Abstract
Objective
This study quantifies the effect of a new dementia special care unit (D-SCU) on the provision of care to all residents in a nursing home (NH).
Method
The authors use data from the On-line Survey Certification and Reporting system to identify free-standing NHs that first reported a D-SCU between 1996 and 2003 (N = 1,519). Fixed-effects models estimate the effect of a new D-SCU on the prevalence of each outcome (physical restraints, feeding tubes, and psychotropic medications) while controlling for secular trends.
Results
For all NHs, the use of physical restraints declined, the use of antipsychotics increased, and other measures remained relatively constant. The introduction of a D-SCU was not associated with changes in trends for any measure.
Discussion
Differences in care processes between NHs with and without D-SCUs are the result of differences in their underlying approach to care, not the result of care practice diffusion from the D-SCU.
Keywords: dementia special care, specialization, nursing homes
First described in the 1960s, dementia special care units (D-SCUs) are among the most popular innovations in nursing homes (NHs) (Maas, Swanson, Specht, & Buckwalter, 1994; Volicer, 2000; Weiner & Reingold, 1989). By 2000, approximately 20% of NHs in the United States reported a D-SCU, nearly 3 times more than the number that reported rehabilitation units, the second most popular form of specialized care (Agency for Healthcare Research and Quality, 2000; Gruneir, Lapane, Miller, & Mor, 2006). D-SCUs are segregated locked units specifically designed for residents with moderate dementia that tend to offer specialized programming and support for residents and their families. Facilities with D-SCUs have consistently been reported to have better quality outcomes than others. Feeding tube use among cognitively impaired residents is less common in NHs with D-SCUs (Mitchell, Kiely, & Gillick, 2003; Mitchell, Teno, Roy, Kabumoto, & Mor, 2003). Not only are physical restraints less frequently used in NHs with D-SCUs, but also these NHs are more likely to report being completely restraint free (Castle, 2000; Castle, Fogel, & Mor, 1997; Castle & Mor, 1998). Residents of NHs with a D-SCU also have a lower risk of hospitalization than do residents of other NHs (Gruneir, Miller, Intrator, & Mor, 2007; Intrator & Mor, 2004).
The consistency of these differences and the fact that they apply to all residents raise questions about why NHs with a D-SCU appear to perform better than NHs without. The general philosophy of D-SCU care is the maximization of quality of life through a reduction in functional deficits and environmental stressors (Maslow, 1994). Although the knowledge and practice strategies associated with such a philosophy may be initially intended for the D-SCU, they ultimately may diffuse to other regions of the NH. Models of innovation diffusion outline key determinants, such as advantage of the innovation over standard practices, compatibility of the innovation with existing values, and relevance of the innovation to existing problems, that conceivably explain the adoption of D-SCU practices by non-D-SCU staff (Greenhalgh, Robert, Macfarlane, Bate, & Kyriakidou, 2004; Sanson-Fisher, 2004). Research on other forms of specialization in the NH, such as the use of hospice, suggests that diffusion of particular practices results in benefits for all residents of the NH and not just the intended recipients (Wu, Miller, Lapane, Roy, & Mor, 2005).
Because the majority of research on correlates of D-SCU presence has been cross-sectional, it is not clear that diffusion of practices explains these differences. This is notable in light of evaluative studies on D-SCUs that have generally found no clear benefit of D-SCU residence (Chafetz, 1991; Holmes et al., 1990; C. D. Phillips et al., 1997; C. D. Phillips, Spry, Sloane, & Hawes, 2000). Surveys of D-SCU administration, structure, and practice have described a fairly heterogeneous group of units and have suggested that a large proportion of these really offer nothing “special” (Holmes & Teresi, 1994; Morris & Emerson-Lombardo, 1994). This, combined with the added cost often charged to D-SCU residents, have led some to suggest that D-SCUs are largely marketing tools intended to attract private-pay individuals (V. L. Phillips, Potter, & Simon, 1998). Furthermore, NHs with D-SCUs are unlike those without D-SCUs. In addition to structural differences, NHs with D-SCUs are also more likely to provide other types of specialized care and to be affiliated with academic institutions (Holmes & Teresi, 1994; Leon, 1994; Leon, Cheng, & Alvarez, 1997). They serve a slightly different group of residents, including a higher concentration of private pay and a lower concentration of Medicaid residents than the average NH (Arling & Williams, 2003; McCann, Bienias, & Evans, 2000).
It is still unclear whether the adoption of a D-SCU influences overall NH quality. If the philosophy of D-SCU care does diffuse to other parts of the NH, then we would expect to see changes in facility level quality measures following D-SCU introduction that are independent of secular changes in care. Our objective is to quantify changes in the care provided to all residents of a NH following the introduction of a newly reported D-SCU. Using annually collected NH data, we identified NHs that first reported a D-SCU between 1996 and 2003 and compared aggregated resident treatment characteristics across years of interest while controlling for secular trends.
Method
Data Sources
The Centers for Medicare and Medicaid Services require that all Medicare- or Medicaid-certified NHs undergo an annual on-site investigation. Following survey completion, all data elements are entered into the On-line Survey Certification and Reporting System (OSCAR; American Health Care Association, 2004). The OSCAR database is the most comprehensive source of NH information and includes data on facility structure, operational characteristics, staffing, and aggregate resident characteristics for nearly all NHs in the United States (96%). NH-level information is available for the years 1992 forward within the OSCAR database, but variables have been added or modified over time. OSCAR data have often been used by others to characterize NHs (Castle & Fogel, 1998; Feng, Katz, Intrator, Karuza, & Mor, 2005; Lapane & Hughes, 2004).
Study Design
We used a panel of OSCAR data to study changes in the NH prevalence of processes of care. We quantified changes in NH-level outcomes after the introduction of a D-SCU by selecting a group of NHs that did not report a D-SCU in 1996 but did report one by 2003. We used fixed-effects models to adjust for background time trends.
Sample
We selected all NHs that had a completed OSCAR survey dated between 1996 and 2004 (N = 18,381). We did not include earlier years because certain variables of interest were not available prior to 1996. Hospital-based NHs were excluded because they tend to differ from free-standing NHs in their resident profile and likelihood of reporting a D-SCU (n = 2,552; Gruneir et al., 2006); NHs with fewer than 20 beds were excluded because they are also less likely to report a D-SCU and because their small denominator may result in unstable estimates of resident proportions (n = 1,116).
Further restrictions were made to accommodate the study design. First, NHs with fewer than three consecutive surveys were excluded (n = 439). Second, because the objective was to compare prevalence of various outcomes before and after D-SCU opening, NHs that reported a D-SCU in the 1st year of available data were excluded (n = 2,171), as were those that never reported a D-SCU by the end of the study period (n = 10,972). We present the differences between these NH groups in Table 1. Finally, because of concerns about the reliability of the D-SCU variable, we excluded NHs with only 1 year of D-SCU data (n = 387) and NHs that reported 100% of all beds as D-SCU beds (n = 44). The final sample included 1,519 NHs.
Table 1.
Comparison of Nursing Homes (NHs) That Reported a Dementia Special Care Unit (D-SCU) at the Start of the Study Period, NHs That Opened a D-SCU During the Study Period, and NHs That Never Opened a D-SCU (Data From 1996 or 1st Year Available)
| NHs With D-SCU at Start of Study Period (n = 2,171) | NHs That Opened D-SCU During Study Period (n = 1,519) | NHs That Never Opened a D-SCU (n = 10,972) | |
|---|---|---|---|
| Number of beds (M, SD) | 141, 78 | 128, 71 | 100, 57 |
| Number of residents (M, SD) | 120, 78 | 110, 68 | 87, 25 |
| Occupancy rate (M, SD) | 82.8, 23.0 | 84.8, 17.5 | 86.4, 18.8 |
| For profit (%) | 64.3 | 65.5 | 75.4 |
| Part of a chain (%) | 57.7 | 56.8 | 53.6 |
| Other specialized units (%) | |||
| Rehabilitation | 7.7 | 3.5 | 2.6 |
| Hospice | 3.1 | 1.0 | 0.9 |
| Resident payment source (M, SD) | |||
| Medicaid | 57.8, 25.6 | 64.0, 22.9 | 65.9, 24.6 |
| Medicare | 8.1, 12.2 | 8.2, 11.6 | 7.6, 11.4 |
| Private | 34.1, 25.5 | 27.8, 21.9 | 26.5, 23.5 |
| Residents with dementia (M, SD) | 49.8, 18.0 | 44.1, 16.9 | 42.8, 18.0 |
Treatment
The underlying concept of specialized care is the enhancement of resident functioning through the creation of an environment that meets the specific needs associated with dementia. D-SCUs are often physically distinct from other parts of the NH and incorporate features to ensure resident safety, create a more homelike environment, and provide appropriate stimuli (Leon, 1994; Zimmerman et al., 1997). Facilities with D-SCUs tend to report higher staffing ratios, more dedicated staff, and more specialized training for their D-SCUs as compared to their general units (Grant, Kane, Potthoff, & Ryden, 1996; Grant, Potthoff, Ryden, & Kane, 1998). For this study, a D-SCU is conceptually defined as any unit that has been developed for the distinct purpose of caring for NH residents with Alzheimer’s disease or other dementia. This is operationalized as any self-identified D-SCU regardless of details. A NH was considered to have opened a D-SCU if one was reported on at least two annual OSCAR surveys.
Outcome Measures
For this study, three process measures of care—physical restraints, feeding tubes, and psychotropic medication use—were examined. We focused on these specific process measures because they are highly relevant to major issues in dementia care (behavior and feeding) and because they are consistent with much of the previous evaluative literature on D-SCUs. Furthermore, uncertainty around the most appropriate interventions for behavioral and physical sequelae of dementia suggests that the use of these process measure will be influenced by facility practice policies and culture (Porell & Carter, 2005). As part of each survey, the number of residents prescribed each measure is recorded. This is divided by the total number of residents in the facility at the time of the survey to calculate the percentage of residents prescribed each care process.
Physical restraints are defined in one authoritative pamphlet as “any manual method or physical or mechanical device, material, or equipments attached or adjacent to the resident’s body that the resident cannot easily remove and that restricts freedom or movement” (Massachusetts Restraint Reduction Task Force, 2000). Arguments in favor of restraint use include fall prevention, control of aggressive behavior, and protection of treatment devices such as feeding tubes (Capezuti, Evans, Strumpf, & Maislin, 1996; Capezuti, Strumpf, Evans, Grisso, & Maislin, 1998). However, the use of physical restraints has also been linked to injury, pressure ulcers, incontinence, cognitive decline, and death (Castle & Mor, 1998; Miles & Irvine, 1992). Recent years have seen a drop in physical restraint use because of the Omnibus Budget Reconciliation Act of 1987 and widespread media attention of the issue. Prevalence of use declined from 22% in 1992 to 16% in 1997, but the decline is not uniform across all types of residents. Residents with cognitive impairments, physical impairments, and a history of falls continue to be at risk of being restrained (Castle et al., 1997).
Feeding tubes are used when residents have a swallowing disorder associated with terminal decline from dementia or are otherwise impaired in their ability to independently feed themselves. Some reports suggest that use of feeding tubes among NH residents is on the rise, particularly among those with advanced cognitive impairment (Mitchell, Teno, et al., 2003). Although feeding tubes are thought to lessen the risk of malnourishment, empirical evidence suggests that they introduce a much greater risk of aspiration (Finucane, Christmas, & Travis, 1999). Furthermore, some have argued that decisions to initiate tube feeds are financially, rather than clinically, motivated because case-mix reimbursement systems often reimburse more for residents with feeding tubes than for comparable residents without feeding tubes (Mitchell, 2003).
Concerns about overuse and misuse of psychotropic medications, in particular as a tool to subdue residents have led some to label these drugs “chemical restraints.” This description encompasses antipsychotic, anxiolytic, and hypnotic or sedative agents. Adverse events associated with use of these medications include falls, extrapyramidal symptoms, and confusion (Beardsley, Larson, Burns, Thompson, & Kamerow, 1989). More recently, atypical antipsychotic medications in older adults have been linked to increased risk of stroke and death (Schneider, Dagerman, & Insel, 2006; Schneider, Tariot, et al., 2006). Research on the prevalence of psychotropic medication use has shown that as many as 30% of residents have received at least one class of drugs, and the most frequently reported are antipsychotics. Various facility characteristics, including proprietary status and staffing ratios, have been shown to be associated with variations in psychotropic medication use (Hughes, Lapane, & Mor, 2000). Furthermore, one study found that staff perceptions of behavior were more predictive of psychotropic medication use than was structured assessment of behavior (Sorensen, Foldspang, Gulmann, & Munk-Jorgensen, 2001).
Analysis
We included NHs that first reported a D-SCU after 1996 but before 2003. This cohort of NHs all eventually opened a D-SCU, and that change is captured for NHs that reported their D-SCU between 1997 and 2002 (n = 1,385) but not those that opened their D-SCU in 2003 (n = 134). Of the 1,519 NHs in the sample, we had complete data on each of the years prior to and following the year that the D-SCU was first reported for 1,240 NHs. To identify crude changes in resident and staffing variables, we used summary statistics to describe each of these 3 years. We also examined trends in the process measures over time for all NHs in the sample.
To estimate the effect of a newly reported D-SCU, we used facility-level fixed-effects models to regress each outcome on the D-SCU indicator variable. The outcomes were left in their continuous form and represented NH-level prevalence of the process measures for each year studied. The D-SCU variable was coded as 0 for all years prior to D-SCU introduction and as 1 for the year of introduction and all years following. We included a set of dummy variables to represent calendar year to control for time trends in the outcome variables. We estimated robust standard errors to correct for within-facility similarities across years. This modeling strategy estimates the change in the NH prevalence of the outcome variable following D-SCU introduction and contrasts that with the change in prevalence among those NHs that did not introduce a D-SCU at the same time. The beta coefficient for the D-SCU term derived from these models (the measure of effect) is interpreted as the effect of D-SCU introduction after adjustment for secular time trends. Because this is a facility fixed-effects model, all state and regional differences are controlled for. The unit of analysis is facility.
We tested several variables, including aggregate resident characteristics (i.e., percentage with dementia, percentage with behavioral problems, payer mix) and staffing levels (i.e., registered nurse, licensed practical nurse, certified nursing aide) but found that none confounded the estimates of association. We also examined facility measures of case mix and found no evidence of confounding. Based on this, we present only the unadjusted estimates.
We also conducted three sensitivity analyses. We were concerned that the year a D-SCU was first reported would be a transitional year for the NH and therefore would not be representative of any eventual changes in practice. Because this could dilute estimates, we constructed the models using a nested coding scheme to isolate the years after introduction. Nested coding is a strategy that allows for direct comparison between levels of a variable rather than multiple comparisons to a single reference category as with dummy variable coding. In this case, we created a variable in which we were able to compare all of the years with a D-SCU to the years without a D-SCU and then all of the years after D-SCU introduction to the year of D-SCU introduction. This allowed us to evaluate whether there were any changes beyond those associated with D-SCU introduction.
We were also concerned that differences in D-SCU implementation would result in different effects on NH outcomes. We attempted two strategies to address this. First, we tried to differentiate NHs by their incentive to open a D-SCU. We hypothesized that a NH in a highly competitive area would be more likely to open a D-SCU as a marketing strategy and therefore less likely to implement substantial changes in care practices, whereas a NH in a less competitive area would not have the same motivation and therefore would be more likely to open a D-SCU with alternate care practices (Banaszak-Holl, Zinn, & Mor, 1996). We defined each NH’s market as its county and operationalized the market’s competitiveness by the Herfindahl Index (HI). The HI is calculated by summing the square of each NH’s market bed share. Higher HI scores represent less competitive (more concentrated) markets, and lower HI scores represent more competitive (less concentrated) markets. Using cutoffs defined by the U.S. Department of Justice (Department of Justice/Antitrust, 2007), we created three groups based on all of the NHs in a NH’s market in the year prior to D-SCU introduction: highly competitive markets (HI < .10, n = 624), moderately competitive markets (.10 ≤ HI < .18, n = 271), and less competitive markets (HI ≥ .18, n = 624). We estimated each of the models stratified by these three HI groups.
Second, we wanted to determine whether the dominance of the D-SCU within the NH would differentially affect outcomes. We were interested in this because we thought that potential diffusion of D-SCU practice to other parts of the NH may be influenced by the size of the D-SCU relative to the entire facility. We operationalized this as the percentage of all beds reported on the D-SCU, as taken from the 1st year the D-SCU was reported. We created three groups as follows: < 15% of beds on D-SCU (n = 385), 15% to 35% of beds on D-SCU (n = 1,000), and ≥ 35% of beds on D-SCU (n = 134). The cutoff points were selected based on other reports that have suggested that D-SCUs generally account for approximately one fourth of beds in a facility. We estimated each of the models stratified by these three groups.
Results
Of the 1,519 NHs that opened a D-SCU during the study period, fewer D-SCUs were opened with each successive calendar year. In 1997, 20.3% of NHs in the study first reported a D-SCU, but only 9.6% of NHs first reported a D-SCU in 2002. Of the 1,240 NHs that had data for the 3 years around D-SCU introduction, comparisons across those years are presented in Table 2. Over time, there were slight increases in occupancy rate (81.3% to 84.9%), in the percentage of residents with dementia (44.3% to 48.6%), and in the percentage of residents with behavioral problems (30.9% to 35.5%). There were no changes in the concentration of private pay residents or in staffing.
Table 2.
Comparison of Resident and Staffing Characteristics in the Years Surrounding the Year When the Dementia Special Care Unit (D-SCU) Was First Reported
| Year Immediately Prior to D-SCU First Reported | Year D-SCU First Reported | Year Immediately After D-SCU First Reported | |
|---|---|---|---|
| Occupancy rate (M, SD) | 81.3, 17.0 | 83.8, 13.4 | 84.9, 13.5 |
| Resident payment source (M, SD) | |||
| Medicaid | 63.9, 22.5 | 63.7, 20.6 | 63.8, 20.1 |
| Medicare | 8.5, 11.7 | 8.3, 10.3 | 8.3, 10.2 |
| Private | 27.6, 20.8 | 28.0, 18.9 | 27.8, 18.5 |
| Residents with dementia (M, SD) | 44.3, 16.6 | 47.1, 15.7 | 48.6, 16.3 |
| Residents with behavioral problems (M, SD) | 30.9, 16.5 | 33.5, 16.2 | 35.5, 16.4 |
| Staffing, FTEs per 100 beds (M, SD) | |||
| Registered nurses | 5.6, 5.6 | 5.7, 4.8 | 5.4, 3.8 |
| Licensed practical nurses | 10.1, 4.7 | 10.6, 5.0 | 10.7, 5.1 |
| Certified nursing aidesa | 31.5, 17.9 | 32.5, 13.8 | 33.5, 17.1 |
Note: N = 1,240. FTE = full-time equivalent. This table includes the 1,240 NHs for which we had complete data on the three years surrounding the introduction of the SCU. There were 279 NHs that did not contribute to this because we did not have data on either the year that the SCU was introduced, the year after the SCU was introduced, or both.
One observation with extreme observation removed from measure.
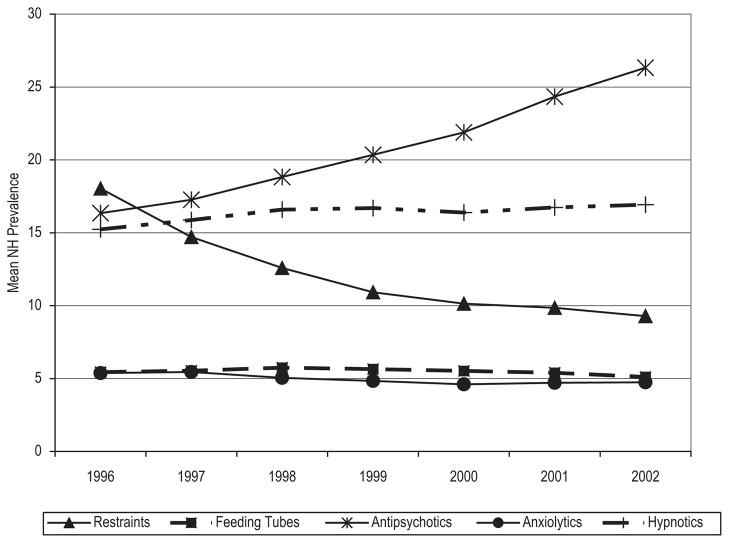
Over the same time period, there was no, or minimal, change in feeding tube, anxiolytic, or hypnotic use. There was a decline in physical restraint use (18.0% to 9.3%) and an increase in antipsychotic use (16.3% to 26.3%) (Figure 1). These same trends were also observed in the models and are presented in Table 3. Model findings suggest that the D-SCU introduction did not modify any of the observed process measure trends (Table 3). Although the prevalence of antipsychotic use showed a slight increase following D-SCU introduction (β = 1.58, 95% confidence interval = 1.12 to 2.03), this one-time increase of less than two percentage points is not suggestive of a meaningful change in light of the stronger time trends.
Figure 1.
Overall Trends in Outcome Use Across the Study Period (1996 to 2002)
Table 3.
Fixed-Effects Model Results—Adjusted Difference in Percentage of Residents Who Experienced Each Care Process
| Restraints
|
Feeding Tube
|
Antipsychotic Medications
|
Antianxiety Medications
|
Hypnotic Medications
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Intercept | 18.0 | 17.3, 18.7 | 5.43 | 5.27, 5.58 | 16.1 | 15.76, 16.46 | 15.22 | 14.84, 15.61 | 5.45 | 5.19, 5.72 |
| Dementia special care unit opened | 0.06 | −0.67, 0.78 | −0.29 | −0.50, −0.09 | 1.58 | 1.12, 2.03 | 0.51 | 0.04, 0.97 | −0.00 | −0.32, 0.32 |
| Year | ||||||||||
| 1996 | REF | REF | REF | REF | REF | |||||
| 1997 | −3.17 | −4.09, −2.52 | 0.16 | −0.07, 0.38 | 0.70 | 0.21, 1.19 | 0.45 | −0.08, 0.98 | 0.04 | −0.34, 0.41 |
| 1998 | −5.42 | −6.33, −4.52 | 0.43 | 0.20, 0.38 | 1.98 | 1.47, 2.50 | 1.21 | 0.65, 1.76 | −0.32 | −0.68, 0.03 |
| 1999 | −7.22 | −8.18, −6.26 | 0.44 | 0.21, 0.68 | 3.25 | 2.70, 3.81 | 1.15 | 0.58, 1.72 | −0.61 | −1.00, −0.23 |
| 2000 | −7.90 | −8.89, −6.90 | 0.36 | 0.12, 0.60 | 4.58 | 4.01, 5.15 | 0.78 | 0.17, 1.38 | −0.84 | −1.24, −0.42 |
| 2001 | −8.28 | −9.32, −7.24 | 0.25 | −0.02, 0.53 | 6.80 | 6.18, 7.42 | 1.19 | 0.56, 1.83 | −0.73 | −1.16, −0.30 |
| 2002 | −8.95 | −10.03, −7.87 | −0.03 | −0.31, 0.25 | 8.62 | 7.94, 9.31 | 1.27 | 0.60, 1.95 | −0.79 | −1.24, −0.33 |
Note: N = 1,519. Separate models were constructed for each outcome variable.
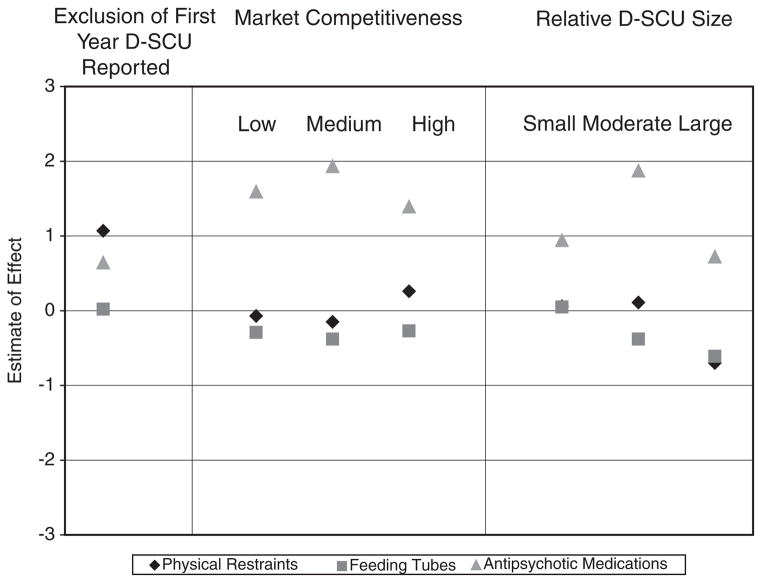
The models with nested coding showed no additional effect on outcomes for the years following the year of D-SCU introduction. Similar null findings were observed for the models at each level of stratification by market competitiveness and at each level of stratification by D-SCU dominance. See Figure 2 for a summary of findings from the sensitivity analyses on each physical restraints, feeding tubes, and antipsychotic medications.
Figure 2.
Estimates of Effect Derived From Sensitivity Analyses
Discussion
We found that the introduction of a D-SCU was not associated with changes in the use of physical restraints, feeding tubes, or psychotropic medications. Over the years of study, the use of physical restraints declined and the use of antipsychotic medications increased, but neither of these trends was influenced by the opening of a D-SCU. These findings persisted after we isolated the years following the year of D-SCU introduction and we attempted to control for market competitiveness and relative size of the D-SCU.
Studies on quality measures and other resident outcomes often include facility D-SCU as either a determinant or a control variable. However, there has generally been little understanding of what this variable really means or why it has consistently been shown to be associated with higher quality. Findings from other research have shown a generally less aggressive approach to care and a stronger emphasis on quality of life through the use of hospice and other supportive services in NHs with D-SCUs (Leon et al., 1997; Morris & Emerson-Lombardo, 1994; Volicer et al., 1994). They have also reported that staff in these NHs have less turnover, more comprehensive training, and greater satisfaction than those in other NHs (Grant et al., 1996; Grant et al., 1998; Mehr & Fries, 1995; Teresi, Grant, Holmes, & Ory, 1998). Previous literature attributed these differences to the presence of the D-SCU, but our findings seem to suggest otherwise. These differences more likely reflect an organizational philosophy or approach that preceded the introduction of the D-SCU and that has its own influence on facility outcomes.
Facilities that choose to adopt innovative strategies likely attain better outcomes than those that do not because they are willing, and able, to make investments where needed (Castle, 2001). It seems likely that any facility with an organizational mandate to innovate is not going to be satisfied with the “status quo” in care and will continue to strive to attain better care practices and outcomes. This is especially important in light of other findings that have shown that NHs that exhibit strategically adaptive behaviors are more economically successful (Zinn, Mor, Feng, & Intrator, 2007) and that NHs with D-SCUs serve a higher proportion of private pay and a lower proportion of Medicaid residents than do others (Zinn & Mor, 1994). This segregation by resources raises questions about the future of NHs that do not innovate and whether this will result in the widening of interfacility disparities in quality of care.
Although we cannot generalize these findings, it may be worthwhile to consider these same issues in other sectors of the health care industry. Hospitals have also undergone various forms of specialization during the past two decades (Eastaugh, 2001). The extent to which this affects patient outcomes is not yet fully clear, and the cross-sectional design of many studies in this area raises the same issues as the literature on NH D-SCUs. For example, even though one study found that hospitals specializing in certain therapies for myocardial infarction had lower mortality and shorter times to treatment than did other hospitals, it also showed important differences between these hospitals in terms of location, teaching status, and presence of onsite cardiac surgery (Nallamothu et al., 2006). Others have shown that clinics identifying themselves as “HIV specialists” were more likely to adopt highly active antiretroviral therapy than are other clinics but that they too differed on several characteristics including staff specialties, provision of support services, and use of guidelines (Wilson et al., 2005). In both cases, effects were independent of volume and indicate that any observed associations with organizational specialization were not the result of greater experience.
Limitations
There are limitations to this study that need to be addressed. First, the OSCAR data are self-reported, so there may be misrepresentation on the D-SCU variable. This may be particularly problematic for NHs that offer specialized dementia programs but do not have segregated units. Because there is limited D-SCU information in the data, we were unable to differentiate D-SCUs by features that may be related to care practices. We attempted to address this with other features that we thought might act as proxies, but this is not perfect. Second, we were not able to include years prior to 1996 because some variables, such as the classes of psychotropic medications, were not yet reported in the OSCAR database. Facilities that first reported an D-SCU after 1996 are relatively late adopters, and it is difficult to know how the inclusion of earlier adopters would have affected our findings (Castle, 2001). Third, we considered only the introduction of new D-SCUs and did not try to study the effect of D-SCU closings. We have not identified any literature that describes how frequently this occurs, but we expect that it is not very common because there may be substantial investment in at least the physical component of the D-SCU. However, we know from other sources that NHs may decide to close D-SCUs when they make a strategic decision to focus on attracting post-acute, short-stay residents. At this point, it is difficult to disentangle coding problems from true closures, but it will be important for future research to look at what precipitates these strategic choices by NHs and if they ultimately affect care.
Finally, we did not control for facility case mix in the models because we did not observe any increase in OSCAR-derived acuity measures over time. It is possible that OSCAR case-mix measures are too crude to capture subtle changes and that resident-level data would have provided finer control of case mix. We were unable to use the Minimum Data Set (MDS) because it was not available for all the years of interest. Other research has shown a moderate correlation between OSCAR- and MDS-based measures (Feng, Grabowski, Intrator, & Mor, 2006). Also, it is important to note that the processes of care that we examined are generally considered undesirable or inappropriate ways to deal with emergent problems. The introduction of practices to improve quality, as measured by processes of care, should lead to decreased use even if resident acuity increases. For example, the implementation of policies that enable nursing staff to avoid the use of physical restraints should result in negligible restraint use regardless of the resident population.
Summary
NHs that report a D-SCU differ from those that do not on various structural, organizational, and population characteristics. Research on several clinical outcomes has consistently shown that NHs with D-SCUs perform better, but it was never clear why this should be the case. Our findings show that the introduction of a new D-SCU in a NH was not associated with changes in specific processes of care relevant to dementia care. This suggests that differences in resident outcomes previously observed between NHs with and without D-SCUs are not because of the presence of the D-SCU itself but may instead be the result of other differences in overall facility operation and practice.
Acknowledgments
We would like to thank R. Tamara Konetzka in the Department of Health Studies at the University of Chicago for her helpful comments on the article. As well, we would like to thank Zhanlian Feng at the Center for Gerontology and Health Care Research at Brown Medical School for his advice on the analysis. This research was supported by an AARP Scholar’s Award to the first author and the National Institute on Aging (Grants AG20557 and AG023622). Andrea Gruneir was a student in the Department of Community Health at Brown Medical School when this research was conducted.
Contributor Information
Andrea Gruneir, Kunin-Lunenfeld Applied Research Unit, Baycrest.
Kate L. Lapane, Brown Medical School.
Susan C. Miller, Brown Medical School.
Vincent Mor, Brown Medical School.
References
- Agency for Healthcare Research and Quality. Research findings no. 6: Special care units in nursing homes—Selected characteristics, 1996. Rockville, MD: Author; 2000. [Google Scholar]
- American Health Care Association. CMS OSCAR data. Washington, DC: Author; 2004. [Google Scholar]
- Arling G, Williams AR. Cognitive impairment and resource use of nursing home residents: A structural equation model. Medical Care. 2003;41(7):802–812. doi: 10.1097/00005650-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Banaszak-Holl J, Zinn JS, Mor V. The impact of market and organizational characteristics on nursing care facility service innovation: A resource dependency perspective. Health Services Research. 1996;31(1):97–117. [PMC free article] [PubMed] [Google Scholar]
- Beardsley RS, Larson DB, Burns BJ, Thompson JW, Kamerow DB. Prescribing of psychotropics in elderly nursing home patients. Journal of the American Geriatrics Society. 1989;37(4):327–330. doi: 10.1111/j.1532-5415.1989.tb05499.x. [DOI] [PubMed] [Google Scholar]
- Capezuti E, Evans L, Strumpf N, Maislin G. Physical restraint use and falls in nursing home residents. Journal of the American Geriatrics Society. 1996;44(6):627–633. doi: 10.1111/j.1532-5415.1996.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Capezuti E, Strumpf NE, Evans LK, Grisso JA, Maislin G. The relationship between physical restraint removal and falls and injuries among nursing home residents. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53(1):M47–M52. doi: 10.1093/gerona/53a.1.m47. [DOI] [PubMed] [Google Scholar]
- Castle NG. Differences in nursing homes with increasing and decreasing use of physical restraints. Medical Care. 2000;38(12):1154–1163. doi: 10.1097/00005650-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Castle NG. Innovation in nursing homes: Which facilities are the early adopters? Gerontologist. 2001;41(2):161–172. doi: 10.1093/geront/41.2.161. [DOI] [PubMed] [Google Scholar]
- Castle NG, Fogel B. Characteristics of nursing homes that are restraint free. Gerontologist. 1998;38(2):181–188. doi: 10.1093/geront/38.2.181. [DOI] [PubMed] [Google Scholar]
- Castle NG, Fogel B, Mor V. Risk factors for physical restraint use in nursing homes: pre- and post-implementation of the Nursing Home Reform Act. Gerontologist. 1997;37(6):737–747. doi: 10.1093/geront/37.6.737. [DOI] [PubMed] [Google Scholar]
- Castle NG, Mor V. Physical restraints in nursing homes: A review of the literature since the Nursing Home Reform Act of 1987. Medical Care Research and Review. 1998;55(2):139–170. 171–176. doi: 10.1177/107755879805500201. [discussion] [DOI] [PubMed] [Google Scholar]
- Chafetz PK. Behavioral and cognitive outcomes of SCU care. Clinical Gerontologist. 1991;11:19–38. [Google Scholar]
- Department of Justice/Antitrust. The Herfindahl-Hirschman Index. Washington, DC: Author; 2007. [Google Scholar]
- Eastaugh SR. Hospital costs and specialization: benefits of trimming product lines. Journal of Health Care Finance. 2001;28(1):61–71. [PubMed] [Google Scholar]
- Feng Z, Grabowski DC, Intrator O, Mor V. The effect of state Medicaid case-mix payment on nursing home resident acuity. Health Services Research. 2006;41(4 Pt 1):1317–1336. doi: 10.1111/j.1475-6773.2006.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Katz PR, Intrator O, Karuza J, Mor V. Physician and nurse staffing in nursing homes: The role and limitations of the Online Survey Certification and Reporting (OSCAR) system. Journal of the American Medical Directors Association. 2005;6(1):27–33. doi: 10.1016/j.jamda.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: A review of the evidence. Journal of the American Medical Association. 1999;282(14):1365–1370. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- Grant LA, Kane RA, Potthoff SJ, Ryden M. Staff training and turnover in Alzheimer special care units: Comparisons with non-special care units. Geriatric Nursing. 1996;17(6):278–282. doi: 10.1016/s0197-4572(96)80241-2. [DOI] [PubMed] [Google Scholar]
- Grant LA, Potthoff SJ, Ryden M, Kane RA. Staff ratios, training, and assignment in Alzheimer’s special care units. Journal of Gerontological Nursing. 1998;24(1):9–16. 59. doi: 10.3928/0098-9134-19980101-08. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Quarterly. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneir A, Lapane KL, Miller SC, Mor V. Dementia special care units in U.S. nursing homes: A current description of distribution and major features. Paper presented at the 59th annual scientific meeting of the Gerontological Society of America; Dallas, TX. 2006. Nov, [Google Scholar]
- Gruneir A, Miller SC, Intrator O, Mor V. Hospitalization of nursing home residents with cognitive impairments: The influence of organizational features and state policies. Gerontologist. 2007;47(4):447–456. doi: 10.1093/geront/47.4.447. [DOI] [PubMed] [Google Scholar]
- Holmes D, Teresi J. Characteristics of special care units in the Northeast Five-State Survey: Implications of different definitional criteria. Alzheimer Disease and Associated Disorders. 1994;8(Suppl 1):S97–S105. [PubMed] [Google Scholar]
- Holmes D, Teresi J, Weiner A, Monaco C, Ronch J, Vickers R. Impacts associated with special care units in long-term care facilities. Gerontologist. 1990;30(2):178–183. doi: 10.1093/geront/30.2.178. [DOI] [PubMed] [Google Scholar]
- Hughes CM, Lapane KL, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Medical Care. 2000;38(12):1164–1173. doi: 10.1097/00005650-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Intrator O, Mor V. Effect of state Medicaid reimbursement rates on hospitalizations from nursing homes. Journal of the American Geriatrics Society. 2004;52(3):393–398. doi: 10.1111/j.1532-5415.2004.52111.x. [DOI] [PubMed] [Google Scholar]
- Lapane KL, Hughes CM. Which organizational characteristics are associated with increased management of depression using antidepressants in US nursing homes? Medical Care. 2004;42(10):992–1000. doi: 10.1097/00005650-200410000-00008. [DOI] [PubMed] [Google Scholar]
- Leon J. The 1990/1991 National Survey of Special Care Units in Nursing Homes. Alzheimer Disease and Associated Disorders. 1994;8(Suppl 1):S72–S86. [PubMed] [Google Scholar]
- Leon J, Cheng C, Alvarez RJ. Trends in special care: Changes in SCU from 1991 to 1995 (‘95/96 TSC) Journal of Mental Health and Aging. 1997;3(2):149–168. [Google Scholar]
- Maas ML, Swanson E, Specht J, Buckwalter KC. Alzheimer’s special care units. Nursing Clinics of North America. 1994;29(1):173–194. [PubMed] [Google Scholar]
- Maslow K. Current knowledge about special care units: Findings of a study by the U.S. Office of Technology Assessment. Alzheimer Disease and Associated Disorders. 1994;8(Suppl 1):S14–S40. [PubMed] [Google Scholar]
- Massachusetts Restraint Reduction Task Force. Physical restraints: A resource guide. Boston: Author; 2000. [Google Scholar]
- McCann JJ, Bienias JL, Evans DA. Change in performance tests of activities of daily living among residents of dementia special care and traditional nursing home units. In: Holmes D, Teresi J, Ory MG, editors. Special care units. New York: Springer; 2000. pp. 141–150. [Google Scholar]
- Mehr DR, Fries BE. Resource use on Alzheimer’s special care units. Gerontologist. 1995;35(2):179–184. doi: 10.1093/geront/35.2.179. [DOI] [PubMed] [Google Scholar]
- Miles SH, Irvine P. Deaths caused by physical restraints. Gerontologist. 1992;32(6):762–766. doi: 10.1093/geront/32.6.762. [DOI] [PubMed] [Google Scholar]
- Mitchell SL. Financial incentives for placing feeding tubes in nursing home residents with advanced dementia. Journal of the American Geriatrics Society. 2003;51(1):129–131. [PubMed] [Google Scholar]
- Mitchell SL, Kiely DK, Gillick MR. Nursing home characteristics associated with tube feeding in advanced cognitive impairment. Journal of the American Geriatrics Society. 2003;51(1):75–79. [PubMed] [Google Scholar]
- Mitchell SL, Teno JM, Roy J, Kabumoto G, Mor V. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. Journal of the American Medical Association. 2003;290(1):73–80. doi: 10.1001/jama.290.1.73. [DOI] [PubMed] [Google Scholar]
- Morris JN, Emerson-Lombardo N. A national perspective on SCU service richness: Findings from the AARP Survey. Alzheimer Disease and Associated Disorders. 1994;8(Suppl 1):S87–S96. [PubMed] [Google Scholar]
- Nallamothu BK, Wang Y, Magid DJ, McNamara RL, Herrin J, Bradley EH, et al. Relation between hospital specialization with primary percutaneous coronary intervention and clinical outcomes in ST-segment elevation myocardial infarction: National Registry of Myocardial Infarction-4 analysis. Circulation. 2006;113(2):222–229. doi: 10.1161/CIRCULATIONAHA.105.578195. [DOI] [PubMed] [Google Scholar]
- Phillips CD, Sloane PD, Hawes C, Koch G, Han J, Spry K, et al. Effects of residence in Alzheimer disease special care units on functional outcomes. Journal of the American Medical Association. 1997;278(16):1340–1344. [PubMed] [Google Scholar]
- Phillips CD, Spry KM, Sloane PD, Hawes C. Use of physical restraints and psychotropic medications in Alzheimer special care units in nursing homes. American Journal of Public Health. 2000;90(1):92–96. doi: 10.2105/ajph.90.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips VL, Potter SJ, Simon SL. Special care units for Alzheimer’s patients: Their role in the nursing home market. Journal of Health and Human Services Administration. 1998;20(3):300–310. [PubMed] [Google Scholar]
- Porell FW, Carter M. Discretionary hospitalization of nursing home residents with and without Alzheimer’s disease: A multilevel analysis. Journal of Aging and Health. 2005;17(2):207–238. doi: 10.1177/0898264304274302. [DOI] [PubMed] [Google Scholar]
- Sanson-Fisher RW. Diffusion of innovation theory for clinical change. Medical Journal of Australia. 2004;180(Suppl 6):S55–S56. doi: 10.5694/j.1326-5377.2004.tb05947.x. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. American Journal of Geriatric Psychiatry. 2006;14(3):191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New England Journal of Medicine. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Foldspang A, Gulmann NC, Munk-Jorgensen P. Determinants for the use of psychotropics among nursing home residents. International Journal of Geriatric Psychiatry. 2001;16(2):147–154. doi: 10.1002/1099-1166(200102)16:2<147::aid-gps286>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Teresi JA, Grant LA, Holmes D, Ory MG. Staffing in traditional and special dementia care units. Preliminary findings from the National Institute on Aging Collaborative Studies. Journal of Gerontological Nursing. 1998;24(1):49–53. doi: 10.3928/0098-9134-19980101-13. [DOI] [PubMed] [Google Scholar]
- Volicer L. Goals of dementia special care units. In: Holmes D, Teresi J, Ory MG, editors. Special care units. New York: Springer; 2000. pp. 93–103. [Google Scholar]
- Volicer L, Collard A, Hurley A, Bishop C, Kern D, Karon S. Impact of special care unit for patients with advanced Alzheimer’s disease on patients’ discomfort and costs. Journal of the American Geriatrics Society. 1994;42(6):597–603. doi: 10.1111/j.1532-5415.1994.tb06856.x. [DOI] [PubMed] [Google Scholar]
- Weiner AS, Reingold J. Special care units for dementia: Current practice models. Journal of Long Term Care Administration. 1989;17(1):14–19. [PubMed] [Google Scholar]
- Wilson IB, Landon BE, Ding L, Zaslavsky AM, Shapiro MF, Bozzette SA, et al. A national study of the relationship of care site HIV specialization to early adoption of highly active antiretroviral therapy. Medical Care. 2005;43(1):12–20. [PubMed] [Google Scholar]
- Wu N, Miller SC, Lapane K, Roy J, Mor V. The quality of the quality indicator of pain derived from the Minimum Data Set. Health Services Research. 2005;40(4):1197–1216. doi: 10.1111/j.1475-6773.2005.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SI, Sloane PD, Gruber-Baldini A, Calkins M, Leon J, Magaziner J, et al. The philosophy of special care in Alzheimer’s special care units. Journal of Mental Health and Aging. 1997;3(2):169–181. [Google Scholar]
- Zinn JS, Mor V. Nursing home special care units: Distribution by type, state, and facility characteristics. Gerontologist. 1994;34(3):371–377. doi: 10.1093/geront/34.3.371. [DOI] [PubMed] [Google Scholar]
- Zinn JS, Mor V, Feng Z, Intrator O. Doing better to do good: The impact of strategic adaptation on nursing home performance. Health Services Research. 2007;42(3 Pt 1):1200–1218. doi: 10.1111/j.1475-6773.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]