Abstract
Telomeric DNA sequences have been at the center stage of drug design for cancer treatment in recent years. The ability of these DNA structures to form four stranded nucleic acid structures, called G-quadruplexes, has been perceived as target for inhibiting telomerase activity vital for the longevity of cancer cells. Being highly diverse in structural forms, these G-quadruplexes are subjects of detailed studies of ligand–DNA interactions of different classes, which will pave the way for logical design of more potent ligands in future. The binding of aminoglycosides were investigated with Oxytricha Nova quadruplex forming DNA sequence (GGGGTTTTGGGG)2. Isothermal Titration calorimetry (ITC) determined ligand to quadruplex binding ratio shows 1:1 neomycin:quadruplex binding with association constants (Ka ) ~ 105M−1 while paromomycin was found to have a two-fold weaker affinity than neomycin. The CD titration experiments with neomycin resulted in minimal changes in the CD signal. FID assays, performed to determine the minimum concentration required to displace half of the fluorescent probe bound, showed neomycin as the best of the all aminoglycosides studied for quadruplex binding. Initial NMR footprint suggests that ligand-DNA interactions occur in the wide groove of the quadruplex. Computational docking studies also indicate that aminoglycosides bind in the wide groove of the quadruplex.
The emergence of G-quadruplexes as potential telomerase inhibitors shows considerable promise for combating cancer (1, 2, 3, 4). The biological relevance of such structures lies in the propensity of many telomeric and promoter sequences to form G-quadruplexes under physiological conditions (5).Increased expression of telomerase, a ribonucleoprotein, has been suggested to be one of the main reasons for the immortality of cancer cells(6, 7, 8, 9).The telomerase induced immortality to cancer cells stems from its ability to act as a reverse transcriptase, elongating curtailed single stranded telomeric ends (10). However for its activity, telomerase requires chromosomal ends to be single stranded. Therefore, ligands that are capable of keeping these ends in non-single stranded forms, such as G-quadruplexes are attractive for cancer treatment (1). While there are a plethora of duplex DNA binding ligands, the number of ligands that bind to G-quadruplexes is limited. This necessitates the search for new compounds to advance G-quadruplex based drug design. Herein, we report the discovery of aminoglycoside (specifically neomycin) ligand binding to oxytricha nova G-quadruplex DNA.
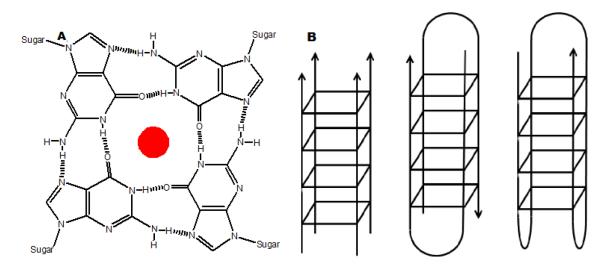
G-quadruplex nucleic acid structures, discovered by Davies and coworkers in 1962, use four DNA/RNA guanine bases to form a planar arrangement connected by eight Hoogsteen paired hydrogen bonds (Figure 1a) (11, 12). The planar arrangement of these guanine bases gives rise to the formation of a G-tetrad, which has a central cavity stabilized by metal cations such as K+ and Na+ (13). These nucleic acid structures are broadly classified into two categories “parallel” and “antiparallel” depending upon the orientation of strands and utilize one or more strands for their formation (Figure 1b). There are many variants of these structures which are often dictated by loops and the nature of stabilizing metal cations.
Figure 1.
(A) diagram of a G-tetrad showing the cyclic array of guanine bases connected by eight hydrogen bonds in Hoogsteen fashion. The cavity in the middle the tetrad is usually occupied by a monovalent or divalent cation represented by the ball in the figure. (B) Representative structures of two broad classes of quadruplexes based upon number of strands involved in their formation.
To date, several types of ligands have been reported to bind to different types of G-quadruplexes. Most of these ligands have a common feature of being planar compounds such as porphyrin derivatives(14, 15, 16), quinacridines(17), anthracenediones(18), triarylpyridines(19), oxazole containing macrocyclic compounds(20), cationic corrole derivatives(21), steroid derivatives(22), and berberine derivatives(23) to name a few. Classical duplex minor groove binders such as distamycin (24, 25) and Hoechst 33258 (26) have also been shown to interact with G-quadruplex structures with less affinity and different modes of interactions than duplex DNA. Interestingly, the majority of the ligands that bind to G-quadruplexes have been proposed to have stacking interactions between G-tetrads or simply binding at the ends. Ligands that selectively recognize G-quadruplex grooves are few even though quadruplex groove recognition is likely to provide much more quadruplex selective ligands (27). The development of ligands that can possibly recognize G-quadruplex through non-stacking mechanisms are essential for complementing existing approaches for recognition of G-quadruplexes.
In recent years, numerous targets of aminoglycosides have been uncovered. This includes group I introns, the rev response element, element of HIV-1 virus, hammerhead ribozymes (28, 29, 30), as well as single stranded nucleic acids (Poly A) (31).We have expanded this list to triple helical nucleic acids comprised of DNA, RNA or hybrid structures (32, 33, 34, 35, 36, 37, 38) and shown that the recognition domain for aminoglycosides is not just limited to RNA targets but to structures that adopt A-form (39, 40, 41, 42). We have also shown that, with synthetic modifications, aminosugars can even be compelled to bind the B-DNA major groove (43, 44, 45, 46, 47). Additionally, the ability of neomycin to assist oligodeoxynucleotide (ODN) delivery in cells(48) has led to the development of ODN-neomycin conjugates as sequence-specific nucleic acid binders(49, 50, 51). This establishes neomycin’s versatility as a nucleic acid binder. We therefore explored if groove recognition of aminoglycosides could be extended to quadruplexes as different quadruplex grooves vary significantly in their width. The flexible nature of aminoglycosides make them better candidates for groove recognition when compared to other DNA groove binders such as distamycin. Besides, quadruplex grooves vary greatly depending upon the structural forms (Figure 1b). For example, a tetramolecular parallel quadruplex has all identical, medium sized grooves, while an antiparallel quadruplex can have grooves of all sizes (wide, medium or narrow), all of which are considerably narrower than a B-DNA major groove. Neomycin in that context was of interest since it prefers to bind grooves that are narrower than B-form DNA major groove (A–form nucleic acid structures).
As a part of our exploration of groove recognition of nucleic acids, we report here that aside from its traditional targets like RNA, there is a considerable affinity of neomycin for G-quadruplex structures. Our studies showed a marked preference of aminoglycosides to the well characterized Oxytricha Nova telomeric sequence which is known to adopt an antiparallel quadruplex structure containing diagonal loop(52). We have used ITC, CD, UV, fluorescence, NMR and computational methods to characterize the binding of neomycin and other aminoglycosides to Oxytricha Nova telomeric quadruplex.
Materials and Methods
Nucleic acid samples
All DNA oligonucleotides were purchased from IDT (Coraville, IA).
The concentrations of the DNA sample solutions were determined by measuring UV absorbance at 85 °C for the quadruplex forming samples, while all other samples were measured at room temperature. Quadruplex structures were formed by heating the stock nucleotide solution in a buffer solution (either K+ or Na+), to 95 °C for 25 min and cooling back to room temperature. The samples were then left to incubate at 4 °C for 2 days to a week. The oligomeric 12 mer duplex (5′-dA12-x-dT12-3′) was synthesized on Expedite Nucleic Acid Synthesis System (8909) using standard phosphoramidite chemistry. Hexaethyleneglycol was used as a spacer (denoted by –X-in the oligomeric DNA sequence). The oligomers were purified on an anion exchange HPLC column (Water Gen-Pak FAX, 4.6×100 mm) with a Tris·HCl buffer system. Buffer A: 25 mM Tris·HCl, 1 mM EDTA, and 10% MeCN (v/v%); Buffer B: Buffer A + 1 M NaCl. Conditions: 2-60% buffer B over buffer A during 0-16 min at a flow rate of 0.75 mL/min. All commercially obtained nucleic acids were used without further purification. The quadruplex formation was confirmed by CD spectroscopy.
Chemicals
Aminoglycosides used in the study were purchased from MP Biomedicals (Solon, Ohio, USA). All chemicals were used without further purification.
Circular dichroism experiments
CD experiments were carried out at 20 °C using a Jasco J-810 spectropolarimeter with a thermo-electrically controlled cell holder. The CD spectra were recorded as an average of 3 or 5 scans. For CD titrations, a concentrated solution of the ligand was serially added to the nucleic acid sample and allowed to equilibrate for 5 min before a scan was taken. The resulting scan was plotted for CD signal changes with respect to wavelength. Data processing was done using Kaleidagraph 3.5 software.
Isothermal titration calorimetry (ITC) experiments
ITC titrations were performed at 20.0 °C on a MicroCal VP-ITC (MicroCal, Inc.; Northampton, MA). In most cases, small aliquots of ligand were injected (thirty injections) from a rotating syringe with a stirring speed of 260 rpm into an isothermal sample chamber containing 1.42 mL of the quadruplex solution at 30μM or 60 μM/strand. Each experiment was followed by a control experiment under the same conditions using buffer solution instead of the quadruplex. The buffer heats were then subtracted. The area under each heat burst curve was integrated and the resulting data points were fitted using the Origin (version 5.0) software.
Fluorescence intercalator displacement (FID)
Fluorescence experiments were performed on Photon Technology International instrument (Lawrenceville, NJ) and on a TECAN Genois −96 well plate reader. All experiments were performed in a temperature range of 20-22 °C. The experiments were performed in a 3-mL quartz cell with 100 mM NaCl, 10 mM sodium cacodylate, and 0.5 mM EDTA buffer at pH 7.0 or with 200μL total volume for 96 well plate experiments. The DNA solutions were prepared at 0.25 μM/quadruplex in 100mM NaCl buffer and mixed with thiazole orange (TO) to a concentration of 0.50 μM. The ligand was serially added to the DNA/TO solution and followed by a 4 minute equilibration time before the fluorescence spectrum was recorded. The TO excitation was performed at 501 nm and the emission was then recorded from 510-650 nm.
UV spectroscopy
All UV spectra were obtained on a 12 cell holder Cary 1E UV-Vis spectrophotometer equipped with temperature controller. Quartz cells with 1cm path length were used for all the experiments. The quadruplex melting was monitored at 260 nm, 235 nm, and 295 nm wavelengths. The DNA samples were heated in the temperature range of 20.0 °C-98.0 °C at the heating rate of 0.2 °C/minute. The resulting temperature-absorbance profiles were plotted using Kaleidagraph 3.5 software. For Tm determination, first derivative analysis was used.
NMR titrations
All NMR experiments were carried on Bruker Avance (500MHz) spectrophotometer equipped with z gradients and temperature control (Department of Chemistry, Clemson University NMR facility). The DNA concentration used in 1H MNR titrations was 0.5 mM per strand. 2D NOESY experiments were carried out at a concentration of 2 mM per strand and a final volume of 250μL. All the experiments were carried out in a Shimegi microvolume NMR tube matched with D2O, and collected at room temperature (~295 K) or at 5°C. All NMR samples contained 10mM Sodium Phosphate, 0.5mM EDTA, 100mM NaCl buffer, pH 7.0. Samples were prepared either as (90% H2O+10%2H2O) or 100% 2H2O.For 1D water suppression, a pulse sequence with presaturation of HOD signal was used. For 1H NMR experiments total of 512 scans were collected. The data was visualized with Bruker X-win Plot 3.5 software. For 2D NOESY experiments, mixing time of 150, 200 and 250 ms was used; acquisition delay was 1.29 sec. Total 64 scans were collected. For NOESY experiments phase sensitive water suppression was utilized as described by Sklenar(53). Data was subsequently 2D transformed, and imported into SPARKY(54) for visualization and resonance assignment. DNA proton resonances were assigned based on NOE patterns and previously published chemical shifts (55, 56).
Moelcular Modeling
All dockings were performed as blind dockings (blind-docking refers to the use of a grid box which is large enough to encompass any possible ligand-receptor complex) using Autodock Vina 1.0(57). AutoDock Vina was chosen because of a] its ability to take advantage of multiple core processors as well as its much more efficient search of the potential energy surface; b] its high accuracy with ligands possessing more than 20 rotable bonds when compared to Autodock 4.2.
Autodock Vina docking was performed using the default “exhaustiveness” value of eight for all molecules except for Neomycin and Paromomycin which were docked with an “exhaustiveness” value of 50. All other parameters were used as defaults. All rotable bonds within the ligand were allowed to rotate freely and the receptor was considered rigid. The Protein Data Bank file (PDB ID: 156D)(58) was used as the DNA quadruplex receptor for all dockings. All ligand structures were created using Discovery Studio® Visualizer 2.5 and then brought to their energetically minimized structures by the Vega ZZ program (59) utilizing a conjugate gradient method with SP4 forcefield. Autodock Tools version 1.5.4(60) was used to convert the ligand and receptor molecules to the proper file formats for AutoDock Vina docking. Validation that AutoDock Vina has the ability to identify the binding site and correctly score the receptor-ligand interactions was confirmed by its ability to accurately predict the differences among experimental binding constants for the series of aminoglycosides analyzed in this investigation. Further confidence is gained from AutoDock Vina’s ability to produce dockings consistent with other available experimental data such as salt dependence. Additionally, AutoDock Vina was used to confirm docking of neomycin to triplex DNA and successfully docked the ligand in the Watson-Hoogsteen groove as reported (61).
Results and Discussion
Selection of Aminoglycosides as G-quadruplex binding ligands
A recent study by Jarstfer and coworkers suggested aminoglycosides as a class of small molecules for telomerase inhibition (62). In addition to this, our initial findings suggested that aminoglycosides (neomycin specifically), apart from their preference for RNA targets, bind to DNA structures. Remarkable stabilization of triple helices of DNA, RNA and hybrid structures prompted us to probe the common feature that makes neomycin bind such novel targets. Our studies suggested that aminoglycosides prefer to bind structures that adopt A-form (42) which are known for the narrowness of their major groove. Since G-quadruplex grooves can adopt varying sizes (narrow/medium/wide), which is dictated by the conformation of the base (syn/anti) participating in the G-tetrad formation, we have investigated if neomycin can possibly fit in the quadruplex grooves. Since neomycin lacks a group that can be detected by spectrophotometric techniques such as UV absorption or fluorescence, we have previously performed studies with ligands where a fluorescent probe (pyrene, acridine) conjugated to neomycin was used to find neomycin’s preference for nucleic acid structures using competition dialysis experiments. The results indicated that apart from its preference for binding to RNA, there is considerable binding of aminoglycoside to G-quadruplex structures as well, particularly antiparallel structure (42). We investigated the binding of neomycin to both parallel an antiparallel structure. Interesting binding properties were found with different types of antiparallel structures. Herein, we present a structurally well characterized Oxytricha Nova sequence, which is known to adopt an antiparallel structure (The findings of other sequences studied will be published elsewhere). The choice of this quadruplex was made since this quadruplex possesses three distinct types of grooves that quadruplex structures have been known to adopt.
ITC titration of neomycin into G-quadruplex and determination of binding parameters of interaction
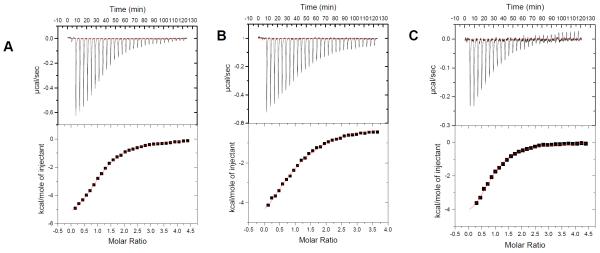
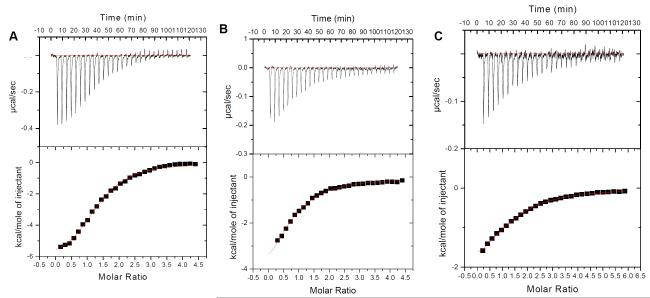
Isothermal Calorimetry (ITC) has been one of the most sought after techniques for studying the interaction of biological molecules with ligands in recent years. This technique allows us to extract various thermodynamic parameters from a single experiment (63, 64, 65). We determined binding of Oxytricha Nova (GGGGTTTTGGGG)2 antiparallel quadruplex with two aminoglycosides, neomycin and paromomycin using ITC. The affinities of the other aminoglycosides were too weak to obtain accurate thermodynamic using ITC. ITC titrations for this quadruplex with neomycin and paromomycin were carried out in sodium and potassium salt buffers. The binding constants (Ka) obtained from these titrations are shown in Table 1, Figure 3-4, S1 (see supplementary information). In the presence of both sodium and potassium salts, with increasing salt concentrations, a slight decrease in the association constant was observed. In the presence of potassium ions, the association constant for the interaction decreased as the salt concentration increased from 30 mM to 90 mM. A similar effect in the association constant of neomycin to the quadruplex was observed in the presence of sodium ions as well. An almost four fold decrease occurred as the sodium salt concentration was varied from 30 mM to 100 mM. However, under similar salt conditions (30 mM), neomycin had a slightly higher affinity in the presence of sodium ions (Ka=3.79×105 M−1), in comparison to potassium ions (Ka=2.76×105M−1).
Table 1.
Association constants obtained using ITC titration of Oxytricha Nova quadruplex (GGGGTTTTGGGG)2 with neomycin and paromomycin under different salts concentrations in buffer 10mM Sodium cacodylate, 0.5mM EDTA at pH 7.0 at 20°C.
| Salt | Ligand | Salt Concentration (mM) |
n (drug/tetraplex) |
KaX105(M−1) |
|---|---|---|---|---|
| Na+ | Neomycin | 30 | 0.97±0.08 | 3.79±0.22 |
| Na+ | Neomycin | 100 | 0.89±0.01 | 1.03±0.50 |
| K+ | Neomycin | 30 | 1.34±0.02 | 2.76±0.21 |
| K+ | Neomycin | 60 | 0.89±0.02 | 1.42±0.10 |
| K+ | Neomycin | 90 | 0.77±0.04 | 1.15±0.08 |
| K+ | Paromomycin | 60 | 1.34±0.03 | 0.87±0.04 |
| K+ | Paromomycin | 90 | 1.07±0.05 | 0.45±0.01 |
C values range from 1 to 11.3. c (Wiseman constant)=Ka·[M]·n Where Ka = Association constant of the ligand [M]= concentration of the macromolecule and n= binding stoichiometry
Figure 3.
ITC profiles for the titration of oxytricha nova quadruplex (GGGGTTTTGGGG)2 with neomycin at 20°C. (A) 10 mM Sodium Cacodylate, 0.5 mM EDTA, and 30 mM NaCl, pH 7.0. (B) 10 mM sodium cacodylate, 0.5 mM EDTA, and 100 mM NaCl, pH 7.0. (C) 10 mM sodium cacodylate, 0.5 mM EDTA, and 30 mM KCl, pH 7.0. The heat burst curves (top panels in the figures) is the result of 10μL injections of a concentrated ligand solution into the DNA solution in buffer conditions as described earlier. The data points (lower panels of figure) reflect the corrected injection heats, which were obtained by subtracting the dilution heats obtained from separate control experiments in which ligand was titrated with buffer only. The red line represents the calculated fits of the data using one binding site model. The data fitting was carried out using Origin 5.0 software.
Figure 4.
ITC profiles for the titration of oxytricha nova quadruplex (GGGGTTTTGGGG)2 with neomycin or Paromomycin at 20°C. (A) neomycin in buffer 10 mM sodium cacodylate, 0.5 mM EDTA, and 60 mM KCl, at pH 7.0. (B) neomycin in buffer 10 mM sodium cacodylate, 0.5 mM EDTA, and 90 mM KCl at pH 7.0. (C) paromomyicn in buffer 10 mM sodium cacodylate, 0.5 mM EDTA, and 60 mM KCl at pH 7.0. The heat burst curves (top panel in the figures) is the result of 10μL injections of a concentrated ligand solution into the DNA solution in buffer conditions as described earlier. The data points (lower panel in the figures) reflect the corrected injection heats, which were obtained by subtracting the dilution heats obtained from separate control experiments in which ligand was titrated with buffer only. The red line represents the calculated fits of the data using one binding site model. The data fitting was carried out using Origin 5.0 software.
In order to determine the number of ion pairs formed during the binding, a plot of association constants versus salt concentrations was made (66, 67) (Figure5). The slope from this log plot reveals the formation of one ion pair. This is in contrast to our findings for triple helical DNA (68) as well as previous work on binding of neomycin class antibiotics to the A-site rRNA construct where formation of three ion pairs was found (69). The formation of one ion pair thus suggests that binding occurs with lesser electrostatic interaction than what has been observed for the recognition of nucleic acid pockets in the aforementioned nucleic acids.
Figure 5.
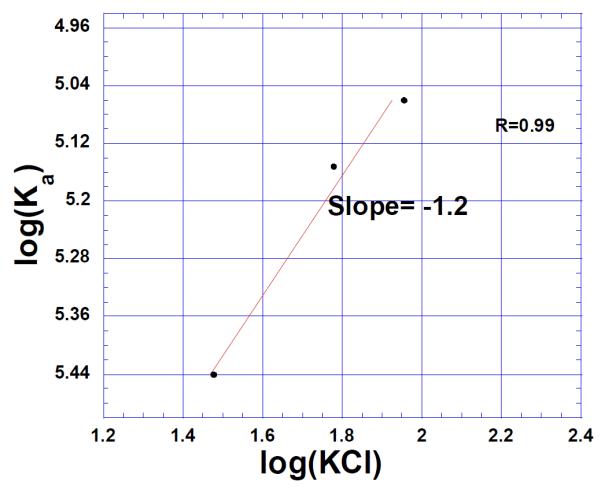
Salt dependence of the neomycin binding to oxytricha nova quadruplex (GGGGTTTTGGGG)2 in 10 mM sodium cacodylate, 0.5 mM EDTA at pH 7.0 (Temperature 20 °C) at varying salt concentrations (30, 60 and 90mM KCl respectively). The experimental data were fit with linear regression and the solid line reflects the resulting curve fit.
The ITC titrations reveal close to a 1:1 binding stoichiometry (ligand to quadruplex) for both neomycin and paromomycin. This binding stoichiometry is of interest given that the quadruplex possesses three types of grooves. The solution structure of this quadruplex has revealed that two strands associate in an antiparallel fashion where thymine loops are diagonally connected (52). The bases around each tetrad are associated in a syn-syn-anti-anti fashion giving rise to a wide, two medium sized and a narrow groove (56, 70). We investigated the shape complementarity of neomycin and paromomycin to the different grooves of quadruplex. Previous studies have shown that neomycin doesn’t stabilize B-DNA owing to wide B-DNA major groove (43), however all four of the quadrupelx grooves are much shallower than a B-form DNA major groove. Additionally, and perhaps more importantly, there are two medium sized grooves; a binding stoichiometry of two ligand molecules per quadruplex would be expected if binding were to occur to these grooves. Recognition of neomycin is thus likely in the narrow or wide groove present. The paromomycin binding also shows 1:1 binding stoichiometry which is suggestive of it binding to the same place as neomycin since the two aminoglycosides are structurally very similar, with neomycin possessing an extra amino group (Figure 2). A weaker binding of paromomycin is similar to observations previously reported, where neomycin shows a much higher increase in nucleic acid affinity than paromomycin (28, 32).
Figure 2.
Structures of the ligands used in the study.
Fluorescence Intercalator Displacement Experiments
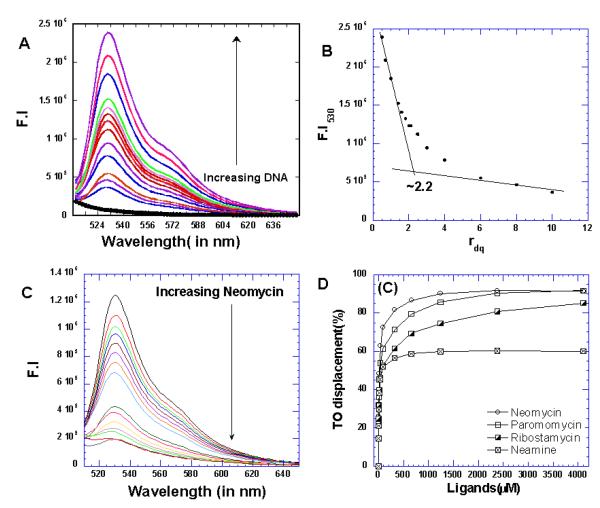
G4DC50 values show Neomycin is better than other aminoglycosides in G-quadruplex binding. Fluorescence indicator displacement assay (FID) developed by Boger (71, 72)allows the study of non fluorescent molecules binding to DNA. This assay has been extended to study the ligand binding to G-quadruplexes as well (73, 74). We chose thiazole orange as our fluorescent probe. Thiazole orange is a more suitable probe than ethidium bromide allowing for determination of high affinity sites as well as its non sequence specificity (71, 74). Additionally, the fluorescence enhancement upon binding to DNA has been observed to be many folds higher for thiazole orange than ethidium bromide (71). Recently the ability of a ligand binding to a G-quadruplex has been expressed in terms of G4DC50 values. These values are representative of ligand concentrations required to displace 50% of the probe bound to the G-quadruplex target in the displacement assays.To ascertain the ratio of fluorescent probe bound to DNA, we carried direct fluorescence titrations where thiazole orange was titrated with concentrated quadruplex DNA solution. The titration revealed that thiazole orange binds to DNA in 2:1 stoichiometric ratio Figure 6A-B. A DNA solution containing two equivalents of thiazole orange was first titrated with the appropriate ligand solution displacing the bound thiaozle orange. As increasing amounts of ligand was added, the fluorescence intensity continued to decrease suggesting displacement of bound thiazole orange Figure 6C. To see if the fluorescence quenching was, indeed, an outcome of aminoglycoside binding, control experiments were run. Titration of thiazole orange with neomycin did not cause any significant drop in the fluorescence intensity. In addition to this a polyamine, spermine, was titrated to displace bound thiazole orange Figure S2 (see supplementary information). Even after addition of large amounts of polyamine, extremely poor thiazole orange displacement resulted.These findings indicate that fluorescence quenching of the probe, thiazole orange, is not the outcome of non specific interactions. Among the aminoglycosides studied, neomycin clearly showed the least amount of ligand required to displace 50% of fluorescent probe. The order of quadruplex preference for four aminoglycosides studied was found to be neomycin>paromomycin>ribostamycin>neamine Table2, Figure 6(D), (please see supplementary information). The DC50 value obtained for this experiment showed clear preference of neomycin for quadruplex (at least four fold higher) than its affinity to duplex DNA. The other aminoglycosides showed even higher DC50 values for duplexes. For quadruplex, the neomycin DC50 (16.6 μM) is almost two fold lower than paromomycin (29.1 μM) and approximately 4.5 fold lower than ribostamycin DC50 (68 μM). A much larger difference is seen with aminoglycoside binding to the DNA triplex, with neomycin preference DC50 (13 μM) almost 15- fold less than paromomycin DC50 (157 μM), and about 35-fold lower than ribostamycin DC50=(459 μM) as observed for the 5′-dA12-x-dT12- x-dT12-3′ triplex (68). While the relative affinities of the drugs to the same target can be compared using this method, one should be cautious in comparing drug binding to different targets, as slight differences in affinities of thiazole orange to duplex DNA, triplex DNA and quadruplex DNA can complicate the analysis. A closer look at the relative affinities of aminoglycosides reveal the same general trend where neomycin was found to have least DC50 value. The findings presented here correlates very well with the ability of these ligands for telomerase inhibition where neomycin was identified as best of all aminoglycosides studied(62).The results presented in Table2 show an increasing DC50 value with decreasing charge on the aminoglycoside suggesting that the charge present on them plays a role in their affinity to the quadruplex. However association constants obtained in increasing salt concentrations displayed little variations in the binding affinity of neomycin. Given that salt dependence studies indicate less charge interactions (formation of three ion pairs for triplex DNA while one ion pair formation for quadruplex DNA) than triplex DNA, shape complementarity seems more likely for quadruplex groove in comparison to triplex DNA or A-site RNA grooves. This is in contrast to the triplex groove recognition by aminoglycosides where both charge/potential complementarity plays a significant role in binding.
Figure 6.
(A) Fluorescence titration thizaole orange with quadruplex DNA 5′-GGGGTTTTGGGG-3′. The thiazole orange solution was titrated with the concentrated DNA solution. (B) The binding stoichiometry plot of thizaole orange with quadruplex DNA. (C) A representative FID displacement titration showing the displacement of fluorescent probe with the addition of concentrated aminoglycoside (neomycin) from the bound quadruplex DNA. (D) The graph showing the displacement of thiazole orange from quadruplex DNA using different aminoglycosides. All experiments were performed in buffer 10mM sodium cacodylate, 0.5mM EDTA, 100mM NaCl at pH 7.0 (T=20°C).
Table 2.
The table of DC50 values obtained from studies on duplex and quadruplex DNAs.
| Aminoglycoside | 5′-GGGGTTTTGGGG-3′ G4DC50(μM) |
5′A12-X-T12-3′ DC50(μM) |
|---|---|---|
| Neomycin | 16.6±2.0 | 68.2±4.0 |
| Paromomycin | 29.1±5.0 | 328.1±64.2 |
| Ribostamycin | 68.1±16.0 | 1037.9±193.0 |
| Neamine | 79.0±24.0 | 520.7±244.4 |
The difference between neomycin and paromomycin is one positive charge, and the binding studies indicate that this positive charge (amine) leads to a two-fold increase in neomycin binding to the quadruplex and a fifteen fold increase in neomycin binding to the triplex. Additionally, as seen with the salt dependence studies, very little dependence on salt is seen with aminoglycoside binding to quadruplex (one ion-pair) as opposed to three ion pairs seen with neomycin bound to a DNA triplex. One can infer that shape complementarity (as opposed to potential complementarity) plays a larger role in aminoglycoside based recognition of the quadruplex grooves, when compared to their binding to the DNA triplex grooves.
CD spectroscopy
We carried out complete CD titrations to find out ligand induced changes in the CD signal to observe any conformation change accompanying the ligand binding. The CD titration of the oxytricha nova quadruplex, 5′-GGGGTTTTGGGG-3′ was carried in the presence of aminoglycosides (Figure 7). The maxima of the CD signals in all experiments carried out were observed at ~295 nm while the minimum is at ~260 nm. This is consistent with the antiparallel structure of the G-quadruplex(75). However, binding of neomycin induces minimal CD changes Figure 7 (left) in the DNA absorption region even after addition of large amounts of ligand (more than three equivalents). A similar CD pattern was also observed for paromomycin, Figure 7(right). These CD results suggest that interaction of these ligands does not perturb the quadruplex structure.
Figure 7.

CD titration of (GGGGTTTTGGGG) 2 quadruplex with Neomycin (left) and paromomycin (right) in buffer 10 mM Sodium Cacodylate and 100 mM NaCl at pH 7.0. Small aliquots of ligand were serially added to the DNA (15/10μM/Str) solution at 20°C. During the titration, the DNA solution was stirred with the added ligand for five minutes followed by another five minutes of equilibration time. The CD signal represents an average of 3 scans.
UV thermal denaturation Studies
UV thermal melting experiments were carried out to find any ligand induced thermal stabilization. The Oxytricha nova quadruplex melted at 55.7°C in cacodylate buffer containing 100mM sodium salt. Quadruplex was thermally denatured with aminoglycosides. At 1:1 ligand to quadruplex ratio, the thermal denaturation profiles showed ~1-2°C stabilization in the presence of neomycin (Figure 8, S3, Please see supplementary information).Similar affinities with DNA triplex lead to >10°C changes in triplex denaturation temperatures. We probed these, somewhat, surprising thermal melting profiles by studying the binding of neomycin at increased temperatures using ITC. In potassium buffer, a general decreasing trend in the association constant was observed as temperature was raised. Almost four fold decrease in the association constant was determined as the temperature of the study was increased from 20°C to 40°C (Figure S4, Table S1, see supplementary information). In the sodium buffer, when we carried the ITC experiment at 40°C, weak and broad heat burst curves were obtained. The resulting enthalpy profile could not be fitted. Because of these limitations, the association constant close to melting temperatures could not be determined. The ITC titrations at different temperatures in potassium buffer suggest a weakened binding at temperatures close to the melting point of the DNA. Additionally, we also probed the thermal melting profile of neomycin binding to quadruplex by van’t Hoff’s method using procedures described by Mergny(76, 77) and Chaires (69). The thermodynamic data was obtained at 1:1 quadruplex to ligand ratio. The neomycin binding was found to be enthalpically favored (as compared to DNA melting alone) by −0.84Kcal.mol−1(Table 3), whereas the Gibbs free energy for the quadruplex dissociation was favored by -0.13Kcal.mol−1 at 55°C. This small favorable dissociation of the quadruplex again shows that binding of neomycin is weak/ negligible at temperatures close to the melting temperatures of the quadruplex. It should, however, be borne in mind that binding that leads to higher melting temperature of the nucleic acid don’t always represent a highly stable system as final state of the entire binding process is determined by overall effect of enthalpic and entropic contributions.
Figure 8.
UV melting profiles of Oxytricha Nova quadruplex (GGGGTTTTGGGG)2 in the presence of various ligands. The oligonucleotides concentration was 10 μM/ str while the ligand concentrations were 1:1 quadruplex/ ligand ratio in buffer 10mM Sodium Cacoldylate, 0.5 mM EDTA and 100 mM NaCl at pH 7.0.The nucleic acid samples were heated at the rate of 0.2 deg/min. The melting temperatures were determined by the first derivative analysis.
Table 3.
Thermodynamic parameters of melting of (GGGGTTTTGGGG)2 quadruplex in buffer 10 mM sodium cacodylate, 0.5mM EDTA, 100 mM NaCl at pH 7.0. [DNA]=5 μM/strand. Please see supplementary information for additional details for calculation of thermodynamic parameters.
| Ligand | ΔH(Kcal/mol) | ΔS(cal/mol) | ΔG(Kcal/mol) at 55°C |
|---|---|---|---|
| None | −133.36 | −380.55 | −8.49 |
| Neomycin(1:1) | −134.20 | −382.7 | −8.62 |
Another interesting aspect of neomycin binding to the quadruplex is the sharpness of the melting profile as compared to the melting of DNA alone (consistent with multiple trials). A sharper transition is indicative of a strongly temperature dependent affinity constant (76) which is concurrent with our findings from ITC measurements.
NMR studies with paromomycin and neomycin
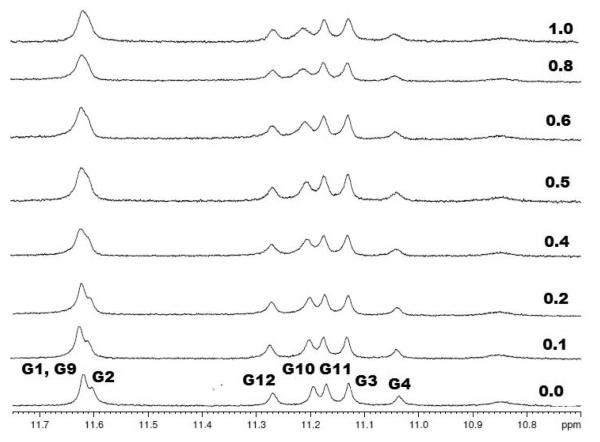
The solution structure of the Oxytricha Nova is well established having been solved in both sodium and potassium salt solutions. Both the sodium and potassium forms of the structure have same overall fold with slight local variations in the T4 loop(55, 56). To investigate the binding of neomycin and paromomycin to quadruplex DNA, the DNA sample was prepared in similar conditions as employed in other biophysical experiments. The chemical shifts for the imino, amino and aromatic protons were assigned using procedures described by Feigon and coworkers(52, 55, 56). The 1H NMR titration was initially carried out with increasing amounts of neomycin. However, even at low ligand concentration (0.5 molar equivalent ligand to DNA) considerable precipitation was seen. We, therefore, carried out 1H NMR titration with structurally similar ligand paromomycin. ITC experiments with paromomycin indicated a slightly lower binding to our DNA target yet demonstrated the same stoichiometry as neomycin. Given its nearly identical structure (the difference being an amino group) as neomycin, we hypothesized a similar mode of binding as what would be expected with neomycin. No precipitation was seen with paromomycin titration into the DNA up to 1.0 molar equivalent of the ligand. The addition of the paromomycin to the DNA caused both shifts in individual resonance positions as well as broadening of the chemical shifts in both imino and aromatic regions of protons. Figure 9 shows the representative titration of paromomycin into DNA.
Figure 9.
1H NMR titration of Oxytricha Nova quadruplex DNA with paromomycin. Increasing amounts of the ligand were serially added to the DNA solution as indicated on each scan. The DNA solution was prepared in 10 mM Sodium Phosphate, 0.5 mM EDTA, 100 mM NaCl at pH 7.0. The titration was carried out at room temperature (22°C).
Several protons resonances demonstrated shifts in both imino and aromatic regions of proton spectra as shown in Figure 9. The chemical shift positions of both imino and aromatic protons of G2 showed the greatest change (Figure 10). The other major shifts were observed for G3, G4 and G10. The G10 imino protons were broadened as well. Interestingly thymine bases that make the loop in this DNA structure showed little effect on them upon paromomycin titration. Titrations with neomycin also showed similar shifts until precipitation was seen (Figure S5, see supplementary information). However, broadening of the imino resonances was more prominent in comparison to paromomycin. To further characterize the mode of binding of paromomycin to DNA, 2D NOESY experiments will be performed and such a structural elucidation of ligand-quadruplex complex is now underway. While a comprehensive analysis is needed to completely characterize the structure of the complex, these initial results clearly demonstrate the binding of paromomycin to DNA in a single stable conformation.
Figure 10.
Changes in the proton resonances at 1:1 DNA ligand ratio of 1H NMR titration of Oxytricha Nova with paromomycin. The changes observed in the imino proton resonances are shown on left and changes in the aromatic proton resonances are presented on the right.
Looking at the groove structures of the four grooves of the quadruplex, the wide groove is composed of bases (G1-G4 )-(G4-G1) while the two medium grooves are composed of bases (G1-G4) –(G9-G12) (G4-G1)-(G12-G9) and the minor groove involves (G9-G12)-(G12-G9) bases. Most of the changes in the chemical shifts of imino and aromatic protons are centered on G1-G4 bases which comprise the wide groove of the quadruplex, suggesting that the drug binding significantly perturbs the wide groove of the quadruplex, as opposed to the other grooves.
Docking studies
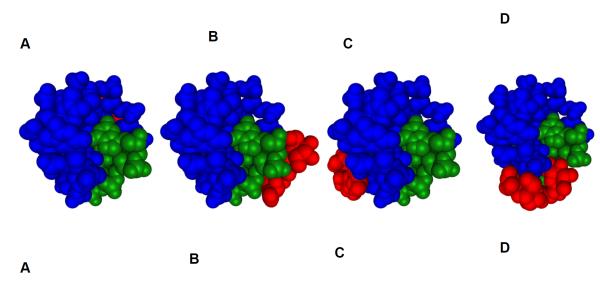
There has been an increasing interest in using docking methods which allow rapid selection of promising candidates from drug libraries (27, 78). AutoDock, initially designed to study protein interactions, has been used to study small molecule nucleic acid interactions revealing accurate reproducibility of crystal structures for both groove binders and intercalators bound to nucleic acids (79). Few laboratories have also benefited from the use of AutoDock for probing small molecule interactions with the G-quadruplex(27, 80, 81).we have performed docking studies using recently introduced AutoDock Vina. According to the molecular modeling performed in this study, neomycin shows marked preference for the wide groove of the oxytricha nova quadruplex as compared to the two medium sized grooves and the narrow groove (Table 4, Figure 11).
Table 4.
Binding energies obtained from docking of neomycin and paromomycin in the different grooves of the quadruplex DNA studied.
| Aminoglycoside | Groove | E Dock (kcal·mol−1) |
|---|---|---|
| Neomycin | Narrow | −4.0 |
| Neomycin | Medium(1) | −5.4 |
| Neomycin | Medium (2) | −5.4 |
| Neomycin | Wide | −7.2 |
| Paromomycin | Wide | −7.0 |
Figure 11.
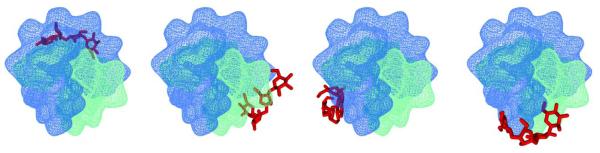
Computer generated models showing two different views of neomycin (red) bound to four different grooves of the Oxytricha nova (A) neomycin docked in the wide groove (B) neomycin docked in the narrow groove (C-D) neomycin docked in the two medium grooves.
When bound to the quadruplex, neomycin can adopt four different conformations that are identical in energy due to the symmetry of the quadruplex’s widest groove. These conformations all share the following characteristics. (1) All rings are essentially parallel to the groove floor except for ring I. (2) In each conformation there exists either one close electrostatic interaction(<3.5 ), consistent with the experimentally determined salt dependence studies (formation of one ion pair), or two more distant contacts (<4.5 ). (3)Each low energy conformation resides in the wide groove. (4) Every conformation has approximately the same energy (−7.2 kcal·mol−1) according to the AutoDock Vina scoring function. The poses for neomycin have been divided into four archetypes (Figure 12). Within these archetypes there is a great deal of flexibility favoring increased electrostatics, Van der Walls or hydrogen bonding interactions.
Figure 12.
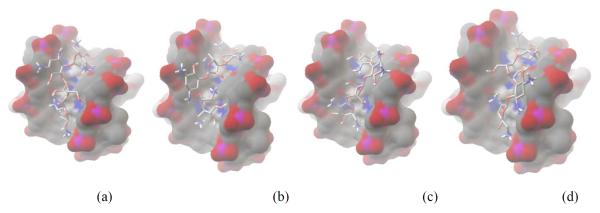
Computer generated model showing the four docked poses of neomycin to quadruplex where (a-d) represent the four poses of equal energy available to neomycin. Binding affinity is −7.2 kcal·mol−1
We also carried out similar docking studies with paromomycin. As expected, due to the structural similarity (Figure 2) between paromomycin and neomycin, the observed low energy binding pocket is also the wide groove of the quadruplex. In comparison to neomycin binding to the quadruplex, it is energetically disfavored by 0.2 kcal·mol−1 (Table 4) corroborating the findings from our calorimetric studies. The absence of one amine group, as compared to neomycin leads to two low energy confirmations possible for paromomycin, as opposed to four in the case of neomycin.
Neomycin’s unfavorable interaction in the narrow groove of the quadruplex likely stems from the extreme narrowness of the groove (end to end distance <4.5 ). Few reports of quadruplex groove binding ligands have been reported (27). It has been suggested that the quadruplex groove recognition is expected to give a higher degree of selectivity over the other DNA structures, yet few groove binders have been identified(27). Polyamides such as distamycin have been shown to bind as two sets of stacked dimers in two of the four identical grooves of (TGGGGT)4 parallel quadruplex(25) with much lower affinities than the duplex. Aminoglycoside based shape complementarity to the qaudruplex large groove opens up new avenues for selective groove recognition of the quadruplexes.
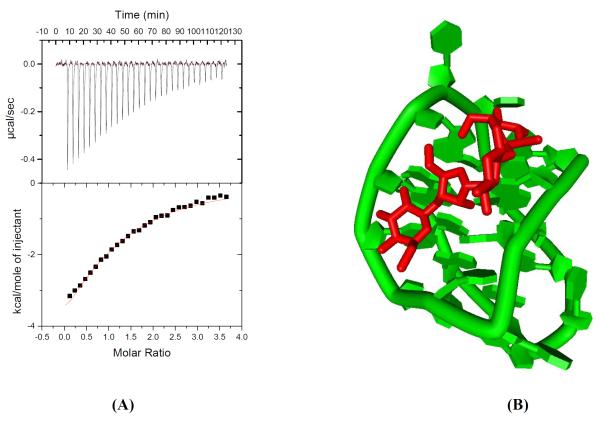
We also carried out docking studies of neomycin binding another antiparallel quadruplex formed by the 22mer DNA sequence (5′-AGGGTTAGGGTTAGGGTTAGGG-3′) mimicking the human telomeric end. This quadruplex, like the (5′-GGGGTTTTGGGG-3′)2 quadruplex also has four grooves (narrow, medium and wide) as revealed by its solution NMR structure. ITC experiments with this 22mer DNA quadruplex with neomycin revealed a 1:1 binding stoichiometry which is same as observed with the Oxytricha Nova quadruplex (Figure 14 A, Table S2). The docking studies showed that similar to Oxytricha Nova, the most favorable binding pose had neomycin in the middle of the wide groove (Figure 14B).
Figure 14.
(A)ITC titration of neomycin into human telomeric quadruplex DNA in buffer 100 mM NaCl,0.5 mM EDTA, 10mM sodium cacodylate at pH 7.0 (T= 20°C). (B) Computer model of neomycin binding in the wide groove of 22mer DNA sequence d-(5′- AGGGTTAGGGTTAGGGTTAGGG-3′) mimicking the human telomeric end. The DNA is shown in the green and neomycin is shown in the red.
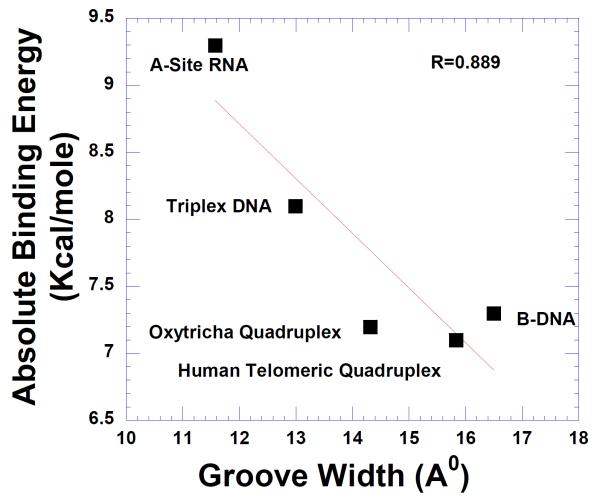
This result also suggests that binding of neomycin is probable in the wide groove quadruplex DNA DNA structures (as opposed to medium/ narrow grooves). To compare the dependence of ligand binding to nucleic acid groove widths, AutoDock analysis of groove binding of neomycin to different nucleic acids was also carried. The results from this analysis are presented in Figure 15.
Figure 15.
A graph showing the binding affinity of neomycin to different nucleic acids with respect to their groove widths. (Please see supplementary information for PDB ID of nucleic acids used in the study; Table S3)
A linear correlation between groove width and neomycin binding was seen. There is a decrease in binding affinity with increasing groove width indicating that neomycin binding to different DNA structures is clearly groove width dependent. As grooves get wider (from A-site RNA to triplex DNA to quadruplex DNA), decrease in the shape complementarity of ligand-nucleic acid leads to a decrease in affinity.
Conclusion
The recent advances in the G-quadruplex recognition by small molecules have prompted us to explore the groove binding nature of aminoglycosides. From our studies, the following conclusions can be drawn:
Neomycin binds Oxytricha Nova quadruplex in 1:1 binding stoichiometry. The direct ITC titration of the Oxytricha Nova quadruplex DNA revealed that neomycin binds with association constants K ~105M−1 a in the presence of both sodium and potassium ions. The ligand favors the Oxytricha Nova quadruplex over the 22-mer human telomeric quadruplex.
The salt dependence studies in the presence of both sodium and potassium ions showed the same trend of a slight decrease in the association constants with an increase in the salt concentrations. This indicates that the binding interaction of neomycin to the quadruplex is moderately driven by electrostatic interactions. The binding stoichiometry of 1: 1 is indicative of a single unique site for neomycin complexation.
FID assays were conducted with the Oxytricha Nova quadruplex. The binding of fluorescent probe indicated a 2:1 ratio of ligand to quadruplex binding. The G4DC50 values obtained from FID titrations show that neomycin is best in displacing the fluorescent probe bound to quadruplex. The other aminoglycosides were much worse in displacing the fluorescent probe bound to these quadruplexes. Neomycin binds approximately five fold better than ribostamycin.
The binding mode of neomycin does not perturb the structure of the quadruplex. The CD experiments showed that binding of either neomycin or any other aminoglycoside brings negligible change in the CD signal.
The binding of ligands is accompanied by small thermal stabilization under the buffer conditions used. UV melting studies show that complexation of any of the ligands studied, result in small increase in the melting temperature of the quadruplex DNA due to decreased binding at temperatures near the melting point of the DNA.
1H NMR titration of the quadruplex DNA with paromomycin suggests ligand binding in the wide groove.
A molecular model generated using AutoDock Vina corroborates the experimental results suggesting that neomycin binds to the wide quadruplex groove with one amino group forming an ion pair with quadruplex phosphate negative charge.
Recognition of quadruplex nucleic acids has mostly been achieved using planar aromatic ligands through stacking interactions. This has been an outcome of lack of structures that could bind in the quadruplex grooves. Quadruplex groove recognition can now be utilized and coupled with ligands that are known to bind through stacking interactions. We have successfully demonstrated that such coupling of two moieties lead to increased binding than individual units for triplex, duplex and hybrid nucleic acid structures.(34, 38, 42) It is probable that such high affinity ligands for specific binding to quadruplexes can be generated by combining two ligands that have their own distinct binding sites. Such efforts are currently underway in our laboratories and will be reported in the near future.
Supplementary Material
Figure 13.

Computer Generated models showing paromomycin bound to quadruplex. (a) and (b) demonstrate the two poses available to paromomycin in the wide groove. Binding affinity is −7.0 kcal·mol−1. (c) A computer generated model showing neomycin (blue) and paromomycin (green) viewed together in the wide groove. Ion pairing interaction of neomycin to phosphate backbone is shown by the solid black line.
Acknowledgement
We thank the NIH (R15CA125724) for financial support. We are thankful to Samantha Cawthorne for help with ITC and Dr. Meredith Newby for help with NMR studies.
Footnotes
Supporting information available. ITC titration data, UV thermal denaturation profiles and NMR spectra of neomycin titration with the quadruplex. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mergny J-, Helene C. G-Quadruplex DNA: A Target for Drug Design. Nature Medicine (New York) 1998;4:1366–1367. doi: 10.1038/3949. [DOI] [PubMed] [Google Scholar]
- 2.Neidle MARS. G-Quadruplexes as Therapeutic Targets. Biopolymers. 2000;56:195–208. doi: 10.1002/1097-0282(2000)56:3<195::AID-BIP10009>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Perry PJ, Jenkins TC. DNA Tetraplex-Binding Drugs Structure-Selective Targeting is Critical for Antitumour Telomerase Inhibition. Mini Reviews in Medicinal Chemistry Mini Reviews in Medicinal Chemistry J1 - Mini Reviews in Medicinal Chemistry. 2001;1:31. doi: 10.2174/1389557013407304. [DOI] [PubMed] [Google Scholar]
- 4.Riou J-, Gomez D, Morjani H, Trentesaux C. Quadruplex Ligand Recognition: Biological Aspects. Quadruplex Nucleic Acids. 2006:154–179. [Google Scholar]
- 5.Oganesian L, Bryan TM. Physiological Relevance of Telomeric G-Quadruplex Formation: A Potential Drug Target. Bioessays. 2007;29:155–165. doi: 10.1002/bies.20523. [DOI] [PubMed] [Google Scholar]
- 6.Camarena FS, Serral GC, Santalo FS. Telomerase and Telomere Dynamics in Ageing and Cancer: Current Status and Future Directions. Clin.Transl.Oncol. 2007;9:145–154. doi: 10.1007/s12094-007-0028-1. [DOI] [PubMed] [Google Scholar]
- 7.Helder MN, Wisman GBA, Van d. Z. Telomerase and Telomeres: From Basic Biology to Cancer Treatment. Cancer Invest. 2002;20:82–101. doi: 10.1081/cnv-120000370. [DOI] [PubMed] [Google Scholar]
- 8.Kelland LR. Telomerase: Biology and Phase I Trials. Lancet Oncol. 2001;2:95–102. doi: 10.1016/S1470-2045(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 9.Kelland L. Targeting the Limitless Replicative Potential of Cancer: The Telomerase/Telomere Pathway. Clin. Cancer Res. 2007;13:4960–4963. doi: 10.1158/1078-0432.CCR-07-0422. [DOI] [PubMed] [Google Scholar]
- 10.Autexier C, Lue NF. The Structure and Function of Telomerase Reverse Transcriptase. Annu. Rev. Biochem. 2006;75:493–517. doi: 10.1146/annurev.biochem.75.103004.142412. [DOI] [PubMed] [Google Scholar]
- 11.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: Sequence, Topology and Structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkinson GN. Quadruplex Nucleic Acids. Royal Society of Chemistry; Cambridge: 2006. Fundamentals of Quadruplex Structures; pp. 1–30. [Google Scholar]
- 13.Hud NV, Plavec J. The Role of Cations in Determining Quadruplex Structure and Stability. Quadruplex Nucleic Acids. 2006:100–130. [Google Scholar]
- 14.Ping Wang LR, Hanping He, Feng Liang, Xiang Zhou, Zheng Tan. A Phenol Quaternary Ammonium Porphyrin as a Potent Telomerase Inhibitor by Selective Interaction with Quadruplex DNA. ChemBioChem. 2006;7:1155–1159. doi: 10.1002/cbic.200600036. [DOI] [PubMed] [Google Scholar]
- 15.Seenisamy J, Bashyam S, Gokhale V, Vankayalapati H, Sun D, Siddiqui-Jain A, Streiner N, Shin-ya K, White E, Wilson WD, Hurley LH. Design and Synthesis of an Expanded Porphyrin that has Selectivity for the c-MYC G-Quadruplex Structure. J. Am. Chem. Soc. 2005;127:2944–2959. doi: 10.1021/ja0444482. [DOI] [PubMed] [Google Scholar]
- 16.Wei C, Jia G, Yuan J, Feng Z, Li C. A Spectroscopic Study on the Interactions of Porphyrin with G-Quadruplex DNAs. Biochemistry (N. Y. ) 2006;45:6681–6691. doi: 10.1021/bi052356z. [DOI] [PubMed] [Google Scholar]
- 17.Hounsou C, Guittat L, Monchaud D, Jourdan M, Saettel N, Mergny J-, Teulade-Fichou M- G-Quadruplex Recognition by Quinacridines: A SAR, NMR, and Biological Study. ChemMedChem. 2007;2:655–666. doi: 10.1002/cmdc.200600286. [DOI] [PubMed] [Google Scholar]
- 18.Zagotto G, Sissi C, Moro S, Ben DD, Parkinson GN, Fox KR, Neidle S, Palumbo M. Amide Bond Direction Modulates G-Quadruplex Recognition and Telomerase Inhibition by 2,6 and 2,7 Bis-Substituted Anthracenedione Derivatives. Bioorg. Med. Chem. 2008;16:354–361. doi: 10.1016/j.bmc.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Waller ZAE, Shirude PS, Rodriguez R, Balasubramanian S. Triarylpyridines: A Versatile Small Molecule Scaffold for G-Quadruplex Recognition. Chemical Communications (Cambridge, United Kingdom) 2008:1467–1469. doi: 10.1039/b718854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jantos K, Rodriguez R, Ladame S, Shirude PS, Balasubramanian S. Oxazole-Based Peptide Macrocycles: A New Class of G-Quadruplex Binding Ligands. J. Am. Chem. Soc. 2006;128:13662–13663. doi: 10.1021/ja064713e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu B, Huang J, Ren L, Weng X, Zhou Y, Du Y, Wu X, Zhou X, Yang G. Cationic Corrole Derivatives: A New Family of G-Quadruplex Inducing and Stabilizing Ligands. Chemical Communications. 2007:3264–3266. doi: 10.1039/b704599a. [DOI] [PubMed] [Google Scholar]
- 22.Brassart B, Gomez D, Cian AD, Paterski R, Montagnac A, Qui K-, Temime-Smaali N, Trentesaux C, Mergny J-, Gueritte F, Riou J- A New Steroid Derivative Stabilizes G-Quadruplexes and Induces Telomere Uncapping in Human Tumor Cells. Mol. Pharmacol. 2007;72:631–640. doi: 10.1124/mol.107.036574. [DOI] [PubMed] [Google Scholar]
- 23.Franceschin M, Rossetti L, D’Ambrosio A, Schirripa S, Bianco A, Ortaggi G, Savino M, Schultes C, Neidle S. Natural and Synthetic G-Quadruplex Interactive Berberine Derivatives. Bioorg. Med. Chem. Lett. 2006;16:1707–1711. doi: 10.1016/j.bmcl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Cocco MJ, Hanakahi LA, Huber MD, Maizels N. Specific Interactions of Distamycin with G-Quadruplex DNA. Nucl. Acids Res. 2003;31:2944–2951. doi: 10.1093/nar/gkg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martino L, Virno A, Pagano B, Virgilio A, Di Micco S, Galeone A, Giancola C, Bifulco G, Mayol L, Randazzo A. Structural and Thermodynamic Studies of the Interaction of Distamycin A with the Parallel Quadruplex Structure [d(TGGGGT)]4. J. Am. Chem. Soc. 2007;129:16048–16056. doi: 10.1021/ja075710k. [DOI] [PubMed] [Google Scholar]
- 26.Maiti S, Chaudhury NK, Chowdhury S. Hoechst 33258 Binds to G-Quadruplex in the Promoter Region of Human c-Myc. Biochem. Biophys. Res. Commun. 2003;310:505–512. doi: 10.1016/j.bbrc.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Cosconati S, Marinelli L, Trotta R, Virno A, Mayol L, Novellino E, Olson AJ, Randazzo A. Tandem Application of Virtual Screening and NMR Experiments in the Discovery of Brand New DNA Quadruplex Groove Binders. J. Am. Chem. Soc. 2009;131:16336–16337. doi: 10.1021/ja9063662. [DOI] [PubMed] [Google Scholar]
- 28.Arya DP. Aminoglycoside-Nucleic Acid Interactions: The Case for Neomycin. Top. Curr. Chem. 2005;253:149–178. [Google Scholar]
- 29.Willis B, Arya DP. Major Groove Recognition of DNA by Carbohydrates. Current Organic Chemistry. 2006;10:663–673. [Google Scholar]
- 30.Willis B, Arya DP. An Expanding View of Aminoglycoside-Nucleic Acid Recognition. Adv. Carbohydr. Chem. Biochem. 2006;60:251–302. doi: 10.1016/S0065-2318(06)60006-1. [DOI] [PubMed] [Google Scholar]
- 31.Xi H, Gray D, Kumar S, Arya DP. Molecular Recognition of Single-Stranded RNA: Neomycin Binding to Poly(A) FEBS Lett. 2009;583:2269–2275. doi: 10.1016/j.febslet.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Arya DP, Coffee RL, Jr., Willis B, Abramovitch AI. Aminoglycoside-Nucleic Acid Interactions: Remarkable Stabilization of DNA and RNA Triple Helices by Neomycin. J. Am. Chem. Soc. 2001;123:5385–5395. doi: 10.1021/ja003052x. [DOI] [PubMed] [Google Scholar]
- 33.Arya DP, Xue L, Tennant P. Combining the Best in Triplex Recognition: Synthesis and Nucleic Acid Binding of a BQQ-Neomycin Conjugate. J. Am. Chem. Soc. 2003;125:8070–8071. doi: 10.1021/ja034241t. [DOI] [PubMed] [Google Scholar]
- 34.Xue L, Charles I, Arya DP. Pyrene-Neomycin Conjugate: Dual Recognition of a DNA Triple Helix. Chem. Commun. 2002;1:70–71. doi: 10.1039/b108171c. [DOI] [PubMed] [Google Scholar]
- 35.Arya DP, Coffee RL, Jr., Charles I. Neomycin-Induced Hybrid Triplex Formation. J. Am. Chem. Soc. 2001;123:11093–11094. doi: 10.1021/ja016481j. [DOI] [PubMed] [Google Scholar]
- 36.Arya DP, Coffee RL., Jr. DNA Triple Helix Stabilization by Aminoglycoside Antibiotics. Bioorganic and Medicinal Chemistry Letters. 2000;10:1897–1899. doi: 10.1016/s0960-894x(00)00372-3. [DOI] [PubMed] [Google Scholar]
- 37.Xue L, Xi H, Kumar S, Gray D, Davis E, Hamilton P, Skriba M, Arya DP. Probing the Recognition Surface of a DNA Triplex: Binding Studies with Intercalatorâ^’Neomycin Conjugates. Biochemistry (N. Y. ) 2010;49:5540–5552. doi: 10.1021/bi100071j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi H, Arya DP. Recognition of Triple Helical Nucleic Acids by Aminoglycosides. Curr Med Chem Anticancer Agents. 2005;5:327–338. doi: 10.2174/1568011054222328. [DOI] [PubMed] [Google Scholar]
- 39.Shaw NN, Arya DP. Recognition of the Unique Structure of DNA:RNA Hybrids. Biochimie. 2008;90:1026–1039. doi: 10.1016/j.biochi.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Shaw NN, Xi H, Arya DP. Molecular Recognition of a DNA:RNA Hybrid: Sub-Nanomolar Binding by a Neomycin-Methidium Conjugate. Bioorg. Med. Chem. Lett. 2008;18:4142–4145. doi: 10.1016/j.bmcl.2008.05.090. [DOI] [PubMed] [Google Scholar]
- 41.Arya DP, Shaw N, Xi H. Novel Targets for Aminoglycosides. In: Arya DP, editor. Aminoglycoside Antibiotics: From chemical biology to drug discovery. Wiley; New York: 2007. pp. 289–314. 1 plate. [Google Scholar]
- 42.Arya DP, Xue L, Willis B. Aminoglycoside (Neomycin) Preference is for A-Form Nucleic Acids, Not just RNA: Results from a Competition Dialysis Study. J. Am. Chem. Soc. 2003;125:10148–10149. doi: 10.1021/ja035117c. [DOI] [PubMed] [Google Scholar]
- 43.Arya DP, Willis B. Reaching into the Major Groove of B-DNA: Synthesis and Nucleic Acid Binding of a Neomycin-Hoechst 33258 Conjugate. J. Am. Chem. Soc. 2003;125:12398–12399. doi: 10.1021/ja036742k. [DOI] [PubMed] [Google Scholar]
- 44.Willis B, Arya DP. Recognition of B-DNA by Neomycin-Hoechst 33258 Conjugates. Biochemistry (N. Y. ) 2006;45:10217–10232. doi: 10.1021/bi0609265. [DOI] [PubMed] [Google Scholar]
- 45.Willis B, Arya DP. Triple Recognition of B-DNA. Bioorg. Med. Chem. Lett. 2009;19:4974–4979. doi: 10.1016/j.bmcl.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 46.Willis B, Arya DP. Triple Recognition of B-DNA by a Neomycin-Hoechst 33258- Pyrene Conjugate. Biochemistry. 2010;49:452–469. doi: 10.1021/bi9016796. [DOI] [PubMed] [Google Scholar]
- 47.Arya DP, Coffee RL, Jr., Xue L. From Triplex to B-Form Duplex Stabilization: Reversal of Target Selectivity by Aminoglycoside Dimers. Bioorg. Med. Chem. Lett. 2004;14:4643–4646. doi: 10.1016/j.bmcl.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Napoli S, Carbone GM, Catapano CV, Shaw N, Arya DP. Neomycin Improves Cationic Lipid-Mediated Transfection of DNA in Human Cells. Bioorg. Med. Chem. Lett. 2005;15:3467–3469. doi: 10.1016/j.bmcl.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 49.Charles I, Xi H, Arya DP. Sequence-Specific Targeting of RNA with an Oligonucleotide-Neomycin Conjugate. Bioconjug. Chem. 2007;18:160–169. doi: 10.1021/bc060249r. [DOI] [PubMed] [Google Scholar]
- 50.Charles I, Arya DP. Synthesis of Neomycin-DNA/peptide Nucleic Acid Conjugates. J. Carbohydr. Chem. 2005;24:145–160. [Google Scholar]
- 51.Charles I, Xue L, Arya DP. Synthesis of Aminoglycoside-DNA Conjugates. Bioorg. Med. Chem. Lett. 2002;12:1259–1262. doi: 10.1016/s0960-894x(02)00157-9. [DOI] [PubMed] [Google Scholar]
- 52.Smith F, Juli Flint W. Quadruplex Structure of Oxytricha Telomeric DNA Oligonucleotides. Nature. 1992;356:164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- 53.Piotto M, Saudek V, Sklenar V. Gradient-Tailored Excitation for Single-Quantum NMR Spectroscopy of Aqueous Solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 54.Goddard TD, Kneller DG. SPARKY 3. University of California; San Francisco: [Google Scholar]
- 55.Smith FW, Feigon J. Strand Orientation in the DNA Quadruplex Formed from the Oxytricha Telomere Repeat Oligonucleotide d(G4T4G4) in Solution. Biochemistry (N. Y. ) 1993;32:8682–8692. doi: 10.1021/bi00084a040. [DOI] [PubMed] [Google Scholar]
- 56.Schultze P, Hud N, Smith F, Feigon J. The Effect of Sodium, Potassium and Ammonium Ions on the Conformation of the Dimeric Quadruplex Formed by the Oxytricha Nova Telomere Repeat Oligonucleotide d(G(4)T(4)G(4)) Nucl. Acids Res. 1999;27:3018–3028. doi: 10.1093/nar/27.15.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trott O, Olson AJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schultze P, Smith FW, Feigon J. Refined Solution Structure of the Dimeric Quadruplex Formed from the Oxytricha Telomeric Oligonucleotide d(GGGGTTTTGGGG) Structure. 1994;2:221–233. doi: 10.1016/s0969-2126(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 59.Pedretti A, Villa L, Vistoli G. VEGA--an Open Platform to Develop Chemo-Bio-Informatics Applications, using Plug-in Architecture and Script Programming. J. Comput. Aided Mol. Des. 2004;18:167–173. doi: 10.1023/b:jcam.0000035186.90683.f2. [DOI] [PubMed] [Google Scholar]
- 60.Sanner MF. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model. 1999;17:57–61. [PubMed] [Google Scholar]
- 61.Arya DP, Micovic L, Charles I, Coffee RL, Jr., Willis B, Xue L. Neomycin Binding to Watson-Hoogsteen (W-H) DNA Triplex Groove: A Model. J. Am. Chem. Soc. 2003;125:3733–3744. doi: 10.1021/ja027765m. [DOI] [PubMed] [Google Scholar]
- 62.Dominick PK, Keppler BR, Legassie JD, Moon IK, Jarstfer MB. Nucleic Acid-Binding Ligands Identify New Mechanisms to Inhibit Telomerase. Bioorg. Med. Chem. Lett. 2004;14:3467–3471. doi: 10.1016/j.bmcl.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 63.Buurma NJ, Haq I. Advances in the Analysis of Isothermal Titration Calorimetry Data for Ligand-DNA Interactions. Methods (Oxford, United Kingdom) 2007;42:162–172. doi: 10.1016/j.ymeth.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Haq I, Jenkins TC, Chowdhry BZ, Ren J, Chaires JB. Parsing Free Energies of Drug-DNA Interactions. Meth. Enzymol. 2000;323:373–405. doi: 10.1016/s0076-6879(00)23374-0. [DOI] [PubMed] [Google Scholar]
- 65.Haq I, Trent JO, Chowdhry BZ, Jenkins TC. Intercalative G-Tetraplex Stabilization of Telomeric DNA by a Cationic Porphyrin. J. Am. Chem. Soc. 1999;121:1768–1779. [Google Scholar]
- 66.Record MTJ, Anderson CF, Lohman TM. Thermodynamic Analysis of Ion Effects on the Binding and Conformational Equilibria of Proteins and Nucleic Acids: The Roles of Ion Association Or Release, Screening, and Ion Effects on Water Activity. Q. Rev. Biophys. 1978;2:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- 67.Jin E, Katritch V, Olson WK, Kharatisvili M, Abagyan R, Pilch DS. Aminoglycoside Binding in the Major Groove of Duplex RNA: The Thermodynamic and Electrostatic Forces that Govern Recognition. J. Mol. Biol. 2000;298:95–110. doi: 10.1006/jmbi.2000.3639. [DOI] [PubMed] [Google Scholar]
- 68.Xi H, Kumar S, Dosen-Micovic L, Arya DP. Calorimetric and Spectroscopic Studies of Aminoglycoside Binding to AT-Rich DNA Triple Helices. Biochimie. 2010;92:514–529. doi: 10.1016/j.biochi.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaul M, Pilch DS. Thermodynamics of Aminoglycoside-rRNA Recognition: The Binding of Neomycin-Class Aminoglycosides to the A Site of 16S rRNA. Biochemistry (N. Y. ) 2002;41:7695–7706. doi: 10.1021/bi020130f. [DOI] [PubMed] [Google Scholar]
- 70.Haider S, Parkinson GN, Neidle S. Crystal Structure of the Potassium Form of an Oxytricha Nova G-Quadruplex. J. Mol. Biol. 2002;320:189–200. doi: 10.1016/S0022-2836(02)00428-X. [DOI] [PubMed] [Google Scholar]
- 71.Boger DL, Fink BE, Brunette SR, Tse WC, Hedrick MP. A Simple, High-Resolution Method for Establishing DNA Binding Affinity and Sequence Selectivity. J. Am. Chem. Soc. 2001;123:5878–5891. doi: 10.1021/ja010041a. [DOI] [PubMed] [Google Scholar]
- 72.Tse WC, Boger DL. A Fluorescent Intercalator Displacement Assay for Establishing DNA Binding Selectivity and Affinity. Acc. Chem. Res. 2004;37:61–69. doi: 10.1021/ar030113y. [DOI] [PubMed] [Google Scholar]
- 73.Allain C, Monchaud D, Teulade-Fichou M- FRET Templated by G-Quadruplex DNA: A Specific Ternary Interaction using an Original Pair of Donor/Acceptor Partners. J. Am. Chem. Soc. 2006;128:11890–11893. doi: 10.1021/ja062193h. [DOI] [PubMed] [Google Scholar]
- 74.Monchaud D, Allain C, Teulade-Fichou M. Development of a Fluorescent Intercalator Displacement Assay (G4-FID) for Establishing Quadruplex-DNA Affinity and Selectivity of Putative Ligands. Bioorg. Med. Chem. Lett. 2006;16:4842–4845. doi: 10.1016/j.bmcl.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 75.Balagurumoorthy P, Brahmachari SK, Mohanty D, Bansal M, Sasisekharan V. Hairpin and Parallel Quartet Structures for Telomeric Sequences. Nucleic Acids Res. 1992;20:4061–4067. doi: 10.1093/nar/20.15.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mergny JL, Lacroix L. Analysis of Thermal Melting Curves. Oligonucleotides. 2003;13:515–537. doi: 10.1089/154545703322860825. [DOI] [PubMed] [Google Scholar]
- 77.Bishop GR, Ren J, Polander BC, Jeanfreau BD, Trent JO, Chaires JB. Energetic Basis of Molecular Recognition in a DNA Aptamer. Biophys. Chem. 2007;126:165–175. doi: 10.1016/j.bpc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Warui DM, Baranger AM. Identification of Specific Small Molecule Ligands for Stem Loop 3 Ribonucleic Acid of the Packaging Signal Î.. of Human Immunodeficiency Virus-1. J. Med. Chem. 2009;52:5462–5473. doi: 10.1021/jm900599v. [DOI] [PubMed] [Google Scholar]
- 79.Holt PA, Chaires JB, Trent JO. Molecular Docking of Intercalators and Groove-Binders to Nucleic Acids using Autodock and Surflex. Journal of Chemical Information and Modeling. 2008;48:1602–1615. doi: 10.1021/ci800063v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan J, Ou T, Hou J, Lu Y, Huang S, Luo H, Wu J, Huang Z, Wong K, Gu L. Isaindigotone Derivatives: A New Class of Highly Selective Ligands for Telomeric G-Quadruplex DNA. J. Med. Chem. 2009;52:2825–2835. doi: 10.1021/jm801600m. [DOI] [PubMed] [Google Scholar]
- 81.Ma Y, Ou T, Hou J, Lu Y, Tan J, Gu L, Huang Z. 9-N-Substituted Berberine Derivatives: Stabilization of G-Quadruplex DNA and Down-Regulation of Oncogene c-Myc. Bioorg. Med. Chem. 2008;16:7582–7591. doi: 10.1016/j.bmc.2008.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.