Abstract
Knowledge of vapor pressure of organic pollutants is essential in predicting their fate and transport in the environment. In the present study, the vapor pressures of 12 halogenated polycyclic aromatic compounds (PACs), i.e. 9-chlorofluorene, 2,7-dichlorofluorene, 2-bromofluorene, 9-bromofluorene, 2,7-dibromofluorene, 2-bromoanthracene, 9-chlorophenanthrene, 9-bromophenanthrene, 9,10-dibromophenanthrene, 1-chloropyrene, 7-bromobenz[a]anthracene and 6,12-dibromochrysene, were measured using the Knudsen effusion method over the temperature range of 301 to 464 K. Enthalpies and entropies of sublimation of these compounds were determined via application of the Clausius–Clapeyron equation. The data were also compared with earlier published literature values to study the influence of halogen substitution on vapor pressure of PACs. As expected, the halogen substitution decreases vapor pressure compared to parent compounds, but does not necessarily increase the enthalpy of sublimation. Moreover, the decrease of vapor pressure also depends on the substitution position and the substituted halogen, and the di-substitution of chlorine and/or bromine decreases the vapor pressure compared to single halogen substituted polycyclic aromatic hydrocarbons. Additionally, the enthalpy of fusion and melting temperature of these 12 PACs were determined using differential scanning calorimetry and melting point analysis.
Keywords: Halogen, Vapor pressure, Polycyclic aromatic hydrocabon, Knudsen effusion method
INTRODUCTION
Chlorinated and brominated polycyclic aromatic hydrocarbons (ClPAHs and BrPAHs) are classes of compounds consisting of polycyclic aromatic hydrocarbons (PAHs) with two or more aromatic rings and one or more chlorine or bromine atoms which substitute hydrogen atoms on the rings. ClPAHs and BrPAHs are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs). The fate and effects of PCDDs, PCDFs, PCBs and PBDEs have been studied in detail for over 30 years due to their possible persistence, and their toxic and carcinogenic properties. Similarly, polychlorinated naphthalenes, one class of ClPAHs, which were produced in large quantity in early 1900s, have also been studied and found to have certain health effects [1]. ClPAHs and BrPAHs with three to five aromatic rings have been reported to occur in urban air [2–5], snow [4], tap water [6], kraft pulp mill waste [7, 8], fly and bottom ash from waste incinerators [9], and electronic waste [10]. Recently, interest has increased in these ClPAHs and BrPAHs due to their biological activity and health effects [11–13]. However, the environmental fate and transport and toxicities of many of these compounds have not been reported in detail.
Vapor pressure is a fundamental property which is key to predicting the vapor phase fate and transport of a substance in the environment. The vapor pressures for parent PAH compounds have been widely studied for the past 50 years, whereas only limited data are available for ClPAHs and BrPAHs [14]. Many experimental techniques have been developed to measure the vapor pressure of PAHs, such as the Knudsen effusion method [15–19], the generator column–high-performance liquid chromatography method [20], the gas saturation method [21], the electronic manometry method [22] and the gas chromatography-retention time method [23, 24]. These methods can also be used to study the vapor pressure of these ClPAHs and BrPAHs.
In the present study, the Knudsen effusion technique was used to measure the solid vapor pressure, its temperature dependence, and enthalpies of sublimation for 12 ClPAHs and BrPAHs, i.e. 9-chlorofluorene, 2,7-dichlorofluorene, 2-bromofluorene, 9-bromofluorene, 2,7-dibromofluorene, 2-bromoanthracene, 9-chlorophenanthrene, 9-bromophenanthrene, 9,10-dibromophenanthrene, 1-chloropyrene, 7-bromobenz[a]anthracene and 6,12-dibromochrysene, none of which have been reported before. Additionally, the melting points and enthalpies of fusion, i.e. phase change characteristics of these ClPAHs and BrPAHs, have also been studied using melting point analysis and differential scanning calorimetry. The vapor pressures of the ClPAHs and BrPAHs that were obtained in these measurements were also compared with the vapor pressures of their parent PAHs and the halogenated PAHs measured earlier by Goldfarb and Suuberg [14], to establish the influence of halogen substitution.
MATERIALS AND METHODS
Materials
The 12 halogenated polycyclic aromatic hydrocarbons (HPAHs) that were examined fall in the molar mass (M) range from 200 g/mol to 386 g/mol. Table 1 summarizes the HPAHs used in the present study. They were used in the measurements without further purification (except during actual vapor pressure measurements, as described below). Since 9,10-dibromophenanthrene and 6,12-dibromochrysene purchased from Aldrich did not have manufacture-provided purity information, the purity of these two compounds were determined by using gas chromatography (GC).
Table 1.
Halogenated polycyclic aromatic hydrocarbons examined.
| CAS Reg. No. | Compound | Min. Purity | M/g/mol | Manufacturer | Molecular structure |
|---|---|---|---|---|---|
| 6630-65-5 | 9-chlorofluorene | 97% | 200.67 | Alfa |
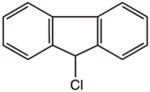
|
| 1133-80-8 | 2-bromofluorene | 95% | 245.11 | Aldrich |
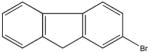
|
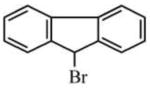
| 1940-57-4 | 9-bromofluorene | 98% | 245.11 | Aldrich |
|
| 7012-16-0 | 2,7-dichlorofluorene | 97% | 235.12 | Alfa |
|
| 16433-88-8 | 2,7-dibromofluorene | 97% | 324.01 | Aldrich |
|
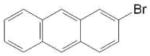
| 7321-27-9 | 2-bromoanthracene | 97% | 257.13 | TCI |
|
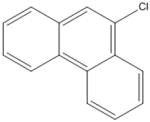
| 947-72-8 | 9-chlorophenanthrene | 98% | 212.681 | Acros |
|
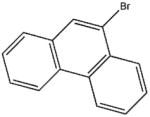
| 573-17-1 | 9-bromophenanthrene | 96% | 257.13 | Acros |
|
| 15810-15-8 | 9,10-dibromophenanthren e | 76%a | 336.02 | Aldrich |
|
| 34244-14-9 | 1-chloropyrene | 98% | 236.7 | Frinton |
|
| 32795-84-9 | 7-bromobenz[a]anthra cene | 98% | 307.18 | TCI |
|
| 131222-99-6 | 6,12-dibromochrysene | 83%a | 386.09 | Aldrich |
|
the purity is characterized by using GC.
Melting temperatures and enthalpies of phase change
Melting temperatures of the samples in Table 1 were measured using a Mettler Toledo MP50 melting point system. 1 mg to 2 mg of each sample was placed in a capillary and heated at 1 ± 0.1 K·min−1. The enthalpies of fusion were measured using a TA Instruments 2910 differential scanning calorimeter (DSC). A 1 mg to 4 mg sample was placed into a hermetically sealed DSC pan and this was scanned in heating mode at a heating rate of 10 K·min−1. The uncertainty in the DSC measurement is approximately ± 5 J·g−1. More details of this technique can be found in previous publications [25–27].
Vapor pressure
The Knudsen effusion technique, which derives from Knedsen’s 1909 Kinetic Theory of Gases, were used to study the vapor pressure of these HPAHs. The Knudsen effusion method is an indirect measurement technique based on the molecular effusion of a vapor through an orifice into a high vacuum chamber and is generally used to measure the vapor pressure of low volatility substances. The present implementation of the Knudsen effusion method is described in a previous publication [25]. Samples were placed inside effusion cells prepared from steel shim stock. The cells were sealed except for a single, circular orifice 0.61 ± 0.02 mm in diameter, and then placed on one arm of a continuously recording microbalance in a high vacuum chamber. The pressure inside of the chamber was reduced to 10−4 Pa in the experiments. The measurements were made under isothermal conditions measured by using a type K thermocouple located above the effusion cell. The reference compound anthracene was periodically employed to test the performance of the Knudsen effusion apparatus and the results were always in good agreement with literature values [16, 20, 28, 29]. More details of this technique may be found in Goldfarb and Suuberg [16] and Oja and Suuberg [18].
RESULTS AND DISCUSSION
Table 2 lists the measured melting temperatures and enthalpies of fusion of these HPAHs. The enthalpies of fusion in the table were calculated by ΔfusS = ΔfusH/Tfus, where Tfus used is the average value of the observed melting temperature range. The melting temperature data were generally in good agreement with the earlier published values, as summarized in the SciFinder compound database (https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf).
Table 2.
Melting temperature and enthalpy of fusion for the 12 halogenated polycyclic aromatic hydrocarbons.
| Compound | Tfus/K | ΔfusH(DSC)/kJ/mol | ΔfusS/kJ/mol/K |
|---|---|---|---|
| 9-chlorofluorene | 360.5 – 361.9 | 14.21 | 39.34 |
| 2-bromofluorene | 381.5 – 383.1 | 16.02 | 41.90 |
| 9-bromofluorene | 373.1 – 374.3 | 13.36 | 35.75 |
| 2,7-dichlorofluorene | 397.6 – 398.5 | 19.44 | 48.84 |
| 2,7-dibromofluorene | 438.5 – 439.5 | 22.08 | 50.30 |
| 2-bromoanthracene | 493.1 – 494.1 | 26.23 | 53.14 |
| 9-chlorophenanthrene | 319.2 – 321.2 | 14.99 | 46.81 |
| 9-bromophenanthrene | 334.5 – 335.8 | 14.91 | 44.49 |
| 9,10-dibromophenanthrene | 450.5 – 452.2 | 16.33 | 36.18 |
| 1-chloropyrene | 388.6 – 389.9 | 11.60 | 29.80 |
| 7-bromobenz[a]anthracene | 425.1 – 426.3 | 23.64 | 55.53 |
| 6,12-dibromochrysene | 542.1 – 544.4 | 23.81 | 43.83 |
Since the measurement uncertainty of differential scanning calorimeter (DSC) is ± 5 J·g−1, the ΔfusH reported here would have an inherent uncertainty of 1.0 kJ/mol to 1.9 kJ/mol, and this carries over into the calculated entropy values.
For halogenated fluorenes, the mono-substitutions of chlorine and bromine lead to lower melting temperatures and enthalpies of fusion compared with their parent PAH, fluorene (389.7 K and 19.58 kJ/mol, respectively) [30, 31], whereas di-substituted fluorenes have higher melting temperatures and enthalpies of fusion (Table 2). Similar to the fluorene derivatives, the mono-halogenated anthracene, phenanthrene, and pyrene compounds have lower melting temperature and enthalpy of fusion compared with their parent PAHs. However, the enthalpy of fusion of 7-bromobenz[a]anthracene is approximately 2 kJ/mol higher than that of benz[a]anthracene (21.38 kJ/mol) [31], while the enthalpy of fusion for 6,12-dibromochrysene is approximately 6 kJ/mol lower than its parent chrysene (29.37 kJ/mol) [31]. Bearing in mind the measurement uncertainty of ± 5 J·g−1 of DSC, the present measured enthalpies of fusion would have an inherent uncertainty of 1.0 kJ/mol to 1.9 kJ/mol.
The entropies of fusion of these HPAHs are generally lower than those of their parent PAHs, except for 9-chlorophenanthrene and 7-bromobenz[a]anthracene. The value for 9-chlorophenanthrene is slightly higher than that of phenanthrene (44.83 J/mol/K) [31], while the value of 7-bromobenz[a]anthracene is approximately 6 J/mol/K higher than that of its parent PAH, benz[a]anthracene (49.23 J/mol/K) [31]. Dannenfelser et al. studied the relationship between the molecular symmetry number, σ, and the entropy of fusion, and demonstrated that ΔfusS = 56.48 − Rlnσ [32], where R is the universal gas constant and R = 8.314 J/mol/K. However, this relationship was observed to not work reliably for these HPAHs.
Table 3 presents the vapor pressure data obtained in the present study. Since the purity of 9,10-dibromophenanthrene is low, 76 %, and the GC analysis results indicates the existence of over 10% of both light and heavy impurities, its vapor pressure was measured after a sample lost over 20 % mass, i.e. after eliminating most of the volatile impurities. The results indicate that the vapor pressure data obtained were repeatable and the linear regression fits the data fairly well. Therefore, its enthalpy and entropy of sublimation data were included in Table 4. For 6,12-dibromochrysene, whose purity is also not high, 83%, the impurities in the sample are mainly light substances, so the vapor pressure data were also obtained after eliminating most of the volatile impurities, and its enthalpy and entropy of sublimation values were also included in Table 4.
Table 3.
Summary of vapor pressure obtained via Knudsen effusion method.
| T/K | P/Pa | T/K | P/Pa | T/K | P/Pa |
|---|---|---|---|---|---|
| 9-chlorofluorene | |||||
| 303.7 | 3.34E-02 | 313.5 | 1.42E-01 | 325.4 | 4.43E-01 |
| 306.6 | 4.91E-02 | 315.4 | 1.53E-01 | 330.5 | 6.81E-01 |
| 308.6 | 6.59E-02 | 318.4 | 2.13E-01 | ||
| 311.5 | 1.01E-01 | 320.4 | 2.62E-01 | ||
| 2-bromofluorene | |||||
| 303.9 | 5.57E-03 | 321.6 | 5.81E-02 | 343.8 | 4.90E-01 |
| 308.9 | 1.34E-02 | 323.5 | 6.92E-02 | 348.8 | 8.51E-01 |
| 311.7 | 1.77E-02 | 328.6 | 1.17E-01 | 353.9 | 1.08E+00 |
| 313.6 | 2.31E-02 | 333.6 | 1.95E-01 | ||
| 318.5 | 3.88E-02 | 338.7 | 3.17E-01 | ||
| 9-bromofluorene | |||||
| 303.7 | 1.07E-02 | 316.6 | 5.65E-02 | 333.7 | 4.07E-01 |
| 306.7 | 1.44E-02 | 318.6 | 7.53E-02 | 338.7 | 6.37E-01 |
| 308.7 | 1.92E-02 | 321.6 | 9.90E-02 | 343.8 | 8.02E-01 |
| 311.6 | 3.17E-02 | 323.6 | 1.41E-01 | ||
| 313.7 | 4.00E-02 | 328.6 | 2.54E-01 | ||
| 2,7-dichlorofluorene | |||||
| 318.5 | 1.30E-02 | 338.6 | 1.09E-01 | 358.9 | 7.36E-01 |
| 323.4 | 2.06E-02 | 343.6 | 1.83E-01 | 364.0 | 1.13E+00 |
| 328.5 | 3.77E-02 | 348.7 | 2.83E-01 | ||
| 333.5 | 6.59E-02 | 353.8 | 4.71E-01 | ||
| 2,7-dibromofluorene | |||||
| 328.7 | 2.97E-03 | 353.9 | 3.97E-02 | 379.2 | 4.44E-01 |
| 333.7 | 4.87E-03 | 358.9 | 6.67E-02 | 384.2 | 6.82E-01 |
| 338.6 | 7.82E-03 | 364.1 | 1.16E-01 | 389.1 | 1.03E+00 |
| 343.7 | 1.15E-02 | 369.1 | 1.77E-01 | ||
| 348.9 | 2.43E-02 | 374.1 | 2.87E-01 | ||
| 2-bromoanthracene | |||||
| 334.8 | 5.41E-03 | 355.0 | 4.15E-02 | 375.3 | 2.87E-01 |
| 339.8 | 9.21E-03 | 360.1 | 7.18E-02 | 380.4 | 4.37E-01 |
| 344.9 | 1.56E-02 | 365.2 | 1.14E-01 | 385.4 | 6.52E-01 |
| 350.0 | 2.61E-02 | 370.4 | 1.84E-01 | 390.4 | 8.87E-01 |
| 9-chlorophenanthrene | |||||
| 301.1 | 9.41E-03 | 308.1 | 1.91E-02 | 316.1 | 4.75E-02 |
| 303.2 | 1.08E-02 | 311.2 | 2.68E-02 | 317.1 | 5.30E-02 |
| 306.1 | 1.52E-02 | 313.2 | 3.35E-02 | 318.1 | 5.90E-02 |
| 9-bromophenanthrene | |||||
| 304.0 | 2.70E-03 | $318.6 | 1.70E-02 | $330.5 | 7.56E-02 |
| 305.9 | 4.71E-03 | $323.6 | 2.69E-02 | $332.6 | 9.73E-02 |
| 308.8 | 7.93E-03 | $325.6 | 3.78E-02 | $333.7 | 1.04E-01 |
| 313.7 | 9.03E-03 | $328.6 | 6.47E-02 | ||
| 1-chloropyrene | |||||
| 333.1 | 6.42E-03 | 353.9 | 6.15E-02 | 374.1 | 4.48E-01 |
| 338.2 | 1.08E-02 | 358.9 | 1.06E-01 | 379.1 | 5.95E-01 |
| 343.1 | 1.80E-02 | 364.0 | 1.77E-01 | 384.1 | 7.55E-01 |
| 348.8 | 4.67E-02 | 369.1 | 2.96E-01 | ||
| 7-bromobenz[a]anthracene | |||||
| 358.9 | 2.27E-03 | 384.2 | 4.03E-02 | 409.2 | 3.94E-01 |
| 364.0 | 5.14E-03 | 389.1 | 6.61E-02 | 414.2 | 6.18E-01 |
| 369.1 | 7.52E-03 | 394.1 | 1.07E-01 | 419.2 | 9.27E-01 |
| 374.2 | 1.28E-02 | 399.2 | 1.63E-01 | ||
| 379.2 | 2.46E-02 | 404.1 | 2.58E-01 | ||
Table 4.
Application of Clausius-Clapeyron equation to present vapor pressure data.
| Compound | Temp range/K | ΔsubH/kJ · mol−1 | ΔsubS/J/mol·K−1 (298.15K) | P/Pa (298.15K) |
|---|---|---|---|---|
| fluorene16 | 298–324 | 87.1 ± 1.9 | 292.1 ± 6.4 | 6.61E-02 ± 5.82E-−07 |
| 9-chlorofluorene | 303–330 | 94.0 ± 3.0 | 315.3 ± 10.0 | 1.86E-02 ± 2.39E-−07 |
| 2-bromofluorene | 303–354 | 93.1 ± 1.6 | 312.3 ± 5.4 | 3.45E-03 ± 2.39E-−08 |
| 9-bromofluorene | 303–344 | 98.4 ± 2.5 | 330.0 ± 8.4 | 5.45E-03 ± 5.59E-−08 |
| 2,7-dichlorofluorene | 318–364 | 95.6 ± 0.6 | 320.6 ± 2.0 | 1.08E-03 ± 2.73E-−09 |
| 2,7-dibromofluorene | 328–389 | 105.1 ± 1.2 | 352.5 ± 4.0 | 5.12E-05 ± 2.36E-−10 |
| anthracene16 | 322–348 | 98.5 ± 3.3 | 330.4 ± 11.1 | 7.71E-04 ± 1.04E-−08 |
| 2-bromoanthracene | 334–390 | 101.3 ± 0.6 | 339.8 ± 2.0 | 6.20E-05 ± 1.48E-−10 |
| phenanthrene15 | 303–333 | 94.9 ± 4.4 | 318.5 ± 14.8 | 1.97E-02 ± 3.68E-−07 |
| 9-chlorophenanthrene | 301–318 | 88.5 ± 1.7 | 296.8 ± 5.7 | 6.17E-03 ± 4.78E-−08 |
| 9-bromophenanthrene | 304–334 | 97.9 ± 4.1 | 328.4 ± 13.8 | 1.49E-03 ± 2.52E-−08 |
| 9,10-dibromophenanthrenea | 353–409 | 114.3 ± 1.5 | 383.4 ± 5.0 | 4.78E-06 ± 2.53E-−11 |
| pyrene16 | 322–381 | 97.8 ± 3.3 | 328.0 ± 11.1 | 5.37E-04 ± 7.31E-−09 |
| 1-chloropyrene | 333–384 | 103.2 ± 2.9 | 346.1 ± 9.7 | 8.70E-05 ± 9.86E-−10 |
| benz[a]anthracene31 | 371–396 | 113.5 ± 2.0 | 380.7 ± 6.7 | 7.30E-06 ± 5.19E-−11 |
| 7-bromobenz[a]anthracene | 358–419 | 123.2 ± 1.3 | 413.2 ± 4.4 | 5.67E-07 ± 2.41E-−12 |
| chrysene16 | 372–409 | 109.9 ± 3.6 | 368.6 ± 12.1 | 2.39E-06 ± 3.16E-−11 |
| 6,12-dibromochrysenea | 409–465 | 141.1 ± 3.2 | 473.3 ± 10.7 | 3.98E-10 ± 3.64E-−15 |
The initial purities of 9,10-dibromophenanthrene and 6,12-dibromochrysene used here were low, 76% and 83% respectively. Hence these entries are based on what was believed to be material that was purified in the course of experiments (see text).
The data of Table 3 were also used to study the influence of halogen substitution. The data were used to calculate that enthalpy (ΔsubH) entropy (ΔsubS) of sublimation, and vapor pressure at 298.15 K assuming the Clausius-Clapeyron equation based upon a constant enthalpy of sublimation,
where T is the absolute temperature of the sample (T < Tfus), and P0 and T0 are values at a particular reference condition. Of course, ΔsubS could be calculated from ΔsubS = ΔsubH/T for any chosen T, given the assumption of constant ΔsubH. The solid state vapor pressures of these HPAHs (PS) in Table 3 can be converted to the sometimes reported subcooled liquid vapor pressures (PL) at any given temperature by
where Tfus is the melting temperature and ΔfusS is the entropy of fusion, and where ΔfusS = ΔfusH/Tfus. All data reported here and used for Table 4 were authentic solid sublimation data.
Table 4 displays the results of fitting the data to the Clausius–Clapeyron equation along with the statistical significance of these results. The enthalpy of sublimation values presented here were calculated from the slope of the Clausius–Clapeyron equation for the temperature range indicated in Table 3. The vapor pressure at 298.15 K was also calculated by using the Clausius–Clapeyron equation and the entropy of sublimation from ΔsubS = ΔsubH/298.15.
Figures 1 to 6 indicate the effect of halogen substitution on the vapor pressures of parent PAHs, i.e. fluorene, anthracene, phenanthrene, pyrene, benz[a]anthracene, and chrysene. The vapor pressures of fluorene, phenanthrene, pyrene, and chrysene were also measured in the present study and the values are in good agreement with earlier published literature values [16–19, 21, 22].
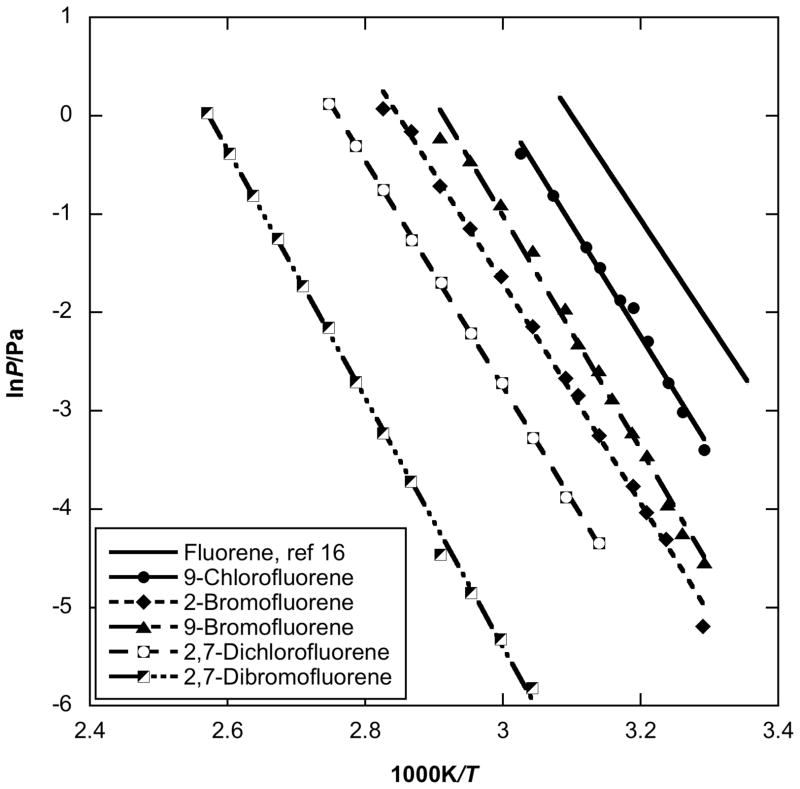
Fig. 1.
Plot of vapor pressure against reciprocal temperature for halogenated fluorenes obtained by using the Knudsen effusion method compared to parent fluorene values.
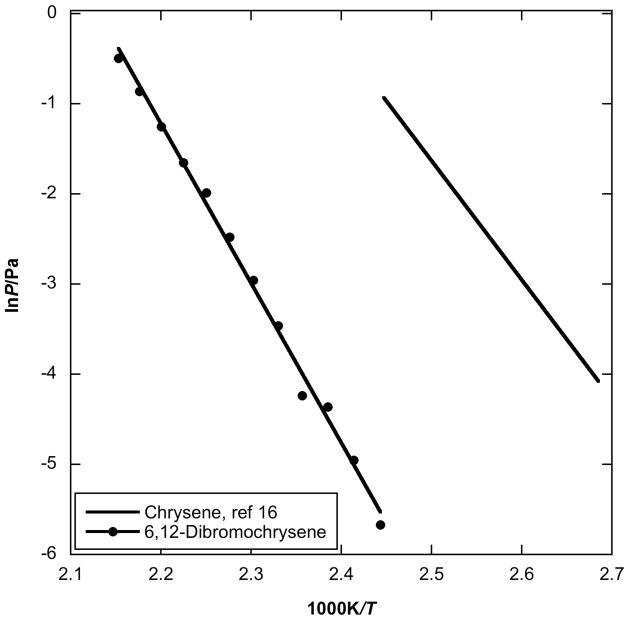
Fig. 6.
Plot of vapor pressure against reciprocal temperature for 6,12-dibromochrysene obtained by using the Knudsen effusion method compared to parent chrysene values.
Starting with the fluorene derivatives (Fig. 1), the vapor pressures of five halogenated fluorenes, i.e. 9-chlorofluorene, 2-bromofluorene, 9-bromofluorene, 2,7-dichlorofluorene and 2,7-dibromofluorene were compared with values of their parent PAH fluorene [16]. The enthalpy of sublimation of fluorene is 87.1 kJ/mol over the temperature range of 298 K to 324 K [16]. The vapor pressures of these halogenated fluorenes are all lower than that of fluorene, while the enthalpy of sublimation values are slightly higher (Table 4). The data indicate an increase in enthalpy of approximately 6 to 11 kJ/mol with the substitution of a single chlorine or bromine onto the fluorene. The vapor pressures of 9-chlorofluorene are approximately 70% lower than those of fluorene in the temperature range of 303 K to 330 K, while the values of 9-bromofluorene are approximately 95% lower. The vapor pressures of 2-bromofluorene are 40 % to 55 % lower than that of 9-bromofluorene over the temperature range of 303 K to 354 K, even though they have the same molecular weight, and the 9-bromofluorene has a slightly higher ΔsubH (though close enough to not necessarily be statistical significant). Note from Table 4 how similar the enthalpies and entropies at 298.15 K of sublimation are for 9-chlorofluorene and 2-bromofluorene, despite the significant difference in the vapor pressure of these compounds. These results illustrate the danger in over-interpretating these enthalpy and entropy values, but they do offer clues as to the relatively greater disorder in the 2-bromofluorene solid state, as compared with the 9-chlorofluorene.. The di-halogen substitution further lowers the vapor pressure of these halogenated fluorenes. The vapor pressure of 2,7-dibromofluorene is approximately 3 orders of magnitude lower and that of 2,7-dichlorofluorene 2 order of magnitude lower than that of fluorene, at room temperature. In this case, the sublimation enthalpy of the disubstituted bromo-compound is significantly higher than the disubstituted chloro-compound.
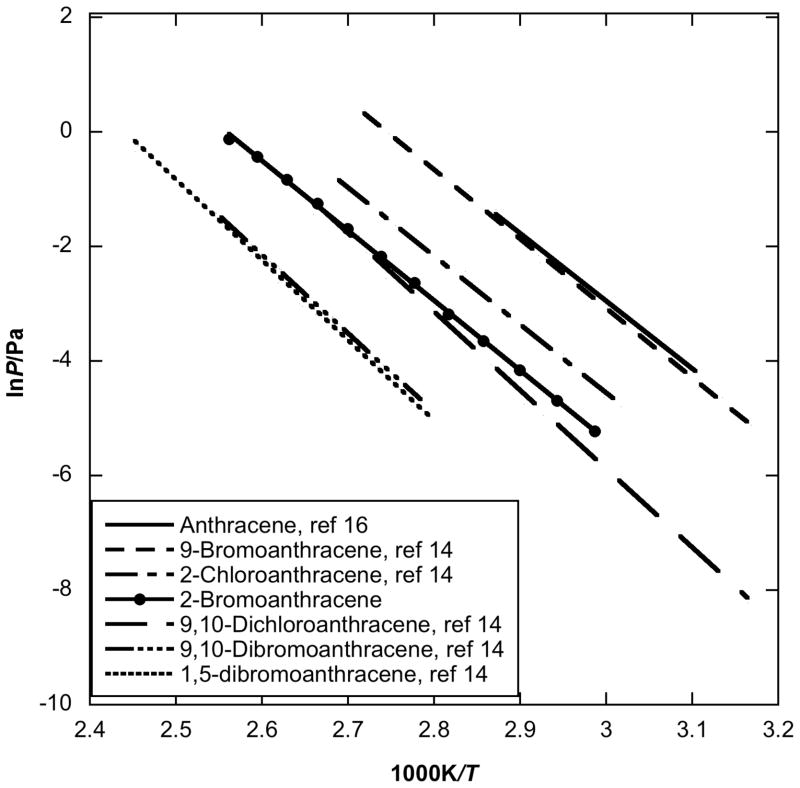
Similar trends are also observed with halogenated anthracenes (Fig. 2). The vapor pressure of 2-bromoanthracene was measured in the present study and was compared with vapor pressure data of anthracene and five other halogenated anthracenes, i.e. 2-chloroanthracene, 9-bromoanthracene, 9,10-dichloroanthracene, 9,10-dibromoanthracene and 1,5-dibromoanthracene measured by Goldfarb and Suuberg [14]. Unlike with the fluorene derivatives, a single chlorine or bromine substitution does not appear to influence the enthalpy of sublimation much. The enthalpies of sublimation of 2-bromoanthracene, 2-chloroanthracene and 9-bromoanthracene, 101.3 ± 0.6 kJ/mol, 99.3 ± 2.7 kJ/mol [14] and 100.5 ± 1.8 kJ/mol [14], respectively, are statistically not significantly different than that of pure anthracene, 98.5 ± 3.3 kJ/mol. However, the earlier reported [14] enthalpies of sublimation of the di-halogen substituted anthracenes, 9,10-dichloroanthracene, 1,5-dibromoanthracene and 9,10-dibromoanthracene, 113.9 ± 4.5 kJ/mol, 116.7 ± 3.0 kJ/mol, 114.2 ± 2.8 kJ/mol, respectively, are all close to each other and approximately 16 kJ/mol higher than that of pure anthracene. The vapor pressure of 2-bromoanthracene is approximately 1 to 2 orders of magnitude lower than those of pure anthracene and 9-bromoanthracene, which are close to one another. Clearly, the 2-position mono-substitution has a larger effect on vapor pressure than does the 9-position substitution. There is no clear trend with position in the case of the di-substituted anthracenes. Clearly, addition of another bromine substituent at the 10-position greatly decreases the vapor pressure of the 9-bomoanthracene. The substitution positions have small effect, as seen when 1,5-dibromoanthracene is compared with 9,10-dibromoanthracene.
Fig. 2.
Plot of vapor pressure against reciprocal temperature for halogenated anthracenes obtained by using the Knudsen effusion method compared to parent anthracene values.
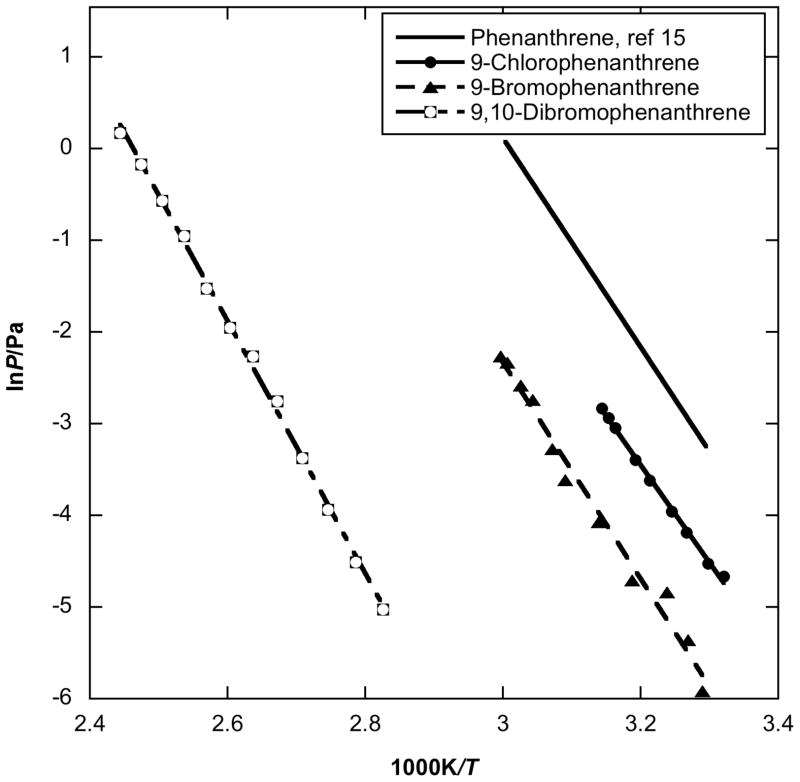
Figure 3 shows the vapor pressure of phenanthrene derivatives, i.e. 9-chlorophenanthrene, 9-bromophenanthrene and 9,10-dibromophenanthrene. Phenanthrene has the same molecular mass and similar structure as anthracene, but the vapor pressure and enthalpy of sublimation, lnP/Pa =(34.387 − 11423/T) and 94.9 kJ/mol, respectively [15], are different from those of anthracene, lnP/Pa =(32.55 − 11834/T) and 98.3 kJ/mol, respectively [16]. The phenanthrene compounds have much higher volatility. Just as in case of anthracene derivatives, the enthalpies of sublimation of 9-chlorophnanthrene and 9-bromophenanthrene are close to their parent PAH (though that of 9-chlorophenanthrene is actually lower), whereas the enthalpy of sublimation of 9,10-dibromophenanthrene is approximately 20 kJ/mol higher than that of pure phenanthrene. The vapor pressures of single chlorine or bromine substituted phenanthrenes on 9-position behave like those of 9-position halogen substituted fluorenes, i.e. bromine substitution lowers the vapor pressure more than chlorine substituion. Additionally, by substitution of another bromine atom onto the aromatic ring, the room temperature vapor pressure of 9,10-dibromophenanthrene becomes approximately 3 orders of magnitude lower than that of 9-bromophenanthrene.
Fig. 3.
Plot of vapor pressure against reciprocal temperature for halogenated phenanthrene obtained by using the Knudsen effusion method compared to parent phenanthrene values.
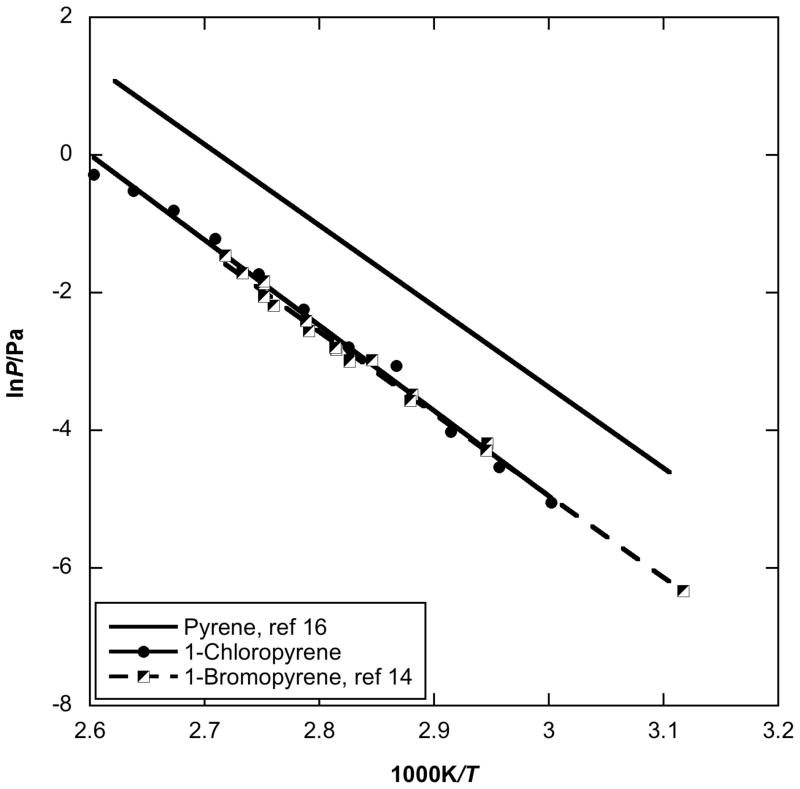
For the pyrene-based PAHs, the vapor pressures of 1-chloropyrene were measured in the present study and compared with those of pyrene [16] and 1-bromopyrene [14] (Fig. 4). The enthalpy of sublimation of 1-chloropyrene is approximately 6 kJ/mol higher than that of parent pyrene, 99.2 kJ/mol [16], while the enthalpy of sublimation of 1-bromopyrene is close to that for pyrene. As distinct from fluorene and anthracene derivatives, a single bromine or chlorine substitution onto the same position has almost the same influence on vapor pressure. Again, the substitutions lower the vapor pressure, relative to the parent PAH.
Fig. 4.
Plot of vapor pressure against reciprocal temperature for halogenated pyrene obtained by using the Knudsen effusion method compared to parent pyrene values.
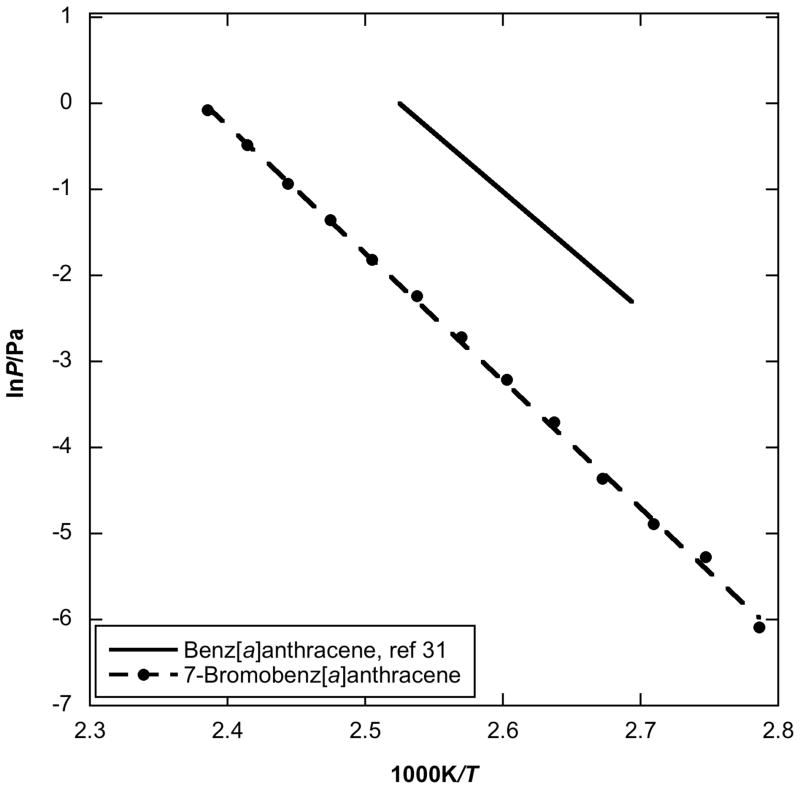
Figure 5 shows the vapor pressure of 7-bromobenz[a]anthracene comparing it with the vapor pressure of its parent benz[a]anthracene [33]. The single bromine substitution onto the 7-position increases the enthalpy of sublimation by approximately 10 kJ/mol (113.5 kJ/mol for benz[a]anthracene, vs. 123.2 kJ/mol for 7-bromobenz[a]anthracene). Moreover, the vapor pressure of 7-bromobenz[a]anthracene is over 1 order of magnitude lower than that of its parent PAH at 298.15 K.
Fig. 5.
Plot of vapor pressure against reciprocal temperature for 7-bromobenz[a]anthracene obtained by using the Knudsen effusion method compared to parent benz[a]anthracene values.
The HPAH with the largest molecular mass studied here is 6,12-dibromochrysene. Figure 6 presents the vapor pressure of 6,12-dibromochrysene in comparison with its parent chrysene [16]. The enthalpy of sublimation of 6,12-dibromochrysene is approximately 30 kJ/mol higher than that of chrysene, (109.9 ± 7.2 kJ/mol). Additionally, the vapor pressure of 6,12-dibromochrysene is approximately 4 orders of magnitude lower than that of chrysene (Table 4). In this instance, the greatly increased sublimation enthalpy requirement shows itself clearly in a lowered vapor pressure.
Overall, the successive substitution of halogens onto PAHs generally decreases the vapor pressure compared to the parent PAH. The decrease of vapor pressure can also depend on the substitution position and the substituted halogen. A mono-bromine 15 substitution generally lowers the vapor pressure more than a mono-chlorine substitution, though the pyrene results show that this need not always be so. A dependence of vapor pressure on bromine and chlorine substitution has been noted for the hexahalogenated dimethyl bipyrroles [34], in which replacement of a chlorine with a bromine atom resulted in a 2.6-fold decrease in vapor pressure at 298.15 K. There can be some position dependence to the effect of substitution. In fluorene and anthracene groups, 2-position substitution decreased the vapor pressure more than 9-position substitution.
The di-substitution of chlorine and bromine further decreases the vapor pressure compared to single halogen substituted PAHs. Crudely speaking, the vapor pressures of di-chlorine substituted PAHs are approximately an order of magnitude lower than that of mono-chlorine substituted PAHs at room temperature, while vapor pressures of dibromine substituted PAHs are approximately 2 orders of magnitude lower than that of mono-bromine substituted PAHs. Similar vapor pressure behavior has also been found in PBDEs [35], in which the subcooled vapor pressure of 4, 4′-dibromodiphenyl ether (BDE15) is approximately an order of magnitude lower than that of 4-bromodiphenyl ether (BDE3) at 298.15K. However, different bromine substitution positions on dibromoanthracene appear to not strongly influence the vapor pressure.
Single halogen substitution can, but does not necessarily increase the enthalpy of sublimation compared to the parent PAHs. With addition of two halogens, the enthalpies of sublimation generally increase significantly. The different substitution positions on dibromoanthracene do not significantly influence the enthalpy of sublimation. It may be concluded that single halogen substitutions often have relatively modest effect on enthalpies of sublimation. With that, the major factor determining vapor pressure can be the influence of substituents on the ordering (i.e. entropy) of the solid state. As the number of halogen substituents on the PAH increases, the energetic effects (i.e. enthalpy) begin to manifest themselves more strongly in determining vapor pressure behavior.
Acknowledgments
The present study was supported by Grant Number P42 ES013660 from the National Institute of Environmental Health Sciences (NIEHS)/NIH, and the contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS/NIH.
The authors appreciate the help of James W. Rice at Brown University in various aspects of the present study and Yongsong Huang for the use of his GC.
Contributor Information
Jinxia Fu, Email: Jinxia_Fu@Brown.edu.
Eric M. Suuberg, Email: Eric_Suuberg@brown.edu.
References
- 1.Domingo JL. Polychlorinated naphthalenes in animal aquatic species and human exposure through the diet: a review. J Chromatogr A. 2004;1054:327–334. doi: 10.1016/j.chroma.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 2.Kitazawa A, Amagai T, Ohura T. Temporal trends and relationships of particulate chlorinated polycyclic aromatic hydrocarbons and their parent compounds in urban air. Environ Sci Technol. 2006;40:4592–4598. doi: 10.1021/es0602703. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson UL, Ostman CE. Chlorinated polycyclic aromatic-hydrocarbons - method of analysis and their occurrence in urban air. Environ Sci Technol. 1993;27:1826–1831. [Google Scholar]
- 4.Haglund P, Alsberg T, Bergman A, Jansson B. Analysis of halogenated polycyclic aromatic-hydrocarbons in urban air, snow and automobile exhaust. Chemosphere. 1987;16:2441–2450. [Google Scholar]
- 5.Ishaq R, Naf C, Zebuhr Y, Broman D, Jarnberg U. PCBs, PCNs, PCDD/Fs, PAHs and Cl-PAHs in air and water particulate samples - patterns and variations. Chemosphere. 2003;50:1131–1150. doi: 10.1016/s0045-6535(02)00701-4. [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi H, Pilkington NH, Otsuki A, Fuwa K. Occurrence of chlorinated polynuclear aromatic-hydrocarbons in tap water. Environ Sci Technol. 1985;19:585–590. doi: 10.1021/es00137a001. [DOI] [PubMed] [Google Scholar]
- 7.Koistinen J, Paasivirta J, Nevalainen T, Lahtipera M. Chlorinated fluorenes and alkylfluorenes in bleached kraft pulp and pulp-mill discharges. Chemosphere. 1994;28:2139–2150. [Google Scholar]
- 8.Koistinen J, Paasivirta J, Nevalainen T, Lahtipera M. Chlorophenanthrenes, alkylchlorophenanthrenes and alkylchloronaphthalenes in kraft pulp-mill products and discharges. Chemosphere. 1994;28:1261–1277. [Google Scholar]
- 9.Horii Y, Ok G, Ohura T, Kannan K. Occurrence and profiles of chlorinated ed and brominated polycyclic aromatic hydrocarbons in waste incinerators. Environ Sci Technol. 2008;42:1904–1909. doi: 10.1021/es703001f. [DOI] [PubMed] [Google Scholar]
- 10.Ni HG, Zeng H, Tao S, Zeng EY. Environmental and human exposure to persistent halogenated compounds derived from E-waste in China. Environ Toxicol Chem. 2010;29:1237–1247. doi: 10.1002/etc.160. [DOI] [PubMed] [Google Scholar]
- 11.Horii Y, Khim JS, Higley EB, Giesy JP, Ohura T, Kannan K. Relative potencies of individual chlorinated and brominated polycyclic aromatic hydrocarbons for induction of aryl hydrocarbon receptor-mediated responses. Environ Sci Technol. 2009;43:2159–2165. doi: 10.1021/es8030402. [DOI] [PubMed] [Google Scholar]
- 12.Ohura T, Morita M, Kuruto-Niwa R, Amagai T, Sakakibara H, Shimoi K. Differential action of chlorinated polycyclic aromatic hydrocarbons on aryl hydrocarbon receptor-mediated signaling in breast cancer cells. Environ Toxicol. 2010;25:180–187. doi: 10.1002/tox.20488. [DOI] [PubMed] [Google Scholar]
- 13.Ohura T, Sawada KI, Amagai T, Shinomiya M. Discovery of novel halogenated polycyclic aromatic hydrocarbons in urban particulate matters: occurrence, photostability, and AhR activity. Environ Sci Technol. 2009;43:2269–2275. doi: 10.1021/es803633d. [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb JL, Suuberg EM. The effect of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic compounds measured via the Knudsen effusion technique. J Chem Thermodyn. 2008;40:460–466. doi: 10.1016/j.jct.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oja V, Suuberg EM. Vapor pressures and enthalpies of sublimation of polycyclic aromatic hydrocarbons and their derivatives. J Chem Eng Data. 1998;43:486–492. [Google Scholar]
- 16.Goldfarb JL, Suuberg EM. Vapor pressures and enthalpies of sublimation of ten polycyclic aromatic hydrocarbons determined via the Knudsen effusion method. J Chem Eng Data. 2008;53:670–676. [Google Scholar]
- 17.Murray JJ, Pottie RF. The vapor pressures and enthalpies of sublimation of five polycyclic aromatic hydrocarbons. Can J Chem. 1974;52:557–563. [Google Scholar]
- 18.Oja V, Suuberg EM. Development of a nonisothermal Knudsen effusion method and application to PAH and cellulose tar vapor pressure measurement. Anal Chem. 1997;69:4619–4626. [Google Scholar]
- 19.Wakayama N, Inokuchi H. Heats of sublimation of polycyclic aromatic hydrocarbons and their molecular packings. J Chem Eng Data. 1967;40:2267–2271. [Google Scholar]
- 20.Sonnefeld WJ, Zoller WH, May WE. Dynamic coupled-column liquid-chromatographic determination of ambient-temperature vapor-pressures of polynuclear aromatic-hydrocarbons. Anal Chem. 1983;55:275–280. [Google Scholar]
- 21.Sato N, Inomata H, Arai K, Saito S. Measurement of vapor-pressures for coal-related aromatic-compounds by gas saturation method. J Chem Eng Jpn. 1986;19:145–147. [Google Scholar]
- 22.Sasse K, Jose J, Merlin JC. A static apparatus for measurement of low vapor-pressures - experimental results on high molecular-weight hydrocarbons. Fluid Phase Equilibr. 1988;42:287–304. [Google Scholar]
- 23.Lei YD, Chankalal R, Chan A, Wania F. Supercooled liquid vapor pressures of the polycyclic aromatic hydrocarbons. J Chem Eng Data. 2002;47:801–806. [Google Scholar]
- 24.Odabasi M, Cetin E, Sofuoglu A. Determination of octanol-air partition coefficients and supercooled liquid vapor pressures of PAHs as a function of temperature: Application to gas-particle partitioning in an urban atmosphere. Atmos Environ. 2006;40:6615–6625. [Google Scholar]
- 25.Fu J, Rice JW, Suuberg EM. Phase behavior and vapor pressures of the pyrene + 9,10-dibromoanthracene system. F luid Phase Equilibr. 2010;298:219–224. doi: 10.1016/j.fluid.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu JX, Suuberg EM. Solid vapor pressure for five heavy PAHs via the Knudsen effusion method. J Chem Thermodyn. 2011;43:1660–1665. doi: 10.1016/j.jct.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Suuberg EM. Vapor pressure of solid polybrominated diphenyl ethers determined via Knudsen effusion method. Environ Toxicol Chem. 2011;30:2216–2219. doi: 10.1002/etc.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender R, Bieling V, Maurer G. The vapour pressures of solids: anthracene, hydroquinone, and resorcinol. J Chem Thermodyn. 1983;15:585–594. [Google Scholar]
- 29.Hansen PC, Eckert CA. An improved transpiration method for the measurement of very low vapor-pressures. J Chem Eng Data. 1986;31:1–3. [Google Scholar]
- 30.Mackay D, Shiu WY, Ma K-C, Lee SC. Physical-chemical properties and environmental fate for organic chemicals. CRC Press; 2006. [Google Scholar]
- 31.Roux MV, Temprado M, Chickos JS, Nagano Y. Critically evaluated thermochemical properties of polycyclic aromatic hydrocarbons. J Phys Chem Ref Data. 2008;37:1855–1996. [Google Scholar]
- 32.Dannenfelser RM. molecular symmetry and related properties. SAR QSAR Environ Res. 1993;1:273–292. [Google Scholar]
- 33.De Kruif C. Enthalpies of sublimation and vapor-pressure of 11 polycyclic-hydrocarbons. J Chem Thermodyn. 1980;12:243–248. [Google Scholar]
- 34.Tittlemier SA, Braekevelt E, Halldorson T, Reddy CM, Norstrom RJ. Vapour pressures, aqueous solubilities, Henry’s Law constants, and octanol/water partition coefficients of a series of mixed halogenated dimethyl bipyrroles. Chemosphere. 2004;57:1373–1381. doi: 10.1016/j.chemosphere.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 35.Tittlemeier SA, Tomy GT. Vapor pressures of six brominated diphenyl ether congeners. Environ Toxicol Chem. 2001;20:146–148. [PubMed] [Google Scholar]