. Early success of the model came from its ability to account accurately for the findings generated from several experiments. Some examples include the following: (a) Simultaneous microstimulation at two points within the SC evokes a single saccade whose amplitude and direction are predicted by the weighted average of the two saccades generated when each site is stimulated independently (Katnani et al. 2009, Robinson 1972). (b) Local inactivation within the SC generates saccades with dysmetria patterns that conform to an averaging hypothesis (Lee et al. 1988). (c) The timing and initial direction of curved saccades, generated by using a double-step paradigm, are accurately predicted by the computation of an averaging scheme (Port & Wurtz 2003).
Experimental findings that dispute the vector-averaging model also exist. For example, stimulation-evoked saccades can have a sigmoidal dependency with current intensity (Van Opstal et al. 1990). Stimulation frequency may also have a similar effect (see table 3 in Stanford et al. 1996, although the authors did not comment on this observation; also see Groh 2011). This relationship reveals a flaw in the averaging computation, because a strict interpretation of this mechanism indicates that a single spike in the colliculus can generate a maximal amplitude vector. The model, however, can be appended with the addition of a parameter to demonstrate amplitude dependency (Van Opstal & Goossens 2008):
where K is a constant that presumably could be an inhibitory threshold. When the total population activity in the SC is low, K can dominate the denominator term and reduce the amplitude of the programmed saccade. If population activity is high, K can be neglected and the computation returns to an original averaging scheme. Another limitation of the averaging mechanism persists in how the computation can be implemented physiologically. Although network architectures that can accomplish normalization have been proposed (Carandini & Heeger 1994, Groh 2001), there is still no substantial anatomical evidence in the oculomotor system to support this structure.
Vector summation is recognized as a more physiological mechanism for decoding a motor command (Georgopoulos et al. 1986). For deciphering SC output for the generation of saccades, it hypothesizes that each active SC neuron contributes a vector that is weighted by the mean firing rate of the cell (Figure 6b). The resulting sum of these weighted vectors produces the desired saccade S⃗ (Van Gisbergen et al. 1987) as
where m⃗n is the vector contribution elicited by cell n and α is a fixed scaling constant. As with the averaging scheme, this simple summation model also does not incorporate any means to explain saccade kinematics. The strength of the model is exhibited by its simplicity, intuitive nature, and ability to produce normometric saccades. Its shortcomings, however, become prevalent when tested with more complex motivations (i.e., simultaneous stimulation of two SC sites and inactivation), but they too can be accounted for by incorporating intracollicular connectivity features such as local excitation and distal inhibition (e.g., Behan & Kime 1996, Isa & Hall 2009, Lee et al. 1997, McIlwain 1982, Meredith & Ramoa 1998, Munoz & Istvan 1998, Pettit et al. 1999, Takahashi et al. 2010). Initial implementation of lateral interaction was shown through an inhibitory tuning parameter (Van Opstal & Van Gisbergen 1989). The addition of inhibition provided a cutoff during the summation of two vectors to simulate weighted averaging saccades seen with simultaneous stimulation. Moreover, a later model that incorporated both visual and motor layers of the SC (Arai et al. 1994) demonstrated that vector summation can generate normometric, averaging, and express saccades. Yet another version (Badler & Keller 2002) additionally emphasized that lateral interactions shift the locus of ensemble activity when a subset of model neurons is “inactivated,” and the resulting end-points of simulated saccades match both experimental data and predictions based on an averaging mechanism.
The discussion of both models to this point has focused on computation of only the desired saccade movement (metric). However, accumulating evidence suggests that the level of activity within the SC does influence the saccade kinematics (see the dual-coding hypothesis discussed in the Eye Movements section above), indicating that the changes in collicular activity across time now become significant to saccade programming. A vector-averaging theory of dynamic ensemble coding has been addressed mainly conceptually (Figure 6c). In essence, the firing rate of SC activity modulates the gain of the brainstem burst generator (Nichols & Sparks 1996, Sparks & Mays 1990). Hence, an attenuated burst, such as after partial inactivation of the SC or during memory-guided saccades, evokes a slower amplitude-matched saccade. With this implementation, not only the metrics, but also the kinematics of a movement could be explained by an averaging scheme. Furthermore, Van Opstal & Goossens (2008) incorporated the notion of gain modulation into a vector averaging computation that utilized instantaneous firing rate across time. As a result, the vector-averaging model provided dynamic estimates of the saccade goal; however, simulations revealed that the computation did not capture saccade kinematics effectively and was relatively insensitive to temporal changes in the SC burst profile.
A detailed and quantified vector summation computation has been developed under the theoretical framework of dynamic ensemble coding (Figure 6d) (Goossens & Van Opstal 2006, Van Opstal & Goossens 2008). The model proposes that a saccade is computed by the vector summation of all individual cell contributions across time:
where NS indicates the number of spikes of cell k (counted from 20 ms before saccade onset to 20 ms before saccade offset), NA equals the total number of cells in the population, δ(x) denotes the Dirac impulse function (i.e., an individual spike), τk,n represents the time of the nth spike of cell k, and s⃗k is a scaled-eye-displacement vector generated by a single spike of cell k (scaling is determined by the model's cell density). Thus, SC neurons now relate the cumulative number of spikes in the active population to the ongoing eye displacement. With such a scheme, the SC output now specifies the desired saccade trajectory, including its kinematics. Simulations of the model revealed several saccade-related properties that other models cannot incorporate without additional assumptions. First, the decoding computation accounts for stretching of horizontal and vertical saccade components necessary for oblique saccades. Second, SC activity encodes the nonlinear main sequence, in contrast to the long-believed idea that the kinematic nonlinearity originates from a local feedback circuit in the brainstem. Thus, the SC acts as a nonlinear vectorial pulse generator where the spatial temporal activity patterns in the motor SC encode desired saccade kinematics, without having to use nonlinear mechanisms such as normalization of activity. Although the model's mechanism reveals advantageous properties, a shortcoming arises in that the computation will always yield a vector sum when tested with the contribution of two sites. To account for averaging saccades, the model must incorporate an additional saturation criterion that could potentially be introduced through modeled lateral interactions.
The evolution of describing ensemble decoding in the SC has produced two conceptually distinct frameworks that continue to be contrasting equivalents in the field. Predictions of the vector-averaging and vector-summation models have provoked many experiments attempting to validate hypotheses and reveal emerging properties, yet a coherent direction has still not been achieved. Different methods are needed to better differentiate between the two models. One such approach may arise from microstimulation experiments that exploit the dependencies of saccade characteristics on stimulation parameters. By systematically lowering the range of stimulation parameters, one may be able to introduce more variability in the resulting saccade output, and characterization of this variability to the known inputs could reveal insightful properties on the spatiotemporal decoding of the SC output (Brecht et al. 2004, Gandhi & Katnani 2009, Katnani & Gandhi 2010).
Nonmotoric Functions of the Superior Colliculus
The intent of this review is to focus primarily on the motoric functions of the SC. This perspective does not diminish a collicular role in mediating other processes, but their inclusion is beyond the scope of this review. Some examples of systems-level phenomena in which the SC have been implicated include multi-modal sensory processing (Stein & Meredith 1993, Stein & Stanford 2008), target selection (Krauzlis et al. 2004, McPeek & Keller 2002), attention (Ignashchenkova et al. 2004, Kustov & Robinson 1996), motor preparation (Dorris et al. 1997, 2007), goal representation (Bergeron et al. 2003, Krauzlis & Carello 2003), and reward-related modulation (Ikeda & Hikosaka 2003). In fact, the potentially simultaneous contributions of multiple higher-level functions often confound interpretations of SC activity during complicated behavioral tasks (Sparks 1999). Future studies will need to address systematically if the SC controls a unifying signal (or command) across sensory, motor, and cognitive processes and whether the specific label attributed to its neural activity is task and training dependent.
Summary Points.
Neurons in the deeper layers of the SC are topographically organized for changes in gaze, but spatial organization for other effectors has not been detected.
Whereas the locus of ensemble activity dictates the size of gaze shift, the extent and complexity of skeletal segments appear best linked with stimulation duration.
Inactivation of the SC produces profound effects on gaze via the saccadic eye component. In contrast, skeletomotor effectors, particularly in mammals, are minimally impaired. These results suggest that the SC is crucial for the control of saccades and that parallel and perhaps redundant pathways can guide skeletal actions.
Neurons in the rostral pole of the SC actually burst for microsaccades and small-amplitude movements. They preserve fixation by balancing premotor activity that encodes for small leftward and rightward saccades across the two rostral SC. This theory is conceptually different from the previously proposed notion that fixation is achieved by signals that stabilize gaze.
Two prevalent computational models exist to explain the mechanisms for decoding SC motor activity: vector averaging and vector summation. Early models of both computations specified only saccade metrics, relying on downstream mechanisms to control kinematics. However, with experimental evidence suggesting that the SC motor activity reflects dynamic processes, both models have transitioned to account for kinematics. Averaging schemes incorporate a dual-coding hypothesis, whereas a recent summation scheme proposes that SC output encodes desired trajectory and velocity. The evolution of each computation has demonstrated strengths and weaknesses, leaving both models, to this point, as contrasting equivalents.
Acknowledgments
We thank Udaya Jagadisan for comments on the manuscript. N.J.G. is supported by the National Eye Institute (R01 EY015485, P30 EY008098) and National Institute of Deafness and Communication Disorders (P30 DC0025205). H.A.K. is supported by an institutional training grant from the National Institute of General Medical Science (T32 GM081760).
Glossary
- SC
superior colliculus
- OT
optic tectum
- Burst generator
neural elements in the paramedian and mesencephalic reticular formations for the generation of horizontal and vertical components of saccades, respectively
- Gaze shift
a change in visual axis small changes are typically completed by a saccade; for large changes, the ocular saccade is nested with a head movement; for even larger changes, other skeletal segments can be integrated with the coordinated eye-head movement
- EMG
electromyography
- Omnipause neuron (OPN)
neuron located along the midline in the paramedian pontine reticular formation (oculomotor pons), discharging at a tonic rate during fixation and becoming quiescent during saccades
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, financial holdings, or any other conflicts of interests that might be perceived as affecting the objectivity of this review.
Literature Cited
- Aizawa H, Wurtz RH. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J Neurophysiol. 1998;79:2082–96. doi: 10.1152/jn.1998.79.4.2082. [DOI] [PubMed] [Google Scholar]
- Anastasopoulos D, Ziavra N, Hollands M, Bronstein A. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res. 2009;193:323–36. doi: 10.1007/s00221-008-1627-y. [DOI] [PubMed] [Google Scholar]
- Anderson RW, Keller EL, Gandhi NJ, Das S. Two-dimensional saccade-related population activity in superior colliculus in monkey. J Neurophysiol. 1998;80:798–817. doi: 10.1152/jn.1998.80.2.798. [DOI] [PubMed] [Google Scholar]
- Arai K, Keller EL, Edelman JA. Two-dimensional neural network model of the primate saccadic system. Neural Netw. 1994;7:1115–35. [Google Scholar]
- Badler JB, Keller EL. Decoding of a motor command vector from distributed activity in superior colliculus. Biol Cybern. 2002;86:179–89. doi: 10.1007/s00422-001-0288-8. [DOI] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol. 2000;84:892–908. doi: 10.1152/jn.2000.84.2.892. [DOI] [PubMed] [Google Scholar]
- Behan M, Kime NM. Intrinsic circuitry in the deep layers of the cat superior colliculus. Vis Neurosci. 1996;13:1031–42. doi: 10.1017/s0952523800007689. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Matsuo S, Guitton D. Superior colliculus encodes distance to target, not saccade amplitude, in multi-step gaze shifts. Nat Neurosci. 2003;6:404–13. doi: 10.1038/nn1027. [DOI] [PubMed] [Google Scholar]
- Brecht M, Singer W, Engel AK. Amplitude and direction of saccadic eye movements depend on the synchronicity of collicular population activity. J Neurophysiol. 2004;92:424–32. doi: 10.1152/jn.00639.2003. [DOI] [PubMed] [Google Scholar]
- Breznen B, Lu SM, Gnadt JW. Analysis of the step response of the saccadic feedback: system behavior. Exp Brain Res. 1996;111:337–44. doi: 10.1007/BF00228723. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AK, Henn V, Cohen B. Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol. 1999;413:55–67. doi: 10.1002/(sici)1096-9861(19991011)413:1<55::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264:1333–36. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, van Gisbergen JA. Perturbation of combined saccade-vergence movements by micro-stimulation in monkey superior colliculus. J Neurophysiol. 1999;81:2279–96. doi: 10.1152/jn.1999.81.5.2279. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Van Gisbergen JA. Stimulation in the rostral pole of monkey superior colliculus: effects on vergence eye movements. Exp Brain Res. 2000;132:72–78. doi: 10.1007/s002219900221. [DOI] [PubMed] [Google Scholar]
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol. 2006;95:3528–42. doi: 10.1152/jn.01320.2005. [DOI] [PubMed] [Google Scholar]
- Chen LL, Tehovnik EJ. Cortical control of eye and head movements: integration of movements and percepts. Eur J Neurosci. 2007;25:1253–64. doi: 10.1111/j.1460-9568.2007.05392.x. [DOI] [PubMed] [Google Scholar]
- Chen LL, Walton MM. Head movement evoked by electrical stimulation in the supplementary eye field of the rhesus monkey. J Neurophysiol. 2005;94:4502–19. doi: 10.1152/jn.00510.2005. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci. 2007;27:7762–76. doi: 10.1523/JNEUROSCI.1124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Olivier E. Priming of head premotor circuits during oculomotor preparation. J Neurophysiol. 2007;97:701–14. doi: 10.1152/jn.00670.2006. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle activity evoked by stimulation of the monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol. 2002a;88:1980–99. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle activity evoked by stimulation of the monkey superior colliculus. II. Relationships with gaze shift initiation and comparison to volitional head movements. J Neurophysiol. 2002b;88:2000–18. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron. 2004;42:831–41. doi: 10.1016/s0896-6273(04)00267-3. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Olivier E, Pelisson D. Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J Physiol. 2004;556:675–81. doi: 10.1113/jphysiol.2004.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–64. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cramer NP, Keller A. Cortical control of a whisking central pattern generator. J Neurophysiol. 2006;96:209–17. doi: 10.1152/jn.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Ulinski PS. Optic tectum of the eastern garter snake, Thamnophis sirtalis. I. Efferent pathways. J Comp Neurol. 1986;245:1–28. doi: 10.1002/cne.902450102. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Sahibzada N, Tsuji K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience. 1986;19:367–80. doi: 10.1016/0306-4522(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Degani AM, Danna-Dos-Santos A, Robert T, Latash ML. Kinematic synergies during saccades involving whole-body rotation: a study based on the uncontrolled manifold hypothesis. Hum Mov Sci. 2010;29:243–58. doi: 10.1016/j.humov.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci. 2007;27:5053–62. doi: 10.1523/JNEUROSCI.4212-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–79. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Lac S, Knudsen EI. Neural maps of head movement vector and speed in the optic tectum of the barn owl. J Neurophysiol. 1990;63:131–46. doi: 10.1152/jn.1990.63.1.131. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Goldberg ME. Saccade-related activity in the primate superior colliculus depends on the presence of local landmarks at the saccade endpoint. J Neurophysiol. 2003;90:1728–36. doi: 10.1152/jn.00016.2003. [DOI] [PubMed] [Google Scholar]
- Elsley JK, Nagy B, Cushing SL, Corneil BD. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J Neurophysiol. 2007;98:1333–54. doi: 10.1152/jn.00386.2007. [DOI] [PubMed] [Google Scholar]
- Everling S, Paré M, Dorris MC, Munoz DP. Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: implications for control of fixation and saccade behavior. J Neurophysiol. 1998;79:511–28. doi: 10.1152/jn.1998.79.2.511. [DOI] [PubMed] [Google Scholar]
- Ewert JP. Tectal mechanisms that underlie prey-catching and avoidance behavior in toads. In: Vañegas H, editor. Comparative Neurology of the Optic Tectum. New York: Plenum; 1984. pp. 247–416. [Google Scholar]
- Freedman EG. Coordination of the eyes and head during visual orienting. Exp Brain Res. 2008;190:369–87. doi: 10.1007/s00221-008-1504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol. 1997;78:1669–90. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol. 1996;76:927–52. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Single and dual microstimulation in the superior colliculus: Effects of stimulation intensity and frequency. Soc Neurosci Abstr. 2009 Program No. 851.7. [Google Scholar]
- Gandhi NJ, Keller EL. Spatial distribution and discharge characteristics of superior colliculus neurons antidromically activated from the omnipause region in monkey. J Neurophysiol. 1997;78:2221–25. doi: 10.1152/jn.1997.78.4.2221. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Keller EL. Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region. J Neurophysiol. 1999;82:3236–53. doi: 10.1152/jn.1999.82.6.3236. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Changing views of the role of the superior colliculus in the control of gaze. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Boston: MIT Press; 2004. pp. 1449–65. [Google Scholar]
- Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol. 2007;98:360–73. doi: 10.1152/jn.00252.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–19. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Bracewell RM, Andersen RA. Sensorimotor transformation during eye movements to remembered visual targets. Vis Res. 1991;31:693–715. doi: 10.1016/0042-6989(91)90010-3. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Dynamic ensemble coding of saccades in the monkey superior colliculus. J Neurophysiol. 2006;95:2326–41. doi: 10.1152/jn.00889.2005. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Kuze B, Brandi AM, Thomas MA, Quenech'du N. Direct projections of omnipause neurons to reticulospinal neurons: A double-labeling light microscopic study in the cat. J Comp Neurol. 2010;518:4792–812. doi: 10.1002/cne.22488. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T, Cooke DF. The cortical control of movement revisited. Neuron. 2002;36:349–62. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- Groh JM. Converting neural signals from place codes to rate codes. Biol Cybern. 2001;85:159–65. doi: 10.1007/s004220100249. [DOI] [PubMed] [Google Scholar]
- Groh JM. Effects of initial eye position on saccades evoked by microstimulation in the primate superior colliculus: implications for models of the SC read-out process. Front Integr Neurosci. 2011;4:130, 1–16. doi: 10.3389/fnint.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and of the parameters of stimulation. Eur J Neurosci. 2001;14:1331–44. doi: 10.1046/j.0953-816x.2001.01744.x. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Kinematics and eye-head coordination of gaze shifts evoked from different sites in the superior colliculus of the cat. J Physiol. 2006;577:779–94. doi: 10.1113/jphysiol.2006.113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal ‘oculomotor’ area in alert cat. II. Unit discharges associated with eye movements and neck muscle activity. Brain Res. 1978;149:313–27. doi: 10.1016/0006-8993(78)90478-x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol. 1990;64:509–31. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;323:940–43. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Microsaccadic suppression of visual bursts in the primate superior colliculus. J Neurosci. 2010;30:9542–47. doi: 10.1523/JNEUROSCI.1137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Moschovakis A. The Superior Colliculus: New Approaches for Studying Sensorimotor Integration. Boca Raton, FL: CRC Press; 2004. p. 324. [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol. 2001;85:804–15. doi: 10.1152/jn.2001.85.2.804. [DOI] [PubMed] [Google Scholar]
- Harris LR. The superior colliculus and movements of the head and eyes in cats. J Physiol. 1980;300:367–91. doi: 10.1113/jphysiol.1980.sp013167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior colliculus control of vibrissa movements. J Neurophysiol. 2008;100:1245–54. doi: 10.1152/jn.90478.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero L, Rodriguez F, Salas C, Torres B. Tail and eye movements evoked by electrical microstimulation of the optic tectum in goldfish. Exp Brain Res. 1998;120:291–305. doi: 10.1007/s002210050403. [DOI] [PubMed] [Google Scholar]
- Hess WR, Burgi S, Bucher V. Motorische funktion des tektalund tegmentalgebietes. Psychiat Neurol. 1946;112:1–52. [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res. 1983;272:368–72. doi: 10.1016/0006-8993(83)90586-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–91. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Saccadic eye movements following injection of lidocaine into the superior colliculus. Exp Brain Res. 1986;61:531–39. doi: 10.1007/BF00237578. [DOI] [PubMed] [Google Scholar]
- Hollands MA, Ziavra NV, Bronstein AM. A new paradigm to investigate the roles of head and eye movements in the coordination of whole-body movements. Exp Brain Res. 2004;154:261–66. doi: 10.1007/s00221-003-1718-8. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–93. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66:205–41. doi: 10.1016/s0301-0082(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Becker W, Kornhuber HH. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern. 1981;39:87–96. doi: 10.1007/BF00336734. [DOI] [PubMed] [Google Scholar]
- Katnani HA, Gandhi NJ. Analysis of current and frequency stimulation permutations in the superior colliculus. Soc Neurosci Abstr. 2010 Program No. 77.9. [Google Scholar]
- Katnani HA, Van Opstal AJ, Gandhi NJ. Evaluating models of decoding superior colliculus activity for saccade generation. Soc Neurosci Abstr. 2009 Program No. 851.8. [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–55. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Comer CM. Visually elicited turning behavior in Rana pipiens: Comparative organization and neural control of escape and prey capture. J Comp Physiol A. 1996;178:293–305. [Google Scholar]
- Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control of three-dimensional head orientation. Science. 2002;295:1314–16. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–32. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol. 2007;97:618–34. doi: 10.1152/jn.00256.2006. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Masino T. Parallel pathways mediating both sound localization and gaze control in the forebrain and midbrain of the barn owl. J Neurosci. 1993;13:2837–52. doi: 10.1523/JNEUROSCI.13-07-02837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Suzuki H. Projections from the functional subdivisions of the frontal eye field to the superior colliculus in the monkey. Brain Res. 1985;327:324–27. doi: 10.1016/0006-8993(85)91528-8. [DOI] [PubMed] [Google Scholar]
- Kostyk SK, Grobstein P. Neuronal organization underlying visually elicited prey orienting in the frog–I. Effects of various unilateral lesions. Neuroscience. 1987;21:41–55. doi: 10.1016/0306-4522(87)90323-x. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Neuronal activity in the rostral superior colliculus related to the initiation of pursuit and saccadic eye movements. J Neurosci. 2003;23:4333–44. doi: 10.1523/JNEUROSCI.23-10-04333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Shared motor error for multiple eye movements. Science. 1997;276:1693–95. doi: 10.1126/science.276.5319.1693. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol. 2000;84:876–91. doi: 10.1152/jn.2000.84.2.876. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Carello CD. Going for the goal. Nat Neurosci. 2003;6:332–33. doi: 10.1038/nn0403-332. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vis Res. 2004;44:1445–51. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332:357–60. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc Natl Acad Sci USA. 1997;94:13299–304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre P, Quaia C, Optican LM. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Netw. 1998;11:1175–90. doi: 10.1016/s0893-6080(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. Removal of two halves restores the whole: reversal of visual hemineglect during bilateral cortical or collicular inactivation in the cat. Vis Neurosci. 1996;13:1143–56. doi: 10.1017/s0952523800007781. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P. Role of the superior colliculus in analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J Comp Neurol. 2001;441:44–57. doi: 10.1002/cne.1396. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Wang H, Crawford DJ. Electrical stimulation of the supplementary eye fields in the head-free macaque evokes kinematically normal gaze shifts. J Neurophysiol. 2003;89:2961–74. doi: 10.1152/jn.01065.2002. [DOI] [PubMed] [Google Scholar]
- McCluskey MK, Cullen KE. Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence of posture. J Neurophysiol. 2007;97:2976–91. doi: 10.1152/jn.00822.2006. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res. 1982;247:243–53. doi: 10.1016/0006-8993(82)91249-5. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Lateral spread of neural excitation during microstimulation in intermediate gray layer of cat's superior colliculus. J Neurophysiol. 1982;47:167–78. doi: 10.1152/jn.1982.47.2.167. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–34. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. J Neurophysiol. 1998;79:1597–602. doi: 10.1152/jn.1998.79.3.1597. [DOI] [PubMed] [Google Scholar]
- Missal M, Lefèvre P, Delinte A, Crommelinck M, Roucoux A. Smooth eye movements evoked by electrical stimulation of the cat's superior colliculus. Exp Brain Res. 1996;107:382–90. doi: 10.1007/BF00230420. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Kitama T, Dalezios Y, Petit J, Brandi AM, Grantyn AA. An anatomical substrate for the spatiotemporal transformation. J Neurosci. 1998;18:10219–29. doi: 10.1523/JNEUROSCI.18-23-10219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Fixation and orientation control by the tecto-reticulo-spinal system in the cat whose head is unrestrained. Rev Neurol. 1989;145:567–79. [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. II. Sustained discharges during motor preparation and fixation. J Neurophysiol. 1991;66:1624–41. doi: 10.1152/jn.1991.66.5.1624. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D, Pélisson D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol. 1991;66:1642–66. doi: 10.1152/jn.1991.66.5.1642. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol. 1993a;70:559–75. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol. 1993b;70:576–89. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995a;73:2313–33. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J Neurophysiol. 1995b;73:2334–48. doi: 10.1152/jn.1995.73.6.2334. [DOI] [PubMed] [Google Scholar]
- Nagy A, Kruse W, Rottmann S, Dannenberg S, Hoffmann KP. Somatosensory-motor neuronal activity in the superior colliculus of the primate. Neuron. 2006;52:525–34. doi: 10.1016/j.neuron.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol. 2000;83:639–51. doi: 10.1152/jn.2000.83.2.639. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol. 2001;86:961–70. doi: 10.1152/jn.2001.86.2.961. [DOI] [PubMed] [Google Scholar]
- Netser S, Ohayon S, Gutfreund Y. Multiple manifestations of microstimulation in the optic tectum: eye movements, pupil dilations and sensory priming. J Neurophysiol. 2010;104:108–18. doi: 10.1152/jn.01142.2009. [DOI] [PubMed] [Google Scholar]
- Nichols MJ, Sparks DL. Component stretching during oblique stimulation-evoked saccades: the role of the superior colliculus. J Neurophysiol. 1996;76:582–600. doi: 10.1152/jn.1996.76.1.582. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Visuomotor fields of the superior colliculus: a quantitative model. Vis Res. 1986;26:857–73. doi: 10.1016/0042-6989(86)90144-6. [DOI] [PubMed] [Google Scholar]
- Paré M, Guitton D. The fixation area of the cat superior colliculus: Effects of electrical stimulation and direction connection with brainstem omnipause neurons. Exp Brain Res. 1994;101:109–22. doi: 10.1007/BF00243221. [DOI] [PubMed] [Google Scholar]
- Peck CK. Visual responses of neurones in cat superior colliculus in relation to fixation of targets. J Physiol. 1989;414:301–15. doi: 10.1113/jphysiol.1989.sp017689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pélisson D, Goffart L, Guillaume A, Catz N, Raboyeau G. Early head movements elicited by visual stimuli or collicular electrical stimulation in the cat. Vis Res. 2001;41:3283–94. doi: 10.1016/s0042-6989(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Helms MC, Lee P, Augustine GJ, Hall WC. Local excitatory circuits in the intermediate gray layer of the superior colliculus. J Neurophysiol. 1999;81:1424–27. doi: 10.1152/jn.1999.81.3.1424. [DOI] [PubMed] [Google Scholar]
- Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol. 2003;90:1887–903. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, King GL, Boisse L, Scott SH, Flanagan JR, Munoz DP. Stimulus-locked responses on human arm muscles reveal a rapid neural pathway linking visual input to arm motor output. Eur J Neurosci. 2010;32:1049–57. doi: 10.1111/j.1460-9568.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol. 1998;79:2097–110. doi: 10.1152/jn.1998.79.4.2097. [DOI] [PubMed] [Google Scholar]
- Quaia C, Lefèvre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Reyes-Puerta V, Philipp R, Lindner W, Hoffmann KP. The role of the rostral superior colliculus in gaze anchoring during reach movements. J Neurophysiol. 2010;103:3153–66. doi: 10.1152/jn.00989.2009. [DOI] [PubMed] [Google Scholar]
- Rezvani S, Corneil BD. Recruitment of a head-turning synergy by low-frequency activity in the primate superior colliculus. J Neurophysiol. 2008;100:397–411. doi: 10.1152/jn.90223.2008. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vis Res. 1972;12:1795–808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control signals. In: Bach-y-Rita P, Lennerstrand G, editors. Basic Mechanisms of Ocular Motility and Their Clinical Implications. Oxford: Pergamon; 1975. pp. 337–74. [Google Scholar]
- Rolfs M. Microsaccades: small steps on a long way. Vis Res. 2009;49:2415–41. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Laubrock J, Kliegl R. Shortening and prolongation of saccade latencies following microsaccades. Exp Brain Res. 2006;169:369–76. doi: 10.1007/s00221-005-0148-1. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp Brain Res. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–33. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh K, Menard A, Grillner S. Tectal control of locomotion, steering, and eye movements in lamprey. J Neurophysiol. 2007;97:3093–108. doi: 10.1152/jn.00639.2006. [DOI] [PubMed] [Google Scholar]
- Sawa M, Ohtsuka K. Lens accommodation evoked by microstimulation of the superior colliculus in the cat. Vis Res. 1994;34:975–81. doi: 10.1016/0042-6989(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Schaefer KP. Unit analysis and electrical stimulation in the optic tectum of rabbits and cats. Brain Behav Evol. 1970;3:222–40. doi: 10.1159/000125475. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural basis of saccade target selection. Rev Neurosci. 1995;6:63–85. doi: 10.1515/revneuro.1995.6.1.63. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–59. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Effects of frontal eye field and superior colliculus ablations on eye movements. Science. 1979;206:590–92. doi: 10.1126/science.115091. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S. Neural control of vocalization in bats: mapping of brainstem areas with electrical microstimulation eliciting species-specific echolocation calls in the rufous horseshoe bat. Exp Brain Res. 1990;79:192–206. doi: 10.1007/BF00228889. [DOI] [PubMed] [Google Scholar]
- Shen K, Paré M. Neuronal activity in superior colliculus signals both stimulus identity and saccade goals during visual conjunction search. J Vis. 2007;7:15, 1–3. doi: 10.1167/7.5.15. [DOI] [PubMed] [Google Scholar]
- Sinha SR, Moss CF. Vocal premotor activity in the superior colliculus. J Neurosci. 2007;27:98–110. doi: 10.1523/JNEUROSCI.2683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AC, Van Gisbergen JA, Cools AR. A parametric analysis of human saccades in different experimental paradigms. Vis Res. 1987;27:1745–62. doi: 10.1016/0042-6989(87)90104-0. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986;66:118–71. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Gandhi NJ. Single cell signals: an oculomotor perspective. Prog Brain Res. 2003;142:35–53. doi: 10.1016/S0079-6123(03)42005-0. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Holland R, Guthrie BL. Size and distribution of movement fields in the monkey superior colliculus. Brain Res. 1976;113:21–34. doi: 10.1016/0006-8993(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci. 1990;13:309–36. doi: 10.1146/annurev.ne.13.030190.001521. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–81. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271:493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol. 1981;19:180–92. doi: 10.1159/000121641. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–66. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181:810–19. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Straschill M, Hoffmann KP. Activity of movement sensitive neurons of the cat's tectum opticum during spontaneous eye movements. Exp Brain Res. 1970;11:318–26. doi: 10.1007/BF01474390. [DOI] [PubMed] [Google Scholar]
- Straschill M, Rieger P. Eye movements evoked by focal stimulation of the cat's superior colliculus. Brain Res. 1973;59:211–27. doi: 10.1016/0006-8993(73)90262-x. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Schiller PH. Eye and head movements evoked by electrical stimulation of monkey superior colliculus. Exp Brain Res. 1975;23:103–12. doi: 10.1007/BF00238733. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol. 2000;83:1283–99. doi: 10.1152/jn.2000.83.3.1283. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Hoffmann KP, Miller LE. Correlation of primate superior colliculus and reticular formation discharge with proximal limb muscle activity. J Neurophysiol. 1999;81:1978–82. doi: 10.1152/jn.1999.81.4.1978. [DOI] [PubMed] [Google Scholar]
- Syka J, Radil-Weiss T. Electrical stimulation of the tectum in freely moving cats. Brain Res. 1971;28:567–72. doi: 10.1016/0006-8993(71)90068-0. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y. Topographic organization of excitatory and inhibitory commissural connections in the superior colliculi and their functional roles in saccade generation. J Neurophysiol. 2010;104:3146–67. doi: 10.1152/jn.00554.2010. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. Head and body movements evoked electrically from the caudal superior colliculus of rats: pulse frequency effects. Behav Brain Res. 1989;34:71–78. doi: 10.1016/s0166-4328(89)80091-9. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS. Two converging brainstem pathways mediating circling behavior. Brain Res. 1986;385:329–42. doi: 10.1016/0006-8993(86)91080-2. [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol. 2000;84:1103–6. doi: 10.1152/jn.2000.84.2.1103. [DOI] [PubMed] [Google Scholar]
- Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A. 2002;188:89–108. doi: 10.1007/s00359-001-0275-5. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen JA, Van Opstal AJ, Tax AA. Collicular ensemble coding of saccades based on vector summation. Neuroscience. 1987;21:541–55. doi: 10.1016/0306-4522(87)90140-0. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Goossens H. Linear ensemble-coding in midbrain superior colliculus specifies the saccade kinematics. Biol Cybern. 2008;98:561–77. doi: 10.1007/s00422-008-0219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JA. A nonlinear model for collicular spatial interactions underlying the metrical properties of electrically elicited saccades. Biol Cybern. 1989;60:171–83. doi: 10.1007/BF00207285. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JA, Smit AC. Comparison of saccades evoked by visual stimulation and collicular electrical stimulation in the alert monkey. Exp Brain Res. 1990;79:299–312. doi: 10.1007/BF00608239. [DOI] [PubMed] [Google Scholar]
- Wagner H. Sound-localization deficits induced by lesions in the barn owl's auditory space map. J Neurosci. 1993;13:371–86. doi: 10.1523/JNEUROSCI.13-01-00371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. I. Hypermetric goal-directed saccades. J Neurophysiol. 2000;83:2260–84. doi: 10.1152/jn.2000.83.4.2260. [DOI] [PubMed] [Google Scholar]
- Walton MMG, Bechara BP, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol. 2007;98:2022–37. doi: 10.1152/jn.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MMG, Bechara BP, Gandhi NJ. Effect of reversible inactivation of superior colliculus on head movements. J Neurophysiol. 2008;99:2479–95. doi: 10.1152/jn.01112.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MMG, Sparks DL, Gandhi NJ. Simulations of saccade curvature by models that place superior colliculus upstream from the local feedback loop. J Neurophysiol. 2005;93:2354–58. doi: 10.1152/jn.01199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MMG, Mays LE. Discharge of saccade-related superior colliculus neurons during saccades accompanied by vergence. J Neurophysiol. 2003;90:1124–39. doi: 10.1152/jn.00877.2002. [DOI] [PubMed] [Google Scholar]
- Werner W. Neurons in the primate superior colliculus are active before and during arm movements to visual targets. Eur J Neurosci. 1993;5:335–40. doi: 10.1111/j.1460-9568.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997a;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]
- Werner W, Hoffmann KP, Dannenberg S. Anatomical distribution of arm-movement-related neurons in the primate superior colliculus and underlying reticular formation in comparison with visual and saccadic cells. Exp Brain Res. 1997b;115:206–16. doi: 10.1007/pl00005691. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. J Neurophysiol. 1972;35:575–86. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]