Abstract
Preserved cognitive performance is a key feature of successful aging. Several theoretical models (compensation, hemispheric asymmetry reduction, and posterior-anterior shift) have been proposed to explain the putative underlying relationship between brain function and performance. We aimed to review imaging studies of the association between brain functional response and cognitive performance among healthy younger and older adults in order to understand the neural correlates of successful cognitive aging. MEDLINE-indexed articles published between January 1989 and May 2008, and bibliographies of these articles and related reviews were searched. Studies that measured brain function using fMRI or PET, evaluated cognitive performance, analyzed how cognitive performance related to brain response, and studied healthy older individuals were included. Forty-seven of 276 articles met these criteria. Eighty-one percent of the studies reported some brain regions in which greater activation related to better cognitive performance among older participants. This association was not universal, however, and was seen mainly in frontal cortex brain response and seemed to be more common among older compared to younger individuals. This review supports the notion of compensatory increases in brain activity in old age resulting in better cognitive performance, as suggested by hemispheric asymmetry reduction and posterior-anterior shift models of functional brain aging. However, a simple model of bigger structure → greater brain response → better cognitive performance may not be accurate. Suggestions for future research are discussed.
Keywords: Aging, fMRI, PET, cognition, brain imaging, frontal cortex
Introduction
A great challenge for future society will be to better understand the aging process. As the proportion of individuals over age 65 grows, it is of utmost socioeconomic importance to promote functional independence and quality of life in this group. Cognitive health has consistently been cited by seniors as important for quality of life (1) and is widely recognized by researchers as an important contributor to late life functioning (2–4). Thus, a key element in dealing with the “graying of the world” must be to discover ways to optimize cognitive performance in old age.
Cognitive performance in most domains declines with age, particularly processes such as psychomotor speed; however, some abilities may remain stable or even improve up to a certain age, such as vocabulary (5). Importantly, there is large heterogeneity in cognitive changes that occur with aging (6). Some seniors are exceptional in their cognitive performance, and understanding this aspect of cognitive aging (as opposed to focusing on pathological change or normal declines) is likely to guide the search for ways to enhance cognitive functioning in aging (3).
Brain health is an important determinant of cognitive health, so a fuller understanding of neural aging, especially those aspects that most influence cognition, is necessary. Much is already known about structural brain changes due to age and age-related diseases (7) and how these relate to cognitive performance (8). We observed, in a comprehensive review of the literature focusing on structural correlates of cognitive performance in healthy elders, that most studies find a positive relationship between regional brain size and cognitive performance (9).
Brain structural integrity, however, is only one neural factor influencing individual differences in cognitive function among older people. Studies of brain function using techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have revealed reliable changes in the level and pattern of brain activity with age. Older age has been associated with lower blood flow and metabolism at rest, particularly in frontal cortex (10). Reduced regional brain response to challenge tasks among older compared to younger participants has also been seen (11). Notably, however, greater regional brain response in older participants also is commonly observed (11). In some instances, pattern of brain response is qualitatively different, for example, when “overactivation” occurs in a homologous region in the opposite hemisphere from the region typically responsive in the young group and is less lateralized (12), referred to as HAROLD: Hemispheric Asymmetry Reduction in OLDer adults (13). In other cases, brain response in posterior regions has been found to be lower in older adults whereas anterior regions show greater response than in younger individuals (14). This relative shift from posterior to anterior involvement has been termed the Posterior-Anterior Shift with Aging (PASA (11)).
An unresolved issue is how these age-related changes in brain responsiveness and altered patterns (e.g., HAROLD or PASA) are associated with cognitive differences between older individuals and with rates of cognitive decline with aging. Age-related alterations in brain function may be associated with poorer cognitive performance, as is seen most dramatically in the case of decreased regional metabolism associated with steep cognitive declines in Alzheimer’s dementia (15). Interestingly, however, there is also evidence that the degree to which older individuals manifest some age-related patterns of brain activity may be associated with better cognitive performance (i.e., compensation (16)). Such findings are in contrast to the idea that alterations of neural function reflect inefficient processing (i.e., de-differentiation (17)).
The purpose of this review was to comprehensively review studies of the association between cognitive performance and brain function among healthy older individuals in order to weigh evidence in support of age-related compensatory versus de-differentiated brain responses, examine factors related to these findings, and evaluate their specificity to old age. Previous reviews of functional imaging changes with age (11, 18) have summarized a subset of findings covered in the present review, but none has focused specifically on correlations between brain function and cognitive performance.
Methods
To retrieve studies for this review, MEDLINE citations (January, 1989 – May, 2008) were surveyed using the National Library of Medicine’s PubMed online search engine using the following search string: ("functional MRI" OR "fMRI" OR "PET" OR "positron emission tomography" OR "cerebral blood flow") AND (brain OR cognition OR cognitive OR cerebral) NOT (dementia OR Alzheimer's OR psychiatric OR disorder OR disease OR impair*) AND (age[Title] OR aging[Title] OR ageing[Title] OR old[Title] OR elderly[Title]). This search revealed 256 articles of potential interest. All references cited in these articles were also reviewed, and we found 20 additional relevant publications.
Inclusion criteria
We applied several selection criteria. To be included in the review, studies had to 1) use fMRI or PET methodology to examine neural functioning, 2) evaluate cognitive performance either on a task given during imaging or on a measure administered on a separate occasion, 3) report results of an analysis examining how differences in cognitive performance related to differences in brain response, and 4) include at least one group of healthy elderly individuals (mean age > 60 years of age).
Review process
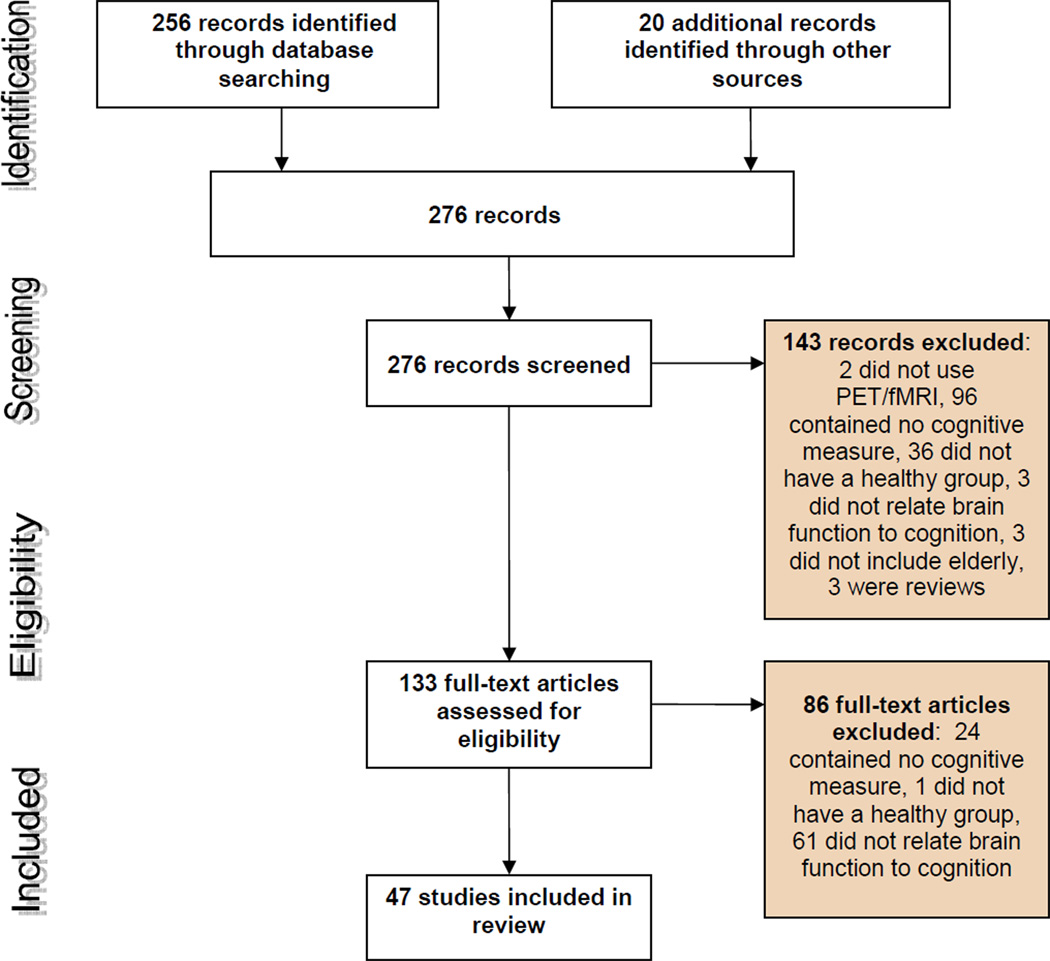
We found 47 reports that met review criteria. Flow of information through different stages of the systematic review is shown in Figure 1.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow of information through the different phases of the systematic review
Each study was examined by two authors (LTE, AS) and the following information was extracted: numbers and characteristics of participants, definition of “healthy” used, neuroimaging method and scanning paradigm employed, and brain regions examined. We also noted whether participants overlapped between samples in the review. Based on study results, we summarized the relationship of age to functional brain imaging measures to provide a context for understanding brain-cognition relationships. We then extracted the measures of cognitive performance used and summarized associations between cognitive performance level and measures of brain functioning. We noted the direction of association and whether this reflected greater response among those with successful versus normal cognitive performance (e.g., positive correlations for accuracy and negative correlations for reaction time both imply that better cognitive performers have greater brain response). When possible, we summarized correlations separately for younger and older groups and noted whether a direct statistical comparison had been made.
Results
Characteristics of Reviewed Studies
Study characteristics and results for the 47 reviewed papers are presented chronologically in Table 1. There was one longitudinal study (19), one study in which cognitive change was examined longitudinally but brain response was measured once (20), and all other investigations were cross-sectional. Among cross-sectional studies, two (21, 22) included only older participants, one examined a large cohort ranging in age from 20 to 87 (23), and the rest (n=41) compared younger adults to at least one group of older individuals. One study (24) reported partial overlap in participants with another study (25).
Table 1.
Chronological Report of Study Characteristics and Results Relevant to Association of Brain Response and Cognitive Performance in 47 Reviewed Studies.
| Citation | Number of Participants |
Characteristics of participants |
Definition of "Healthy" |
Type of Imaging |
Paradigm tested | Brain Region(s) Examined |
Age Difference(s) in Brain Function |
Success/ Performance Determination |
Success/ Performance Correlation or Effect(s) |
|---|---|---|---|---|---|---|---|---|---|
| Cabeza et al., 1997, Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study | Y: 12, O: 12 | Y: mean age = 26, age range = 19–31, 6M:6F, mean years education = 17.8; O: mean age = 70, age range = 67–75, 5M:7F, mean years education = 16.0 | No history of neurological or psychiatric illness or use of medication or condition that could affect CBF. | PET | Episodic encoding and retrieval (both recognition and retrieval) of word pairs. Contrast(s): partial least squares analysis of age group differences relative to encoding vs. retrieval and recognition vs. recall | Whole brain analysis | Encoding vs. retrieval: Y > O: superior anterior cingulate, L temporal cortex (Y: ENC > RET, O: RET > ENC), inferior anterior cingulate, RET medial frontal pole (Y: RET > ENC, O: ENC ≥ RET); O > Y: B insula, RET occipital cortex (O: ENC > RET, Y: ENC ≤ RET), cuneus/precuneus, L prefrontal cortex (O : RET > ENC, Y: ENC ≥ RET). | Delayed recall accuracy | Mostly non-signficant correlations, but in Y and O combined: NEG correlation ( ) with recall in R insula. ) with recall in R insula. |
| Madden et al., 1997, Selective and divided visual attention: Age-related changes in regional cerebral blood flow measured by PET | Y: 12, O: 12 | Y: mean age = 24.33, age range = 21–28, 12M:0F; O: mean age = 65.5, age range = 60–77, 12M:12F; all had completed some post-secondary education | No major health problems on screening questionnaire. negative neurologic screening exam. MMSE of >28 and < 5 on Beck depression inventory. MRI free of any atrophy or structural abnormalities. | PET | Visual attention tasks: Passive (look at letter array and alternate button press), Central (look for one of 2 target letters in center of array), Divided (look for one of two target letters anywhere in 9 letter array). Contrast(s): Central vs. Passive, Divided vs. Passive, Divided vs Central | Whole brain analysis | Central vs Passive: activations: Y > O for anterior cingulate, deactivations: Y > O for R middle frontal gyrus; Divided vs Passive: activations: Y > O for B fusiform gyrus, O > Y for L middle frontal gyrus, deactivations: Y = O; Divided vs Central: activations: Y > O in BA 18, O > Y in anterior cingulate, L superior frontal gyrus, R middle frontal gyrus, deactivations: Y > O in L middle frontal gyrus, O > Y in L insula. | Mean reaction time on correct trials |
Y:
Central vs. Passive: NEG correlation with RT in R superior temporal and middle frontal gyrus; Divided vs. Passive: POS correlation with RT in L superior parietal, anterior cingulate, and inferior occipital gyrus, NEG correlation in R frontal and B inferior temporal; Divided vs. Central: POS correlation in L superior parietal and L occipitotemporal, NEG correlation in R superior frontal gyrus. O:
Divided vs. Passive: POS correlation with RT ( ) L frontal, R superior parietal, L cerebellum, L inferior temporal cortex, NEG correlation with RT ( ) L frontal, R superior parietal, L cerebellum, L inferior temporal cortex, NEG correlation with RT ( ) in B medial temporal gyrus, R superior temporal, midline superior frontal gyrus; Divided vs. Central: POS correlation with RT ( ) in B medial temporal gyrus, R superior temporal, midline superior frontal gyrus; Divided vs. Central: POS correlation with RT ( ) in B superior parietal lobe and L BA 6, NEG correlation with RT ( ) in B superior parietal lobe and L BA 6, NEG correlation with RT ( ) in B medial temporal gyrus, L insula, anterior cingulate, superior temporal gyrus. ) in B medial temporal gyrus, L insula, anterior cingulate, superior temporal gyrus. |
| Grady et al., 1998, Age-related changes in regional cerebral blood flow during working memory for faces | Y: 13, O: 16 | Y: mean age = 25, mean years of education = 16, 10M:3F; O: mean age = 66, mean years of education = 16, 11M:5F | Excluded those with diseases or medications that might affect brain function. | PET | Working memory task for faces (delayed match-to-sample). Contrast: working memory vs. sensorimotor control; partial least squares analysis used to examine effect of differing delays. | Whole brain analysis | Overall effects: O > Y in L dorsolateral prefrontal activation and R extrastriate (both activation and deactivation); Y > O in B inferior prefrontal activation and R prefrontal, L premotor, R perisylvian and midline cingulate deactivation. | Reaction time |
Y and O: POS correlations in R orbitofrontal and extrastriate, NEG correlations in perisylvian, dorsomedial prefrontal, and posterior cingulate ( ); Y more POS slope than O: R middle temporal and medial prestriate; O more POS slope than Y: B dorsolateral PFC, L hippocampus, and B fusiform ( ); Y more POS slope than O: R middle temporal and medial prestriate; O more POS slope than Y: B dorsolateral PFC, L hippocampus, and B fusiform ( ). ). |
| Hazlett EA, Buchsbaum et al., 1998, Age-related shift in brain region activity during successful memory performance | N=70 (10 per decade of age); Also subset of Y: 16, O: 16 | overall age range = 20–87, 35M:35F, Subsample: Y: mean age = 33.4, age range = 20–49; O: mean age = 73.5, age range = 60–87, mean years of education = 16.1 | Medical and psychiatric examination. Exclusions; psychoactive medication use, substance abuse/dependence, psychiatric illness, positive urine drug screen. Cognitively normal. | PET | Serial Verbal Learning test (SVLT) with 5 lists of 16 words (from 4 semantic categories) each presented visually, read aloud by the participant, and recalled 5 times. Contrast(s): N/A. | 15 cortical ROIs. Also, voxel-wise statistical parametric mapping. | NEG association with metabolic rate in superior, middle, and inferior frontal gyrus; POS association in occipital lobe. On voxel-wise, regions of greatest NEG correlation were medial frontal/cingulate gyrus and anterior tip of temporal lobe. | Within Y and O groups, created subgroup with GoodRecall (>13.3 words) and PoorRecall (<12.2 words). Within GoodRecall and PoorRecall subgroups, Y = O for number of words recalled. |
Y: GoodRecall > PoorRecall in frontal; O: GoodRecall > PoorRecall ( ) in occipital. Frontal/occipital ratio favored frontal in Y
GoodRecall vs. PoorRecall, favored occipital in O
GoodRecall vs. PoorRecall ( ) in occipital. Frontal/occipital ratio favored frontal in Y
GoodRecall vs. PoorRecall, favored occipital in O
GoodRecall vs. PoorRecall ( ) ; GoodRecall > PoorRecall in L frontal/occipital ratio regardless of age. ) ; GoodRecall > PoorRecall in L frontal/occipital ratio regardless of age. |
| Madden, Gottlob et al., 1999, Aging and recognition memory: Changes in regional cerebral blood flow associated with components of reaction time distributions | Y: 12, O: 12 (same as Madden, Turkington, et al., 1999) | Y: mean age = 23.17, 6F:6M, age range = 20-2; O: mean age = 71.0, 7F:5M, age range = 62–79, mean age = 71.0; All participants had finished high school. | No major health problems on screening questionnaire. Negative neurologic screening exam. MMSE of ≥28 and ≤ 6 on Beck depression inventory. MRI free of abnormalities. | PET | Three task conditions for word learning and recognition: Encoding (intentional; living/nonliving judgment), Baseline (told that words will not be tested; capitalization judgment), and Retrieval (yes/no recognition judgment); Contrast(s): Encoding vs. Baseline and Retrieval vs. Baseline. | ROIS in which a significant correlation with mean reaction time had been observed in Madden, Turkington, et al., 1999. | None reported | Ex-Gaussian curve fitted to reaction time distribution yielded measures of mu (mean of the Gaussian component; leading edge) and tau (mean of the exponential component; tail). | Y: Retrieval vs. Baseline: POS correlation with mu (but not tau) in R middle frontal gyrus. O: Encoding vs. Baseline: POS correlation with tau in R middle frontal gyrus and L parahippocampal gyrus; Retrieval vs. Baseline: POS correlation with mu in R middle frontal gyrus and with tau in R BA 10, NEG correlations with mu in R inferior parietal lobule, with tau in R cuneus, and with both mu and tau in L transverse temporal gyrus |
| Madden, Turkington et al., 1999, Adult age differences in the functional neuroanatomy of verbal recognition memory | Y: 12, O: 12 | Y: mean age = 23.17, 6F:6M, age range = 20–29; O: mean age = 71.0, 7F:5M, age range = 62–79, all participants had finished high school. | No major health problems on screening questionnaire. Negative neurologic screening exam. MMSE of ≥28 and ≤ 6 on Beck depression inventory. MRI free of abnormalities. | PET | Three task conditions for word learning and recognition: Encoding (intentional; living/nonliving judgment), Baseline (told that words will not be tested; capitalization judgment), and Retrieval (yes/no recognition judgment); contrast(s): Encoding vs. Baseline and Retrieval vs. Baseline | Whole brain analysis; success analysis only for values at local maxima of regions of significant rCBF. | Encoding minus Baseline: O > Y in thalamus; Retrieval minus Baseline: Y > O in thalamus, O > Y in B prefrontal cortex. | Reaction time difference between experimental and baseline conditions. | Baseline and Encoding condition: O:
POS correlation of RT difference with rCBF difference in left parahippocampal gyrus and R middle frontal gyrus ( ); Baseline and Retrieval condition: Y:
POS correlation in R middle frontal gyrus; O:
POS correlation in R middle frontal gyrus ( ); Baseline and Retrieval condition: Y:
POS correlation in R middle frontal gyrus; O:
POS correlation in R middle frontal gyrus ( ), NEG correlations in several posterior regions ( ), NEG correlations in several posterior regions ( ) ) |
| McIntosh et al., 1999, Recruitment of unique neural systems to support visual memory in normal aging | Y: 10, O: 9 | Y: mean age = 23, age range = 20–30; O: mean age = 65, age range = 60–79 | In good health and in normal range on MMSE and a vocabulary test. | PET | Discrimination of spatial frequency between two sine wave gratings presented either 500 msec (short ISI) or 4000 msec (long ISI) apart (pretesting to match difficulty/accuracy between groups). Contrast(s): partial least square of brain-behavior correlations that were common to or distinguished ISI conditions and group (old vs. young) | Whole brain analysis | None reported (only brain-behavior patterns examined) | Discrimination threshold (lower is better) |
Y and O: Short ISI: NEG correlation with discrimination threshold ( ) in occipital, POS correlation ( ) in occipital, POS correlation ( ) in striatum, inferior prefrontal and inferotemporal. Long ISI: reverse pattern compared to above. O only: Short ISI: NEG correlation with discrimination threshold ( ) in striatum, inferior prefrontal and inferotemporal. Long ISI: reverse pattern compared to above. O only: Short ISI: NEG correlation with discrimination threshold ( ) in temporal and dorsal occipital, POS correlation ( ) in temporal and dorsal occipital, POS correlation ( ) in posterior thalamus and dorsomedial prefrontal cortex. Long ISI: reverse pattern compared to above. Y: opposite and attentuated difference between ISI conditions. ) in posterior thalamus and dorsomedial prefrontal cortex. Long ISI: reverse pattern compared to above. Y: opposite and attentuated difference between ISI conditions. |
| Reuter-Lorenz et al., 2000, Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET | Verbal task: Y: 8, O: 16; Spatial task: Y: 10, O: 10 | Y: verbal: mean age = 23.3, age range = 21–30, mean years education = 15, 8F:0M; Spatial: mean age = 21.2, age range = 18–25, mean years education = 15; O: verbal: mean age = 69.9, age range = 62–75, mean years education = 16; Spatial: mean age = 67.4, age range = 62–73, mean years education = 16 | Right handed, negative neurological histories, normal or corrected to normal vision. O participants excluded from analysis if had spatial performance > 1SD below Y group. | PET | Verbal and spatial delayed match to sample tasks; Contrast(s): Delayed verbal match vs. immediate verbal match; Delayed spatial match vs. immediate spatial match. | 85 (verbal) and 98 (spatial) bilateral ROIs from literature, divided into anterior and posterior ROIs. For correlations, laterality difference score calculated for anterior, posterior, and all ROIs. | Verbal: Y > O: difference between L and R across all anterior ROIs (L > R in Y, L = R in O, although this exact pattern only seen in one subregion - Broca's area); Spatial: Y > O: difference between L and R across all anterior ROIs (R > L in Y, R = L in O, although this exact pattern only seen in one subregion - R supplementary motor area). | Composite measure of Z-score for average reaction time subtracted from the z-score for percent correct, as well as each of these separately. Additional analysis using a median split based on reaction time. | Verbal: RT median split:  in L-R laterality of dorsolateral prefrontal cortex (SCA = bilateral, NCA = R only); SCA = NCA in Broca's area laterality (both bilateral). Spatial: SCA = NCA. Whole group: Verbal: O:
POS correlation of z score composite with all L ROIs, L posterior ROIs ( in L-R laterality of dorsolateral prefrontal cortex (SCA = bilateral, NCA = R only); SCA = NCA in Broca's area laterality (both bilateral). Spatial: SCA = NCA. Whole group: Verbal: O:
POS correlation of z score composite with all L ROIs, L posterior ROIs ( ), NEG correlation of RT with L Broca's ( ), NEG correlation of RT with L Broca's ( ); Y: no significant correlations with composite, NEG correlation left lateral supplementary motor area; Spatial: No significant correlations. ); Y: no significant correlations with composite, NEG correlation left lateral supplementary motor area; Spatial: No significant correlations. |
| Rypma and D'Esposito, 2000, Isolating the neural mechanisms age-related changes in human working memory | 3 experiment s: Exp. 1: Y: 6, O: 6; Exp. 2: Y: 7, O: 6; Exp. 3: Y: 6, O: 6 | Exp. 1- Y: age range = 21–30, 3M:3F, mean years education = 15.4; O; age range= 61–82, 3M:3F, mean years education = 17.6; Exp. 2- Y: age range = 19–26, 4M:3F, mean years education = 16 years; O: age range = 55–83, 2M:4F, mean years education = 14.5; Exp. 3- Y: age range = 20–29, 4M:2F, mean years education = 15.5; O: age range = 66–71, 3M:3F, mean years education = 16.8 | No medical, neurological, or psychiatric illness or any prescription medication. O had no evidence of dementia and MMSE > 26 and Beck depression inventory < 10. | fMRI | Event-related verbal delayed response task with a small (2 letters) or large (6 letters) memory load (Exp 1 and 2); event-related object or spatial or object+spatial match to sample task (Exp 3); Contrast(s): encoding period vs. baseline, maintenance period vs. baseline, retrieval period vs. baseline. | Dorsolateral (Brodmann’s areas 9 and 46) and ventrolateral (Brodmann’s areas 44, 45 and 47) prefrontal cortex; averaged across L and R hemispheres. | Y > O in dorsolateral prefrontal cortex during response period in high load condition in all three experiments. | Mean reaction time. |
All 3 Expts:
O:
NEG correlation of RT and dorsolateral prefrontal activation during the response period ( ); Y:
POS correlation of RT and dorsolateral prefrontal activation during the response period. ); Y:
POS correlation of RT and dorsolateral prefrontal activation during the response period. |
| Reuter-Lorenz et al., 2001 (42), Neurocognitive ageing of storage and executive processes | Y: 12, O: 12 (combined with subjects from Reuter-Lorenz et al., 2000 for success analysis) | Y: age range = 19–30, 12M:0F; O: age range = 61–72, 12M:0F | Right handed, negative neurological histories, normal or corrected vision. O participants excluded if spatial performance > 1SD below Y group. | PET | Verbal delayed match to sample task; Contrast(s): Delayed verbal match vs. immediate verbal match. | ROIs based on results of Reuter-Lorenz et al, 2000. | O > Y: R dorsolateral prefrontal cortex, R area 44, R parietal areas 40 and 7; Y > O: L area 44. | Reaction time |
O:
NEG correlation with RT ( ) in R dorsolateral prefrontal cortex. ) in R dorsolateral prefrontal cortex. |
| Cabeza R et al., 2002, Aging gracefully: Compensatory brain activity in high-performing n older adults | Y: 12, O: 16 (SCA: 8, NCA: 8) | Y: mean age = 25.3, age range = 20–35, 7M:5F; SCA: mean age = 68, age range = 64–78, 4M:4F; NCA: mean age = 69.9, age range = 63–74, 4M:4F | No neurologic or psychiatric history. None on medication or with medical condition affecting cerebral blood flow. | PET | Episodic memory for recently studied words. Contrast(s): Recall vs. source memory and source memory vs. recall. | Prefrontal regions activated by entire group. | None reported | Divided into SCA vs. NCA based on composite memory score of subtests from Wechsler Memory Scale and California Verbal Learning Test. | Recall-source contrast: Y > SCA = NCA in L dorsolateral prefrontal, Y =  in left ventrolateral; Source-recall contrast: Y > SCA = NCA in R dorsolateral prefrontal, Y < NCA = SCA in right anterior prefrontal, SCA > Y = NCA in left anterior prefrontal. in left ventrolateral; Source-recall contrast: Y > SCA = NCA in R dorsolateral prefrontal, Y < NCA = SCA in right anterior prefrontal, SCA > Y = NCA in left anterior prefrontal. |
| Grady et al., 2002, The effects of encoding task on age-related differences in the functional neuroanatomy of face memory | Y: 12, O: 11 | Y: mean age = 23.2, age range = 20–28, 6M:6F, mean years education = 17; O: mean age 70, age range = 62–79, 6M:5F, mean years education = 15.7 | No diseases or medications that affect brain function; no abnormalities on MRI; MMSE and vocabulary in normal range. | PET | Episodic encoding and retrieval for faces. Levels of processing manipulation during encoding (3 levels: intentional, incidental with orientation judgment, incidental with pleasantness judgment). Retrieval phase involved forced-choice recognition tasks. Contrast(s): partial least squares analysis of patterns associated with encoding vs. control, retrieval vs. control, encoding vs. retrieval. | Whole brain analysis | Brain regions that distinguish all encoding vs. control (inferior prefrontal and L amygdala) and all recognition vs. control (B prefrontal, L premotor, cingulate gyrus) in Y only distinguish intentional and deep vs. control in O. Thus, Y > O in these regions during shallow encoding and recognition of shallowly encoded words (confirmed in contrast of encoding vs. recognition). Recognition: O > Y in L anterior temporal and premotor cortex; Y > O in R dorsolateral prefrontal. Encoding vs. recognition: Y > O in L prefrontal and anterior cingulate (Y: E > R, O: R > E). | Partial least square analysis of recognition accuracy. | Encoding and Recognition: Y:
POS correlation with accuracy in B hippocampus, orbitofrontal cortex, L temporal pole, O:
POS correlation with accuracy ( ) in B posterior temporal and occipital and R prefrontal cortex, Both Y and O: Deep incidental encoding: POS correlation ( ) in B posterior temporal and occipital and R prefrontal cortex, Both Y and O: Deep incidental encoding: POS correlation ( ) with L prefrontal (2 regions), L posterior temporal; Shallow incidental encoding: POS correlation ( ) with L prefrontal (2 regions), L posterior temporal; Shallow incidental encoding: POS correlation ( ) with extrastriate regions. ) with extrastriate regions. |
| Iidaka, Okada et al., 2002, Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI | Y: 12, O: 12 | Y: mean age = 25.1, age range = 19–36, 6M:6F, mean years education = 15.8; O: mean age = 65.2, 6M:6F, age range = 62–72, mean years education = 16.0 | No history of neurological diseases, psychiatric diseases, or drug or alcohol abuse based on interview. No medications that could affect CBF. T2 MRI free of incidental infarctions. | fMRI | Emotional face perception: judging the gender of faces with negative (angry or disgusting), positive (happy), or neutral emotional valence; Contrast(s): negative vs. baseline (judging relative size of rectangles), positive vs. baseline, neutral vs. baseline; negative vs. neutral, positive vs. neutral, and negative vs. positive also assessed for age analysis. | Whole brain analysis: single group results used as masks for comparison of age groups; success analyses conducted in amygdala, hippo-campus and parahippo-campal gyrus. | Negative condition: Y > O: L amygdala; Positive condition: Y > O: R parahippocampal gyrus, R lingual gyrus, R angular gyrus; Neutral condition: Y > O: B midbrain, L lingual gyrus; task-independent activity of R hippocampus POS correlated with age in O group only. | Score on the first principal component of four neuropsychological tests (digit symbol, trail-making, word recall, and figure recall). |
O:
POS correlation between global neuropsych and overall activation of R parahippocampus (only word recall individually significant) ( ); Y: no significant correlations. ); Y: no significant correlations. |
| Madden, Langley et al., 2002, Adult age differences in visual word identification: Functional Neuroanatomy by PET | Y: 12, O: 12 | Y: mean age = 23.58, age range = 20–29, 6M:6F, education = 15.67; O: mean age = 65.0, age range = 62–70, 6M:6F, education = 16.75 | No current health problems or previous significant medical events (e.g., head injury with loss of consciousness > 5 min., stroke, TIA, MI, and heart surgery), determined by questionnaire. MMSE ≥ 27. MRI reviewed to rule out significant abnormality. | PET | Lexical decision task (word/nonword discrimination) with 3 conditions of different duration and presentation rates. Contrast(s): Lexical decision condition vs. nonsemantic (simple visual search) condition, long duration lexical decision vs. short duration, short presentation rate lexical decision vs. long presentation rate. | Whole brain analysis: single group results used as masks for comparison of age groups; success analyses only conducted in local maxima of age effects. | Lexical decision minus Baseline: Y > O in activation of L striate cortex (BA 17) and deactivation of parietal cortex, O > Y in L inferior temporal cortex (BA 37). No age differences in effect of duration or presentation rate. | Pearson correlations between standardized RTs and normalized count values for the left striate and left inferior temporal voxels. |
Y:
POS correlation of RT with rCBF in L striate; O: small, non-significant POS correlation in this region, that was not significantly different from Y ( ). ). |
| Madden, Turkington et al., 2002, Aging and attentional guidance during visual search functional neuroanatomy by PET | Y: 12, O: 12 | Y: mean age = 23.0, 5M:7F, age range = 20–27; O: mean age = 63.5, 5M:7F, age range = 60–77, all subjects had at least high school education | No current health problems or previous significant medical events, as determined by a screening questionnaire. MMSE > 27. No significant atrophy or structural abnormality on MRI. | PET | Visual search task with 3 conditions: Feature (target distinguished by color from all 17 distractors), Guided (target + 2 distractors in same color), and Conjunction (target + 8 distractors in same color); Contrast(s): conjunction vs. feature, guided vs. feature, conjunction vs. guided. | Whole brain analysis with age analysis only in areas of significant rCBF task response. | Conjunction minus feature: Y > O in R striate and extrastriate cortex; O > Y deactivation of R middle temporal gyrus; Conjunction minus guided: Y > O in R striate cortex and B ventral extrastriate cortex; Guided minus feature: O > Y deactivation in R middle temporal gyrus and B anterior cingulate. | Accuracy of visual search. |
Y > O: more POS correlation in Y in ventral processing stream and R middle frontal gyrus, more NEG correlation in Y in R middle temporal gyrus; O: many other regions of POS correlation ( ). ). |
| Mattay, Tessitore et al., 2002, Neurophysiological correlates of age-related changes in human motor function | Y: 10, O: 12 | Y: mean age = 30, age range = 24–34, 9M:1F; O: age range = 50–74, mean age = 59, 7M:5F | No history of neurologic, psychiatric, or medical problems; no current medications. No significant changes above normal age-related on T1 and T2 MRI. | fMRI | Visually paced "Push button" motor task with dominant hand alternating with a rest state. Contrast(s): motor task vs. rest. | Whole brain analysis. | O > Y: B primary motor, R primary sensory, R lateral premotor area, B supplementary motor, L putamen, B parietal cortex, B cerebellum. | Mean reaction time |
O:
NEG correlations with respect to RT ( ) in B primary motor cortex, B lateral premotor area, midline supplementary motor area, L parietal cortex, L occipital cortex, R cerebellum. ) in B primary motor cortex, B lateral premotor area, midline supplementary motor area, L parietal cortex, L occipital cortex, R cerebellum. |
| Nielson et al., 2002, Differences in the functional neuroanatomy of inhibitory control across the adult life dpan | Part 1: Y: 10, MA: 7, YE: 9, O: 8; Part 2: Young Old Good (YO/G): 4, Young Old Poor (YO/P) 6; Old Old Good (OO/G) 6, Old Old Poor (OO/P) 4. | Part 1: Y: mean age = 25.5, age range = 18–31, mean years education = 15.5, 6M:4F; MA: mean age = 43.3, age range = 33–55, mean years education = 17.1, 3M:4F; YE: mean age = 68.9, age range = 62–72, mean years education = 16.9, 1M:8F; O: mean age = 75.1, age range = 73–78, mean years education = 19.3, 4M:4F. Part 2: YO/G: mean age = 64.3, mean years education = 18.3; YO/P: mean age = 62.5, mean years education = 17.2; OO/G: mean age = 73.5, mean years education = 17.7; OO/P: mean age = 76.0, mean years education = 19.8 | No medications, substances or diseases affecting performance or imaging; no history of or current psychological or neurological conditions; O > 50 had to have MMSE of > 26 and Geriatric Depression Scale < 10. | fMRI | Go/No Go task in which response is made to alternating stimuli (X or Y) thus requiring inhibition of response to previously correct stimulus. Contrast: correct targets vs. baseline and correct lures vs. baseline. | Part 1: 43 clusters, Part 2: 27 clusters, all those that met criteria for significant activation in at least one group. | Part 1: Inhibition (NoGo): Y> MA, YE, and O in R middle frontal and fusiform gyri; MA < Y, YE, and O in striatal-thalamic clusters; O > Y, MA, YE in R BA 6, multiple L prefrontal clusters. Targets (Go): Y > MA, YE, and O in basal ganglia, thalamus, R cingulate, L fusiform; Part 2: OO > YO in 2 of 17 R hemisphere clusters (middle frontal gyrus and inferior parietal lobule) and 7 of 10 L hemisphere clusters (parietal, prefrontal, and thalamic). | Median split of overall inhibition performance in subgroup of 20 older participants: good performers ≤ 5 errors of commission (> 78% correct), poor performers > 6 errors (< 78% correct). |
 in B presupplementary motor area and L thalamus; performance effect in R presupplementary motor most pronounced in OO group. Across entire sample: POS association between R parietal activation and better inhibition performance, target performance, and faster target RT ( in B presupplementary motor area and L thalamus; performance effect in R presupplementary motor most pronounced in OO group. Across entire sample: POS association between R parietal activation and better inhibition performance, target performance, and faster target RT ( ). NEG correlation in L middle frontal gyrus: increased activation associated with slowed RT to targets ( ). NEG correlation in L middle frontal gyrus: increased activation associated with slowed RT to targets ( ). ). |
| Rosen et al., 2002 (74), Variable effects of aging on frontal lobe contributions to memory | Y: 8, O: 14 (SCA: 7, NCA: 7) | Y: mean age = 24, age range = 19–33, 5M:3F, mean years education = 17.3; O: mean age = 71, age range = 61–81, 3M:11, mean years of education: 16.3 | Screen for neurologic, psychiatric, vascular risk factors, or medication known to affect vascular reactivity or cognition. | fMRI | Verbal encoding task (block design). Contrast(s): Semantic vs. non-semantic encoding (level of processing effect). | L and R inferior PFC, anterior / medial frontal (based on areas of activity in combined group). | SCA = Y in left inferior and anterior / medial PFC; SCA > Y in right inferior PFC. NCA = Y in all 3 regions. | Memory screening (2 years prior): high and low memory groups sig. different on proportion correct on four memory tests Ranges not reported. |
 in the anterior / medial frontal, L inferior frontal, and R inferior frontal. in the anterior / medial frontal, L inferior frontal, and R inferior frontal. |
| Stebbins et al., 2002, Aging effects on memory encoding in the frontal lobes | Y: 15, O: 15 | Y: mean age 25.3, age range = 22–32, 3M:12F, mean years education = 16.7; O: mean age = 76.5, age range = 65–87, 2M:13F, mean years education = 17.8 | No history of neurological illness; O were from the Religious Orders study and had medical and neurological examination and no neuropsychological abnormalities. | fMRI | Incidental word encoding during either semantic (abstract vs. concrete) or non-semantic (uppercase vs. lowercase) decisions. Contrast(s): semantic vs. non-semantic encoding. | Whole brain analysis in each group masked by ROIs traced on 4 slices from each individual: B superior, middle, inferior frontal and cingulate gyri | Y > O: L superior and middle frontal activation (overall: L > R in Y, L = R in O). | Neuropsychological measures of: general mental status, immediate and delayed story recall, processing speed, working memory, reasoning; education, word knowledge. |
Y:
POS correlation with working memory in L cingulate; O:
POS correlation ( ) with working memory in L inferior and middle frontal gyri, and with immediate recall memory in R inferior frontal gyrus and L middle gyrus. ) with working memory in L inferior and middle frontal gyri, and with immediate recall memory in R inferior frontal gyrus and L middle gyrus. |
| Daselaar SM et al., 2003, Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects | Y: 17, O: 40 (Normal Memory O: 19, Reduced Memory O: 21) | Y: mean age = 32.7, age range = 30–35, 17M:0F, education score (7 point scale) = 5.9; O: age range 63–71, 40M:0F; Normal Memory O: mean age = 66.4, mean education score= 5.6; Reduced Memory O: mean age = 66.2, mean education score = 5.3 | None taking psychoactive medications, no neurologic and/or psychological impairment reported on general health questionnaire. O > 25 on MMSE. No anatomic abnormalities atypical for age on structural MRI. | fMRI | Incidental episodic encoding for words (pleasantness judgments), intermixed with baseline task (left/right button press); Contrast(s): successfully encoded vs. baseline; correctly rejected vs. baseline (retrieval attempt); correctly recognized vs. correctly rejected (retrieval success); correctly rejected vs. correctly recognized. | Whole brain analysis | None reported | Divided O participants into Reduced Memory O versus Normal Memory O based on whether performance on scanner task was less than or greater than the mean of the Y group. | Encoding: Y = Normal Memory O > Reduced Memory O ( ) in perirhinal/parahippocampal cluster. Slightly less left lateralization in Normal Memory O. Retrieval Attempt: Reduced Memory O > Normal Memory O ( ) in perirhinal/parahippocampal cluster. Slightly less left lateralization in Normal Memory O. Retrieval Attempt: Reduced Memory O > Normal Memory O ( ) = Y in L Insula, R Middle Temporal, L Supramarginal, Cerebellum, and Occipital cortex; Normal Memory O > Y = Reduced Memory O ( ) = Y in L Insula, R Middle Temporal, L Supramarginal, Cerebellum, and Occipital cortex; Normal Memory O > Y = Reduced Memory O ( ) in L Inf Frontal Gyrus. Retrieval Success: Reduced Memory O > Y in L Inf Frontal Gyrus. ) in L Inf Frontal Gyrus. Retrieval Success: Reduced Memory O > Y in L Inf Frontal Gyrus. |
| Grady, McIntosh, & Craik, 2003, Age-related differences in the functional connectivity of the hippocampus during memory encoding | Y: 11, O: 12 | Y: mean age = 23, age range = 19–28, 6M:6F, mean years education = 16; O: mean age = 66, age range = 58–73, 6M:6F, mean years education = 15 | No diseases or medications that affect brain function; no abnormalities on MRI; MMSE and vocabulary in normal range | PET | Intentional encoding of words and line drawings during a semantic task (living/non-living); Contrast(s): partial least squares analysis of relationship of object encoding or word encoding activity to recognition accuracy | Regions of significant correlation with right hippocampal seed regions (chosen because of relationship to performance in both groups) | None reported | Recognition accuracy (proportion hits minus proportion false alarms) |
Y only: Object encoding: POS correlations with accuracy (and R hippocampus activity) in L hippocampus, ventrolateral prefrontal, inferior temporal, ventral extrastriate, R dorsolateral prefrontal, L parietal, NEG correlations with accuracy in dorsomedial extrastriate, superior temporal gyrus, posterior cingulate; O only: Object encoding: POS correlations ( ) in prefrontal, superior temporal, and inferior parietal, NEG correlations ( ) in prefrontal, superior temporal, and inferior parietal, NEG correlations ( ) in ventral extrastriate. Y > O (positive in Y, NEG in O [ ) in ventral extrastriate. Y > O (positive in Y, NEG in O [ ]): Object encoding: R thalamus, L sensorimotor, and R superior temporal. Word encoding: ventral frontal, ventral extrastriate, inferior temporal. O > Y (POS in O [ ]): Object encoding: R thalamus, L sensorimotor, and R superior temporal. Word encoding: ventral frontal, ventral extrastriate, inferior temporal. O > Y (POS in O [ ], NEG in Y): Word encoding: dorsolateral prefrontal, dorsal occipital, and superior temporal. Y = O: Object encoding: POS correlations in R ventrolateral prefrontal; Word encoding: POS correlations in B middle frontal gyri, R insula, NEG correlations in orbitofrontal cortex, precuneus, and dorsal anterior cingulate. ], NEG in Y): Word encoding: dorsolateral prefrontal, dorsal occipital, and superior temporal. Y = O: Object encoding: POS correlations in R ventrolateral prefrontal; Word encoding: POS correlations in B middle frontal gyri, R insula, NEG correlations in orbitofrontal cortex, precuneus, and dorsal anterior cingulate. |
| Gron et al., 2003, Variability in memory performance in aged healthy individuals: An fMRI study | O: 24 | O: mean age = 64.4, age range = 56–76, 13M:11F | No pathology on medical history, radiological, neurological, and neuropsychological testing and no subjective memory complaints. | fMRI | Intentional encoding of abstract patterns and recall of the pattern. Contrast(s): Initial learning vs. late learning; maximum recall block vs. initial recall block. | Whole brain analysis | Initial learning: POS correlation with age (OO > YO) in anterior cingulate; Maximum recall: NEG correlation with age (YO > OO) in R pulvinar and R precuneus, POS correlation (OO > YO) in medial frontal gyrus. | Area under the curve of number correct over repeated recall blocks. | O: Initial learning: POS correlation with performance ( ) in occipital regions (including middle occipital gyrus to medial occipito-temporal including lingual, fusiform, and parahippocampal gyri, R hippocampus, L inferior and middle frontal gyrus, anterior cingulate, and L anterior thalamus. Maximum recall: POS correlation with performance ( ) in occipital regions (including middle occipital gyrus to medial occipito-temporal including lingual, fusiform, and parahippocampal gyri, R hippocampus, L inferior and middle frontal gyrus, anterior cingulate, and L anterior thalamus. Maximum recall: POS correlation with performance ( ) in B dorsolateral and anterior prefrontal, R inferior frontal gyrus, R lateral middle temporal gyrus, R hippocampus, medial superior temporal gyrus, R lateral amygdala. ) in B dorsolateral and anterior prefrontal, R inferior frontal gyrus, R lateral middle temporal gyrus, R hippocampus, medial superior temporal gyrus, R lateral amygdala. |
| Langenecker and Nielson, 2003, Frontal recruitment during response inhibition in older adults | Y: 11, O: 11 (O were subsample from Langenecker et al., 2002 tested a second time) | Y: mean age = 28.1, mean years education = 17, 4M:7F; O: mean age = 72.8, mean years education = 18, 3M:8F | Normal cognitive and emotional status (MMSE > 26, GDS < 10), plus a neuropsychological battery for older participants only. | fMRI | Go/No Go task in which response is made to alternating stimuli (X or Y) thus requiring inhibition of response to previously correct stimulus. Contrast(s): correct targets vs. baseline and correct lures vs. baseline. | 26 clusters that met significance criteria in at least one group. | O > Y: L inferior frontal gyrus, L inferior parietal lobule, L claustrum, and L putamen, R medial and middle frontal gyri. 19 out of 26 clusters not significantly different upon retest in older participants. | Percent correct inhibition. | Weak POS correlations in R medial frontal gyrus and R supramarginal gyrus in O ( ), but sample was too small to adequately investigate this idea. ), but sample was too small to adequately investigate this idea. |
| Scarmeas et al., 2003, Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects | Y: 19, O: 17 | Y: mean age = 23, age range = 19–28, 7M:12F, mean years education = 16.7; O: mean age = 71, age range = 59–81, 8M:9F, mean years of education = 15 | Screen for medical, neurologic (especially dementia), psychiatric, and neuropsychological disorders, and medications with vascular or cognitive effects. Screening MRI of the brain. | PET | Serial recognition memory task: encoding and recognition of list of unique shapes (list length individually titrated to 75% accuracy) vs. nonmemory control (same shape presented on each trial). Contrast(s): shape learning / recognition vs. non-memory repeated object learning / recognition. | Whole brain analysis | None reported | First principal component of New Adult Reading Test-North American Version, WAIS-R vocabulary subtest, and years of education used as a measure of cognitive reserve (CR). |
Y: POS correlation with CR in R Postcentral Gyrus, and R Inferior Temporal Gyrus, O:
POS correlation with CR in R Cuneus, Posterior Cingulate, and R Cuneus ( ); and NEG correlation with CR in R Superior Temporal Gyrus, L Claustrum-Insula, R Superior Temporal Gyrus, L Inf Parietal Lobule, Cingulate Gyrus, Inf Frontal Gyrus, and L Parahippocampal Gyrus ( ); and NEG correlation with CR in R Superior Temporal Gyrus, L Claustrum-Insula, R Superior Temporal Gyrus, L Inf Parietal Lobule, Cingulate Gyrus, Inf Frontal Gyrus, and L Parahippocampal Gyrus ( ); O more NEG slope than Y: R Inf Temporal, Postcentral; O more POS slope than Y: L Cuneus ); O more NEG slope than Y: R Inf Temporal, Postcentral; O more POS slope than Y: L Cuneus |
| Lustig & Buckner, 2004 (75), Preserved neural Correlates of priming in old age and dementia | Y: 34, O: 33 | Y: mean age = 22.3, age range = 18–34, 18M:16F; O: mean age = 78.3, age range = 61–93, 12M:21F, mean years education = 13.7 | Clinical dementia rating scale = 0 | fMRI | Repetition priming for words during a semantic classification task. Contrast(s): repeated vs. new words. | Two regions of L inferior frontal gyrus (BA 45/47 and 44/6). | No differences in brain response in either ROI to repeated vs. new words (both groups showed reductions), nor on whole brain analysis, nor in homologous R hemisphere ROIs. | Magnitude of repetition-induced reduction in reaction time |
Y = O: POS correlations between reduced activity and reduced RT ( ) in BA 45/47 ROI ) in BA 45/47 ROI |
| Colcombe, Kramer et al., 2005, The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans | Y: 20, O: 40 (SCA: 20, NCA: 20) | Y: mean age = 23.5, age range = 19–28, 11M:9F; O: mean age = 67.5, age range = 52–87, 22M:18F; SCA: mean age = 67.6, mean education = 16.3, 10M:10F; NCA: mean age = 67.4, mean education = 16.5, 12M:8F | Screen for neurologic disease, and ability to be tested in MRI. O with MMSE < 27 excluded. Vision test < 20/30 were corrected. | fMRI | Flanker test (identify direction of central arrow surrounded by congruent or incongruent distracter arrows) event-related with long intertrial interval. Contrast: incongruent vs. baseline. | Regions of significant activation across groups in incongruent trials vs. baseline. | O > Y: B middle frontal gyrus, anterior cingulate / supplementary motor area. | Median split of percent increase in reaction time for incongruent over congruent trials. |
 : L middle frontal gyrus. : L middle frontal gyrus. |
| Fera F. et al., 2005 (76), Neural mechanisms underlying probabilistic category learning in normal aging | Y: 18, O: 15 | Y: mean age = 25.5, mean years education = 16.7, 9M:9F; O: mean age = 67.1, mean years education = 16.8, 9M:6F | No history or current neurological, medical or psychiatric conditions, no current medications. | fMRI | Probabilistic category learning: “weather prediction” test; Contrast: weather prediction vs. perceptual motor control across four epochs. | Whole brain analysis | Y > O: Caudate, anterior and posterior cingulate, B prefrontal; O > Y: B parietal. | Percentage correct and reaction time in each of 4 epochs. | Whole brain correlational analysis and only POS correlations are shown. Y:
POS correlations with RT in prefrontal, premotor, medial frontal, cingulate, caudate, occipital, parietal, and thalamus; positive correlation with accuracy in dorsolateral and inferior prefrontal, caudate, parietal, Broca's area, and thalamus; O:
POS correlation with RT in prefrontal, parietal, thalamus and occipital ( ); POS correlation with accuracy in prefrontal, caudate, presupplementary motor and parietal ( ); POS correlation with accuracy in prefrontal, caudate, presupplementary motor and parietal ( ). ). |
| Grady, McIntosh, & Craik, 2005, Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults | Y: 12, O: 12 | Y: mean age = 25.6, age range = 21–29, 6M:6F, mean years education = 16.6; O: mean age = 70.4, age range = 58–85, 7M:5F, mean years education = 14.3 | No diseases or medications that affect brain function; no abnormalities on MRI; MMSE and vocabulary in normal range. | PET | Recognition memory for words or line drawings encoded during a semantic (living/nonliving) or perceptual (case of words or size of picture) task. Contrast(s): recognition of semantically-encoded items vs. silent reading/naming control; recognition of perceptually-encoded items vs. silent reading/naming control. | Whole brain analysis (partial least quares covariance analysis). | Y > O: L middle temporal gyrus, hippocampus, and R inferior parietal (Y activations, O no response), L superior temporal, R inferior temporal (Y no response, O deactivation); O > Y: rostral anterior cingulate, L parahippocampus, R inferior frontal gyrus (O activations, Y no response or deactivation). | Mean number of hits |
Y = O:
POS correlation with performance ( ) in anterior and posterior cingulate gyrus, R caudate nucleus, NEG correlations ( ) in anterior and posterior cingulate gyrus, R caudate nucleus, NEG correlations ( ) in posterior frontal, L temporal, ad R occipital and parietal areas; O > Y (POS in O [ ) in posterior frontal, L temporal, ad R occipital and parietal areas; O > Y (POS in O [ ] and NEG or small in Y) in B anterior and inferior prefrontal cortex, L dorsomedial preforntal, B middle temporal, and L visual cortex; Y > O (POS or small in Y and NEG in O [ ] and NEG or small in Y) in B anterior and inferior prefrontal cortex, L dorsomedial preforntal, B middle temporal, and L visual cortex; Y > O (POS or small in Y and NEG in O [ ]) in R motor cortex, R parahippocampal gyrus, inferior parietal. ]) in R motor cortex, R parahippocampal gyrus, inferior parietal. |
| Rosano C, Aizenstein H. et al., 2005 (77), Functional neuroimaging indicators of successful executive control in the oldest old | Y: 20, O: 8 | Y: mean age = 23.0, 9M:11F, mean years education =14.5; O: mean age = 81.5, 5M:3F, mean years education = 13.7 | Normal range on a cognitive battery; Excluded for psychological and CNS medical problems, older were excluded for significant neurological or neurodegenerative diseases. | fMRI | POP (Preparing to Overcome Prepotency) task: pre-cue fixation, cue to expect to make either a congruent button press (same direction as arrow; low load) or an incongruent button press (opposite direction from arrow; high load); Contrast(s): average signal change in high-load vs. low-load trials for preparation (first 9s of trial) or decision (last 9s of trial) phase. | Dorsolateral prefrontal cortex: BA 9, 45, 46 (preparation), anterior cingulate cortex (decision), and posterior parietal cortex: BA 7, 40 (preparation) | Y > O in BA 7, 9, and 46, but no age × load interactions. | High load minus low load accuracy and reaction time. |
Y and O: POS correlation of load-related increases of activation with stability of performance accuracy across load in R BA 7, 40, and with stability of RT across load in R 46; O, but not Y, showed POS relationship between increased activation and RT stability across load in BA 40 ( ). ). |
| Rypma et al., 2005, Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI | Y: 8, O: 6 | Y: mean age = 19.5, age range = 19–26, 4M: 4F, mean years of education = 16.0; O: mean age = 70.2, age range = 55–83, 3M:4F, mean years of education = 14.5 | Screen for hypertension, other medical, neurological, or psychiatric disorder and use of prescription medication; MMSE > 26, BDI < 10. | fMRI | Item-recognition task with set size 1–8 letters (slow event-related design). Contrast(s): mean activity vs. baseline during Encoding, Delay, and Response periods for each memory load. | Dorsal (BA 9 & 46) and ventral (BA 44, 45, & 47) prefrontal cortex (anatomically defined) | O = Y in relationship with load during all periods in dorsal and ventral PFC (Dorsal: increase with load in both groups in Delay period only; Ventral: decrease with load during Encoding in both groups, increase with load during Delay and Response in both groups) | Composite performance score of accuracy and reaction time (both Y and O) using z-scores; Y: range = 1.58 to −1.46; O: range = 2.49 to −2.94 |
Y:
NEG correlation with performance, O:
POS correlation with performance during Encoding and Response for dorsal PFC; during Encoding and late-Delay for ventral PFC ( ). Encoding differences mainly due to accuracy; Response differences mainly due to RT. ). Encoding differences mainly due to accuracy; Response differences mainly due to RT. |
| Stern et al., 2005, Brain networks associated with cognitive reserve in healthy young and old adults | Y: 20, O: 17 | Y: mean age = 23.4 8M:12F, mean years education = 15.7; O: mean age = 70.9, 7M:10M, mean years education = 15 | No medical, neurological, or psychiatric conditions. No cognitive impairment or dementia. | PET | Continuous recognition task for nonverbal shapes with list length of one (easy condition) or titrated length so that performance was at 75% accuracy (hard condition); Contrast(s): change in network activation from the easy encoding/recognition to the hard encoding/recognition conditions. | Whole brain covariation analysis. | O > Y: R hippocampus, posterior insula, thalamus, and B operculum (O show increases with task difficulty and Y show decreases); Y > O: R lingual gyrus, inferior parietal lobe, L posterior cingulate, B calcarine cortex (Y show increases with task difficulty and O show decreases.) | Cognitive reserve factor score from years of education and performance on the New Adult Reading Test and Vocabulary subtest of the WAIS-R. |
Y:
POS correlation of cognitive reserve with brain response within the regions that differed by age; O:
NEG correlation ( ) ) |
| Tessitore et al, 2005, Functional changes in the activity of brain regions underlying emotion processing in the elderly | Y: 12, O: 15 | Y: mean age = 25, 6M:6F, age range = 20–29; O: mean age = 67, 8M:7F, age range = 60–80 | No history of neurological, psychiatric, or medical problems. No current medication except birth control and hormone replacement. | fMRI | Emotional facial expression matching (angry or afraid). Contrast(s): Emotion matching vs. shape matching | Whole brain analysis | Y > O: R amygdala, B posterior fusiform; O > Y: B ventral and L medial prefrontal | Reaction time and accuracy |
Y:
POS correlations of RT with L dorsal prefrontal, B posterior fusiform; O:
POS correlations of RT with L dorsal and medial prefrontal and R inferior occipital ( ) ) |
| Persson et al., 2006, Structure-function correlates of cognitive decline in aging | O: 40 (Declining: 20, Stable: 20 | Declining: mean age = 65.3, 7M:13F, mean years education = 10.4; Stable: mean age = 66.0, 7M:13F, mean years education = 10.0 | No reported neurological problems; MMSE ≥ 25; no group differences in vascular risk factors | fMRI | Incidental encoding of words during a categorization task (abstract vs. concrete). Contrast(s): encoding vs. fixation | Regions of interest based on activation in both groups: L dorsal frontal, L inferior frontal, R dorsal frontal, R ventral frontal | None reported | Stable: unchanging performance on 3 memory tasks over 10 year period (3 evaluations), Declining: sig. decline at both evaluations, ending at a sig. lower memory performance (normal range) |
Declining > Stable ( ): R ventral prefrontal ): R ventral prefrontal |
| Rabbitt et al, 2006, Losses in gross brain volume and cerebral blood flow account for age related difference in speed but not in fluid intelligence | N = 69 | O: 29M:40F, male mean age = 72.7, female mean age = 73.1 | Absence of diabetes, hypertension, or neurological conditions, absence of MRI abnormalities | MRI measure of arterial blood flow | Rest | Total blood flow into the brain, not regional | Not given | Performance on 10 neuropsychological tests |
POS correlation of CBF to performance on 8 out of 10 tests. ( ) After controlling for age within this elderly group, only 3 tasks (two speed and one executive) were still related ) After controlling for age within this elderly group, only 3 tasks (two speed and one executive) were still related |
| Van der Veen, Frouke et al., 2006, Effects of aging on recognition of intentionally and incidentally stored words: An fMRI study | Y: 12, O: 12 | Y: mean age = 25.1, 12M:0F, age range = 23–27; O: mean age = 64.7, 12M:0F, age range = 63–67, subjects matched for level of education | No history of medical, neurological or psychiatric illness, current use of psychoactive medication, diastolic BP> 95, use of recreational drugs or excessive alcohol, no history of claustrophobia. | fMRI | Verbal episodic retrieval for intentionally-encoded, incidentally-encoded, and novel words. Contrast(s): event-related analysis of correctly recognized vs. correctly rejected (succesful retrieval); correctly recognized intentionally- vs. incidentally-encoded words. | Whole brain analysis and ROIs based on previous studies. | Correctly recognized vs. correctly rejected: O > Y: Whole brain analysis: B medial prefrontal gyrus; ROI analysis: 2 clusters R parahippocampal gyrus; Incidental vs Intentional: Y > O: Whole brain analysis: middle occipital gyrus (Y: incidental > intentional, O: intentional > incidental | Percentage correctly recognized items |
O:
POS correlations with accuracy ( ) in L parahippocampal gyrus and L lingual gyrus (from whole brain analysis) and B parahippocampal gyrus (from ROI analysis). NEG correlations with accuracy ( ) in L parahippocampal gyrus and L lingual gyrus (from whole brain analysis) and B parahippocampal gyrus (from ROI analysis). NEG correlations with accuracy ( ) in B posterior cingulate gyrus and B inferior parietal gyrus. Y: no significant correlations. ) in B posterior cingulate gyrus and B inferior parietal gyrus. Y: no significant correlations. |
| Bernard et al., 2007, Neural correlates of age-related verbal episodic memory decline: A PET study with combined subtraction/correlation analysis | Y: 12, O: 12 | Y: mean age = 22.5, 6M:6F; O: mean age = 59, age range = 55–63, 6M:6F (O < Y for years of education) | No medical, psychiatric, or neurological disorders; unmedicated; no memory complaint, no abnormalities on MRI imaging. | PET | Intentional verbal encoding and reading task, both with living/nonliving judgment; Stem-cued recall task for the intentionally-encoded words and stem-completion task. Contrast(s): encoding vs. reading; cued-recall vs stem-completion | Whole brain analysis | Encoding: no significant differences; Recall: no significant differences | Cued recall accuracy |
Y:
POS correlation with accuracy in left parahippocampal gyrus; O:
POS correlations with accuracy ( ) in R inferior frontal gyrus, L hippocampus; O > Y: R inferior frontal gyrus ) in R inferior frontal gyrus, L hippocampus; O > Y: R inferior frontal gyrus |
| Brassen et al., 2007, Structure–function interactions of correct retrieval in healthy elderly women | Y: 14, O: 14 | Y: mean age = 25.6, age range = 21–33, 0M:14F; O: mean age = 64.9, age range = 60–71, 0M:14F | No history of neurological or psychiatric illness; normal blood pressure; no medications or conditions that could affect CBF; normal values (no less than 1SD below mean) on neuropsychological battery and normal MMSE. | fMRI | Episodic encoding and retrieval of emotionally-valenced and neutral words. Contrast(s): correct recognition vs. incorrect recognition of neutral words. | Whole brain analysis. Group difference analysis masked by within group results. Success analysis only in regions of group effect. | Y > O: R superior frontal gyrus; O > Y: B middle temporal gyrus. | Amount of correctly recognized/rejected words |
O:
POS correlation with accuracy ( ) in L middle temporal gyrus (trend toward R middle temporal gyrus), Y: POS correlation with accuracy in R prefrontal cortex. ) in L middle temporal gyrus (trend toward R middle temporal gyrus), Y: POS correlation with accuracy in R prefrontal cortex. |
| Gutchess et al, 2007, Contextual interference in recognition memory with age | Y: 20, O: 20 | Y: mean age = 21.05, 10M:10F, age range = 18–28, mean years of education = 14.88; O: mean age = 68.10, 6M:14F, age range = 60–84, mean education = 14.97 | In good neurological, physical, and psychological health, free of medications or conditions affecting cerebral blood flow, at least 27 out of 30 on MMSE | fMRI | Recognition memory for intentionally-encoded objects with familiar or unfamiliar backgrounds. Contrast(s): rejection of novel objects in familiar contexts: new object / old background minus new object / new background for correct rejections | Whole brain and ROIs in prefrontal cortex; for performance associations, medial superior prefrontal and R middle frontal ROIs | Y > O: B dorsolateral prefrontal, L anterior middle frontal, anterior cingulate, posterior cingulate, L calcarine/lingual gyrus, L angular/middle temporal gyrus | Recognition discrimination scores (A'; both continuous and median split) | Poor Recognition O < Good Recognition O < Y in R dorsolateral prefrontal; Poor Recognition O < Good Recognition O = Y in medial and right middle frontal gyrus ( ); Correlations: Y: No relationship; O: positive correlation in B dorsolateral prefrontal ( ); Correlations: Y: No relationship; O: positive correlation in B dorsolateral prefrontal ( ) ) |
| Langenecker et al., 2007, An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: A study in the basal ganglia | Y: 11, O: 11 (same as Langenecker et al., 2003) | Y: mean age = 28.1, age range = 25–32, 4M:7F, mean years education = 17; O: mean age = 72.8, age range = 67–77, 3M:8F, mean years education = 18 | No history of psychiatric, neurological or other medical factors influencing cognition; MMSE > 27, Geriatric Depression Scale < 9, O had normal performance for age on a neuropsychological battery. | fMRI | Go/No Go task in which response is made to alternating stimuli (X or Y) thus requiring inhibition of response to previously correct stimulus. Contrast: correct targets vs. all others. | Caudate and putamen / globus pallidus. | Y > O: caudate, O > Y: putamen/globus pallidus. | Y and O split into 2 groups based on median percent correct target trials. |
O: poor performers > good performers ( ) in B putamen/globus pallidus; Y: poor performers > good performers in L putamen/globus pallidus and R caudate. ) in B putamen/globus pallidus; Y: poor performers > good performers in L putamen/globus pallidus and R caudate. |
| Madden, Spaniol et al., 2007, Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study | Y: 16, O: 16 | Y: mean age = 23.4, 8F:8M, age range = 19–28, mean years education: 16.7; O: mean age = 67.0, 8F:8M, age range = 60–82, mean years education = 17.5 | No significant health problems or medication known to affect cognitive functioning or cerebral blood flow reported on screening questionnaire. MMSE ≥ 27, and Beck Depression Inventory ≤ 9. T2 MRI images judged to be free of significant abnormalities. | fMRI (and DTI) | Visual search task with two conditions: Guided (target had 3/4 chance of being uniquely colored compared to 3 distractors) or Neutral (target had 1/4 chance of being uniquely colored); duration of display greater for O than Y; Contrast(s): area under curve of percentage signal change (for 12s after trial onset compared to the 6s before trial onset) for each condition (Guided or Neutral) and trial type (target uniquely colored or not). | 13 bilateral cortical and subcortical regions of interest: | O > Y in frontal (frontal eye fields, middle frontal gyrus) and parietal (angular and supramarginal gyri and superior parietal lobule) ROIs regardless of condition or trial type. | Target type effect on reaction time (percentage difference in RT between targets that were uniquely colored vs. not). | Guided condition: O > Y: more POS correlation in in frontal eye fields and superior parietal lobule ( ); Y > O: more POS correlation in fusiform gyrus (SCA = NCA). ); Y > O: more POS correlation in fusiform gyrus (SCA = NCA). |
| Rypma, Eldreth et al., 2007, Age-related differences in activation-performance relations in delayed-response tasks: A multiple-component analysis | Y: 8, O: 6 | Y: mean age = 19.5, 4M:4F, age range = 19–26, mean years education = 16.0; O: mean age = 70.2, 3M:3F, age range = 55–83, mean years education = 14.5 | No age-related disorders such as hypertension, or any other medical, neurological, or psychiatric disorder based on neurologist screening of histories; no use of prescription medication, MMSE >26, and BDI < 10. | fMRI | Delayed response task: encoding, maintenance, and retrieval of sets of letters that varied in size between 1 and 8 letters. Contrast(s): Encoding vs. Baseline, Delay vs. Baseline, Response vs. Baseline. | Dorsal prefrontal cortex: middle and superior frontal gyri (BA 9 & 46) and Ventral prefrontal cortex: inferior frontal gyrus (BA 44,45, & 47). | None reported. | Reaction time (RT) and accuracy across memory loads. | Dorsal PFC: Encoding:
Y = non-significant NEG correlation with accuracy; O = POS correlation ( ) with accuracy; Retrieval: Y = POS correlation with RT; O = POS correlation with accuracy ( ) with accuracy; Retrieval: Y = POS correlation with RT; O = POS correlation with accuracy ( ), non-significant NEG correlation with RT ( ), non-significant NEG correlation with RT ( ). Ventral PFC: Encoding: O = POS correlation ( ). Ventral PFC: Encoding: O = POS correlation ( ) with accuracy; Delay: Y = NEG correlation with accuracy; Retrieval: O = POS correlation with RT ( ) with accuracy; Delay: Y = NEG correlation with accuracy; Retrieval: O = POS correlation with RT ( ). ). |
| Zarahn et al., 2007, Age-related changes in brain activation during a delayed item recognition task | Y: 40, O: 18 | Y: mean age = 25.1, 31M:9F, mean years education = 15.7; O: mean age = 74.4, 7M:11F, mean years education = 15.3 | No psychiatric or neurological illness, non-demented | fMRI | Delayed response task: encoding, maintenance, and retrieval of sets of letters that varied in size (1,3,6 letters). Contrast(s): Load-dependent and load-independent changes during encoding, delay, and response | Whole brain covariation analysis. | Age effects only for load-dependent changes in brain response during the delay period: O > Y for two spatial networks, one including left premotor cortex and one including right parahippocampal gyrus | Discriminability at set size 6; 1/RT slope, and 1/RT intercept |
O:
NEG correlation between RT slope and expression of elderly-specific latent factor that included parahippocampus ( ); O > Y: More NEG correlation of load-dependent encoding response and both discriminability and RT slope, more NEG correlation of load-independent delay response and both RT slope and RT intercept ( ); O > Y: More NEG correlation of load-dependent encoding response and both discriminability and RT slope, more NEG correlation of load-independent delay response and both RT slope and RT intercept ( ) ) |
| Beason-Held et al., 2008, I. Longitudinal changes in aging brain function | O: 25 (8 year longitudinal study) | O: mean age at baseline = 67.8, 15M: 10F, mean years education = 17.3 | No history of central nervous system, major psychiatric, or severe cardiovascular disorder; deemed cognitively normal at 9 year follow-up. | PET | Recognition memory for intentionally-encoded words or figures and a rest condition. Contrast(s): Year9 vs. Year1 for each condition separately; conjunction of similar Year 9 vs. Year 1 effects for all tasks; Year 9 vs. Year 1 effect for each condition vs. both others. | Whole brain analysis | Common to all conditions: longitudinal decreases in R superior and medial frontal, superior and insular temporal, anterior and middle cingulate, thalamus, and caudate; increases in B superior and R prefrontal white matter, posterior L hippocampus, inferior parietal, middle occipital gyrus, and putamen. | Change in accuracy (sensitivity) over 8 years. |
O: Verbal memory: POS correlation of accuracy change with blood flow change ( ) in inferior temporal and inferior parietal (greater decrease in flow related to greater decrease in performance), pre/postcentral gyrus (increased performance related to increased flow), NEG correlation of accuracy change with blood flow change ( ) in inferior temporal and inferior parietal (greater decrease in flow related to greater decrease in performance), pre/postcentral gyrus (increased performance related to increased flow), NEG correlation of accuracy change with blood flow change ( ) in superior temporal gyrus (increased performance related to decreased flow), NEG correlation with RT change ( ) in superior temporal gyrus (increased performance related to decreased flow), NEG correlation with RT change ( ) in parahippocampal gyrus. Figural memory: NEG correlation with accuracy ( ) in parahippocampal gyrus. Figural memory: NEG correlation with accuracy ( ) in precentral gyrus, NEG correlation with RT change ( ) in precentral gyrus, NEG correlation with RT change ( ) in insula, and superior and middle temporal gyri, POS correlation with RT ( ) in insula, and superior and middle temporal gyri, POS correlation with RT ( ) in middle frontal gyrus. ) in middle frontal gyrus. |
| Heuninckx et al., 2008, Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons | Y: 12, O: 26 | Y: mean age = 22.4, 6M:6F, age range = 20–25; O: mean age = 65.7, 14M:12F, age range = 62–72 | No history of neurological disease or use of psychoactive or vasoactive medication. MMSE ≥ 26. | fMRI | Paced (slower for O), cyclical hand and foot movements that were in same direction (isodirectional: both flexed to both extended) or opposite directions (nonisodirectional:hand flexed/foot extended to hand extended/foot flexed) and rest. Contrast(s): isodirectional vs. rest; nonisodirectional vs. rest. | Whole brain analysis | Isodirectional: O > Y in L anterior insular cortex; Nonisodirectional: O > Y in L anterior insula, frontal gyrus pars opercularis, inferior frontal gyrus pars triangularis, middle frontal gyrus, superior frontal sulcus and gyrus, superior temporal gyrus, angular gyrus, superior parietal gyrus, fusiform gyrus, and inferior post central gyrus, R paracentral lobule, parahippocampal gyrus, posterior cerebellum, B lingual gyrus, anterior cerebellum. | Coordination accuracy (inverse of phase error between hand and foot). | Isodirectional: O:
POS correlations ( ) with coordination in regions where Y = O in activation: L precentral and postcentral gyri, trend in R anterior cerebellum, Y: no correlations. Nonisodirectional: O:
POS correlations ( ) with coordination in regions where Y = O in activation: L precentral and postcentral gyri, trend in R anterior cerebellum, Y: no correlations. Nonisodirectional: O:
POS correlations ( ) in regions where Y = O in activation: L superior postcentral gyrus/sulcus, inferior postcentral gyrus, R supplementary motor area and L SMA, L cingulate motor area, and in regions where Y > O in activation: L inferior frontal gyrus pars opercularis and pars triangularis, anterior insula, superior parietal gyrus, superior frontal sulcus and gyrus, middle frontal gyrus, anterior cerebellum and R posterior cerebellum; Y: no correlations. ) in regions where Y = O in activation: L superior postcentral gyrus/sulcus, inferior postcentral gyrus, R supplementary motor area and L SMA, L cingulate motor area, and in regions where Y > O in activation: L inferior frontal gyrus pars opercularis and pars triangularis, anterior insula, superior parietal gyrus, superior frontal sulcus and gyrus, middle frontal gyrus, anterior cerebellum and R posterior cerebellum; Y: no correlations. |
| Rajah & McIntosh, 2008, Age-related differences in brain activity during verbal recency memory | Y: 8, O: 8 | Y: mean age = 25.6, 1M:7F, age range = 21–35; O: mean age = 72.7, 6M:2F, age range = 62–80 | No medical, neurological, or psychiatric disorders, all MMSE scores greater than 27 out of 30 | fMRI | Recognition or recency judgments for intentionally encoded lists of words with or without semantic associations to each other compared to a baseline alphabetizing task. Contrast(s): Partial Least Squares analysis of Retrieval vs. Alphabetizing, Recency vs. Recognition, Recency and Alphabetizing vs Recognition, and Difficult vs. Easy | Whole brain; success analysis only in regions of significant age effects | O > Y: R parahippocampus, R parietal, L precuneus, R prefrontal for Recency and Recognition | Accuracy and reaction time separately for recognition and recency judgments | Recognition: O:
POS correlation with accuracy and NEG with RT in R prefrontal regions ( ); NEG correlation with accuracy and POS correlation with RT in R parahippocampus ( ); NEG correlation with accuracy and POS correlation with RT in R parahippocampus ( ); Y:
NEG correlation with accuracy and POS correlation with RT in R prefrontal; NEG correlation with accuracy and NEG correlation with RT in R parahippocampus. Recency: O: no correlation with accuracy (SCA=NCA) and NEG correlation with RT in R prefrontal regions ( ); Y:
NEG correlation with accuracy and POS correlation with RT in R prefrontal; NEG correlation with accuracy and NEG correlation with RT in R parahippocampus. Recency: O: no correlation with accuracy (SCA=NCA) and NEG correlation with RT in R prefrontal regions ( ); NEG correlation with accuracy and POS correlation with RT in R parahippocampal ( ); NEG correlation with accuracy and POS correlation with RT in R parahippocampal ( ); Y:
POS correlation with accuracy and NEG with RT in R parahippocampal; NEG correlation with accuracy and POS correlation with RT in R prefrontal (in BA 9) ); Y:
POS correlation with accuracy and NEG with RT in R parahippocampal; NEG correlation with accuracy and POS correlation with RT in R prefrontal (in BA 9) |
| Stern et al., 2008, A common neural network for cognitive reserve in verbal and object workiing memory in young but not old. | Letter task: Y: 40, O: 18; Shape task: Y: 24, O: 21 | Letter task: Y: mean age = 25.1, 30M:10F, mean years education: 15.7; O: mean age = 74.4, 7M:11F, mean years education = 15.3. Shape task: Y: mean age = 24.0; 12M:12F; mean years education = 14.7; O: mean age = 75.8, 11M:10F, mean years education = 15.8 | No neurologic or psychiatric illness; no dementia | fMRI | Delayed response task: encoding, maintenance, and retrieval of sets of letters or shapes that varied in size (1,3,6 letters; 1,2,3 shapes). Contrast(s): Load-related changes during encoding, delay, and response | Whole brain covariation analysis. | Not given | Cognitive reserve: equal weighting of WAIS-R Vocabulary and National Adult Reading Test |
Y: Letter and shape tasks during encoding, POS and NEG correlations of cognitive reserve with activity in a network including superior and medial frontal; in letter encoding only, NEG correlation of reserve with activity in a network including frontal, parietal, temporal, and basal ganglia; O: For letter encoding, POS correlation of cognitive reserve with activity in a network including frontal, parietal, temporal, and basal ganglia ( ); for letter retention, POS correlation with activity in a network including frontal, cingulate, and parietal; for letter retrieval ( ); for letter retention, POS correlation with activity in a network including frontal, cingulate, and parietal; for letter retrieval ( ), mix of NEG and POS correlations with regional activity in network including frontal and thalamus ( ), mix of NEG and POS correlations with regional activity in network including frontal and thalamus ( and and  ); No significant correlations with the task-independent network seen in Y, but tendency towards opposite correlations of cognitive reserve during shape task encoding phase. ); No significant correlations with the task-independent network seen in Y, but tendency towards opposite correlations of cognitive reserve during shape task encoding phase. |
| Wierenga et al., 2008, Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks | Y: 20, O: 20 | Y: mean age = 25.1, age range = 20–34, 10M:10F, mean years education = 15.85; O: mean age = 74.9, age range = 68–84, 10M:10F, mean years education = 15.65 | No history of neurological disease, dementia, mild cognitive impairment-amnestic type, cardiovascular disease, uncontrolled hypertension, psychiatric diagnosis; no psychoactive medications. | fMRI | Overt picture naming for animals, tools and vehicles and a passive viewing condition. Contrast(s): Event-related analysis of naming vs. passive viewing. | Whole brain analysis; success analysis only in frontal and subcortical regions. | O > Y: B rostral cingulate, R anterior cingulate, B posterior supplementary motor area, B motor cortex, R Broca's homologue, R inferior frontal gyrus, R sensorimotor cortex, B perisylvian cortex, and L lingual gyrus. | Naming accuracy and response latency; also subdivided into high accuracy (> 90%) and low accuracy (< 87%) due to a bimodal distribution. |
Y:
POS correlations with accuracy in L superior frontal gyrus, L middle frontal gyrus, L anterior cingulate, R neostriatum, NEG correlations in R inferior frontal and superior frontal gyrus; O:
POS correlations with accuracy ( ) in R thalamus, L lentiform nucleus, R inferior frontal gyrus, NEG correlations with accuracy ( ) in R thalamus, L lentiform nucleus, R inferior frontal gyrus, NEG correlations with accuracy ( ) in R precentral gyrus and L middle frontal gyrus, NEG correlations with response latency ( ) in R precentral gyrus and L middle frontal gyrus, NEG correlations with response latency ( ) in L neostriatum, L thalamus, B anterior superior frontal gyrus; High O: POS correlations with accuracy (VSCA > SCA) in B inferior frontal gyrus, R BA 45, B medial frontal gyrus, L superior frontal gyrus, B thalamus, L lentiform nucleus, NEG correlations (SCA > VSCA) in R superior frontal gyrus and R precentral gyrus; Low O: POS correlations ( ) in L neostriatum, L thalamus, B anterior superior frontal gyrus; High O: POS correlations with accuracy (VSCA > SCA) in B inferior frontal gyrus, R BA 45, B medial frontal gyrus, L superior frontal gyrus, B thalamus, L lentiform nucleus, NEG correlations (SCA > VSCA) in R superior frontal gyrus and R precentral gyrus; Low O: POS correlations ( ) in L inferior frontal, R thalamus, and lentiform nucleus, NEG correlations ( ) in L inferior frontal, R thalamus, and lentiform nucleus, NEG correlations ( ) in L insula, R inferior frontal ) in L insula, R inferior frontal |
NOTE: B=bilateral, BA=Brodmann’s area, BDI=Beck Depression Inventory, CNS=central nervous system, DTI=diffusion tensor imaging, ENC=encoding, F=female, fMRI=functional magnetic resonance imaging, GDS=Geriatric Depression Scale, ISI=inter-stimulus interval, L=left, M=male, MI=myocardial infarction, MMSE=Mini-Mental Status Exam, NCA=normal cognitive aging, NEG=negative, O=old, PET=positron emission tomography, POS=positive, R=right, rCBF=regional cerebral blood flow, RET=retrieval, ROI=region of interest, RT=reaction time, SCA=successful cognitive aging, SD=standard deviation, TIA=transient ischemic attack, WAIS-R=Wechsler Adult Intelligence Scale-Revised, Y =young.
Summary statistics for the reviewed studies are given in Table 2. The review included more studies using fMRI than PET, and sample sizes were typical of many functional neuroimaging investigations. A large majority of reports employed medical, neurological, and psychiatric screening criteria to assure a healthy sample, most also employed cognitive screening (e.g., Mini-Mental State Examination (26)), about half also excluded subjects based on current medication usage, and a smaller number had an additional criterion that the structural MRI be normal (21, 22, 27–40). Education level, when reported, was generally high (median four years of college) and comparable between younger and older groups. All studies had both male and female participants except for two that enrolled only men (41, 42) and one that included only women (43). Gender ratio was comparable between old and young groups in most reports. Participants’ ethnicity was not reported in any reviewed studies and so, could not be summarized.
Table 2.
Summary of Study Characteristics for Reports in the Review
| Study Characteristic | Summary | |||
|---|---|---|---|---|
| Sample Characteristic | Young Sample | Old Sample | ||
| Median | Range | Median | Range | |
| Number of Participants | 12 | 0–40 | 14 | 6–69 |
| Gender Ratio (Men/Women) | 1 (mean: 1.3) | 0–9 | 1 (mean: 0.97) | 0–3 |
| Mean Age (years) | 24 | 19.5–33.4 | 69.9 | 59–81.5 |
| Minimum Age (years) | 20 | 18–30 | 61 | 50–73 |
| Maximum Age (years) | 29 | 25–49 | 78 | 63–93 |
| Mean Education (years) | 16 | 14.5–17.8 | 16 | 10.2–19.3 |
| Image Modality (no. of studies) | 28 fMRI, 19 PET | |||
| Exclusion Criteria (no. of studies)* | 45 neurological, 37 psychiatric, 36 medical, 35 cognitive, 25 medications, 16 radiological abnormalities | |||
| Challenge Task (no. of studies)* | 1 rest, 21 episodic memory (phase: 13 encoding, 15 retrieval [method: 4 recall, 10 recognition]; stimuli: 13 verbal, 3 figural, 2 faces, 2 abstract), 8 working memory (stimuli: 7 verbal, 1 spatial, 1 facial), 5 inhibitory, 2 motor, 2 language, 2 visual search, 2 nondeclarative memory, 1 visual attention | |||
| Brain Regions Examined (no. of studies) * | 1 whole brain (single value), 28 voxel-wise with whole brain coverage, 24 region of interest | |||
| Measure of Cognitive Performance (no. of studies) * | 35 accuracy, 20 reaction time, 4 composite of accuracy and reaction time; 38 based on scanner task performance, 9 based on tasks given outside of scanner | |||
Note that the number of studies does not always add up to 47 across the categories because a study could be counted in more than one category.
The reviewed studies employed a variety of cognitive paradigms: episodic learning and memory tasks were most frequently used, followed by working memory tasks, and those assessing inhibitory processing. Less commonly examined domains included motor function, language, nondeclarative memory, visual search, and visual attention.
In terms of analysis strategies, age effects were often examined in a voxel-wise fashion across the entire brain. In many other studies, mean response in a priori regions of interest determined either by previous studies or by significant within-group differences were used in the age analysis. In many studies, brain-behavior correlations were only examined in the subset of regions in which an age difference in mean brain response was observed.
The cognitive performance variable examined differed among studies, but was most often accuracy of task performance, followed by mean reaction time. Most investigations used task performance during imaging as the measure of cognitive performance.
Correlation of Brain Function and Cognitive Performance
Greater brain response generally was associated with better cognitive performance in the reviewed studies: there was at least one finding of such an association in all but 6 of the 47 studies (25, 44–48)(See Table 3). In 19 studies, only positive associations between magnitude of brain response in the older group and cognitive performance were found; 19 others found both positive and negative associations. Thus, across many different types of tasks, more cognitively successful seniors had greater brain response in at least one brain region compared to their lower-performing peers. Positive correlations, when observed, were frequently found in frontal lobe regions and negative correlations were rare in this region: 19 studies reporting correlations within the frontal lobes found positive associations, 6 found negative correlations, and 9 found some evidence for both positive and negative associations. Findings within parietal and temporal cortex were similarly skewed in favor of positive associations. In occipital cortex and in hippocampal / medial temporal cortex, an equal number of studies reported each direction of correlation with performance. Among the 22 studies in which the analysis could have revealed brain-behavior correlations in any region (i.e., analysis was not restricted to particular areas), there were 9 studies that found positive associations in frontal cortex (one negative); for all other regions, positive correlations were observed in 5 studies or fewer. Thus, positive associations were more commonly found in frontal cortex than in other regions even among studies in which there was a chance of finding such correlations in any brain region.
Table 3.
Summary of Brain-Behavior Relationships in the Reviewed Studies
| Brain Response | |||
|---|---|---|---|
| Successful Performers > Normal Performers |
Normal Performers > Successful Performers |
Mixed Findings |
|
| Any region | 38 | 25 | 19 |
| All examined regions* | 19 | 6 | 19 |
| Frontal cortex | 19 | 6 | 9 |
| Temporal cortex | 7 | 3 | 5 |
| Parietal cortex | 10 | 4 | 2 |
| Occipital cortex | 6 | 6 | 2 |
| Hippocampal / Medial Temporal | 5 | 5 | 0 |
| Basal ganglia | 4 | 2 | 0 |
| Thalamus | 2 | 4 | 1 |
| Posterior cingulate | 3 | 1 | 0 |
| Cerebellum | 3 | 1 | 0 |
Six investigations found only negative correlations (i.e., greater brain response in poorer performing elders); these involved reaction time to incongruent vs. congruent visual distracters (44), go/no-go task accuracy evaluated across the whole brain (25) and in the basal ganglia (45) (participants overlapped between these studies), relationship of cognitive reserve to age-differential networks during learning and recognition of difficult versus easy word lists (46), reaction time during a facial emotion matching task (47), and relationship of reaction time slope and discriminability to load-dependent and load-independent brain response in spatial networks responsive during different phases of a working memory task (48).
Given known differences in cognitive and brain aging patterns between men and women (49, 50), we examined whether gender ratio in the older study group appeared to be related to the direction of associations between brain function and cognitive performance. We divided studies into three categories based on whether the older sample was predominantly male (>67% men; n=10), predominantly female (>67% women; n=10), or gender-balanced (≤67% men or women; n=26). Studies with greater or equal numbers of women in the older sample were slightly more likely to observe positive compared to negative correlations (predominantly female: 70% positive vs. 30% negative, gender-balanced: 73% positive vs. 50% negative), whereas those with more men had mixed findings (100% positive vs. 70% negative).
Two studies had a longitudinal component, permitting inferences about the temporal relationship between changes in cognitive performance and changes in brain function. In a study with repeated assessment of both cognition and brain function (19), older adults were assessed at baseline and after 8 years with PET imaging during a recognition memory test. Overall cognitive performance did not change significantly in this sample, but longitudinal increases and decreases of brain activity were observed. Positive relationships between brain response changes and performance changes were seen in temporal and parietal regions; and negative associations were found in temporal and frontal regions. The findings suggest some degree of reorganization of cortical networks of older individuals in the context of preserved cognitive performance, with both beneficial and detrimental regional changes observed. In a retrospective design in which cognition, but not brain response, was measured longitudinally, Persson et al (20), compared measures of brain response between two groups of individuals – those who had stable memory performance over the previous decade and those who had declined (although none had declined out of the normal range). Brain response during verbal encoding was measured with fMRI, and analyses of brain-behavior relationships were conducted within frontal regions activated by both groups. Greater response was found in right ventral prefrontal cortex among individuals whose memory performance had declined compared to those whose performance had remained stable.
Uniqueness of Brain-Behavior Correlation Pattern in Older Individuals
Thirty-three studies included a young comparison group in which the relationship between brain response and cognitive performance was also examined. Many studies found strong correlations in both younger and older groups. Most identified some brain regions in which similar brain-behavior correlations were observed in both age groups, but many also reported regions in which correlations were significant only in one group. An interesting category of finding was the observation of brain regions in which the relationship between performance and brain response was opposite in direction in the two age groups. In 13 studies (40%), the correlation with performance was much more strongly positive in the old than in the young group, and in many cases the relationship was negative in younger individuals (30, 31, 51–60). In contrast, positive correlations among the young group and negative associations among the old also were observed in some studies; this was somewhat more likely to be the case in posterior and medial temporal brain regions (30, 31, 35, 46, 48, 54, 55, 57, 59, 60). In a small number of studies (32, 41, 61, 62), brain-behavior correlations were much more pronounced in the old group than the young, but the opposite pattern (no correlations in old and multiple significant correlations in young) was not seen in any study.
Relationship of Brain-Behavior Correlations to Nature of Age-Related Changes
An aim of this review was to understand whether older individuals who display qualitatively different patterns of brain response perform better than those with a more youthful pattern. Although few studies examined this directly (48, 58), we were able to classify age effects into those that were broadly supportive of HAROLD and PASA and then examine the direction of brain-behavior associations in these studies. We found 14 studies in which group aging results were broadly consistent with either HAROLD (n=7) or PASA (n=6) or both (n=1). Of these, 10 studies reported positive correlations between brain function and cognitive performance in some region and 7 reported negative correlations in some region. Six studies found positive correlations in frontal cortex (4 found negative correlations) and no other region had more than 3 reports of significant function-cognition relationships, either positive or negative. Thus, studies that demonstrated altered brain patterns in older individuals consistent with HAROLD and PASA models were slightly more likely to find these changes to be beneficial rather than detrimental, but findings of negative relationships were also prevalent.
Discussion
In our review of studies addressing how brain function relates to cognitive performance among older individuals, we found frequent support for the notion that increased brain responsiveness is associated with better cognitive performance. Positive correlations between frontal cortex brain response and performance were much more frequently reported than negative correlations, and positive associations were more often observed in frontal cortex than in other regions. Positive correlations also outnumbered negative ones in parietal and temporal cortices, whereas findings were more mixed for both occipital cortex and medial temporal lobe. The predominance of positive correlations in frontal cortex is consistent with findings of our review of the relationship between brain structure and cognitive performance in late-life, in which frontal findings were also the most numerous and consistent (9). Interestingly, although greater size of medial temporal lobe was also fairly consistently linked to greater cognitive success (9), the directionality of relationships between brain function in this region and cognitive performance was mixed. This suggests that a simple model of: bigger structure → greater brain response → better cognitive performance may not be accurate, and points out the necessity to measure both brain structure and function in the same study in order to test more complete models.
Positive correlations with performance, particularly in frontal cortex, have previously been interpreted as compensatory in nature (13). Specifically, it has been theorized that frontal cortex must become more active in order to support good performance in the face of deficient responses elsewhere (63). Although many studies observed positive correlations with performance, most did not directly test how this might be related to deficient brain response in other regions. It was also not always clear how performance of the highest performing seniors compared to that of high performing younger adults. Thus, it was difficult to know whether brain response in regions of positive correlation with cognitive performance was truly compensatory or, instead, represented an attempt at compensation that was only partially successful. It was also difficult to evaluate whether there was a benefit to qualitatively different patterns of activation as opposed to quantitative differences in response in the same brain regions (48). Among studies that demonstrated a group effect of age consistent with HAROLD or PASA, positive associations between brain response in these regions were more frequently observed than negative ones. Mixed results were found in two studies examining this question more directly using working memory tasks; Reuter-Lorenz et al (58) found that older adults with a more bilateral activation pattern performed more quickly, but Zarahn et al (48) found only one qualitatively different pattern of brain response among older adults, which was associated with worse performance. Clearly, further work is needed to develop techniques to classify the pattern of brain activity among individual participants and examine cognitive consequences of those patterns.
The exceptions to findings of positive correlations between brain response level and cognitive performance, perhaps suggestive of de-differentiated responses, are potentially illuminating. Most notable is a quasi-longitudinal study in which individuals with the greatest cognitive decline had the most subsequent frontal overactivity (20). If excess frontal response is compensatory, one might expect to observe it most in individuals who maintain good cognitive performance. However, as the authors point out, it could be that extra brain response in frontal cortex is only recruited when other systems begin to fail and that decliners’ brains were attempting a reorganization (in response to hippocampal and white matter deficits observed in this group) while those who were stable did not require it (yet). Of the six cross-sectional studies that found only negative associations between brain response and cognitive performance, three involved inhibitory processing and/or overcoming prepotent responses. Frontal overactivity may be detrimental for this particular type of cognitive activity. Another pattern that emerged was one of negative correlations with performance in posterior regions but positive correlations in anterior regions. This appears to be consistent with the concept that individuals who show the PASA pattern have the best cognitive performance. Still, if one postulates that anterior shift occurs in response to loss of posterior function, it is perhaps surprising that those with the most residual posterior function would have the worst cognitive performance. Perhaps earlier sensory processes require a more focal response that, once weakened in the course of aging, cannot be compensated for by greater posterior activation. This might suggest that only those individuals who can strengthen and broaden their anterior response and reciprocally quiet their posterior response can continue to perform at a high level.
The above discussion highlights the largest gap in our knowledge regarding the link between brain function and cognition in old age – i.e., how it may change along the course of development. Many theories posit a dynamic process (e.g., (63, 64)), in which functional increases occur in response to structural changes that decrease focal responses elsewhere. Direct evidence addressing this dynamic process is extremely limited. Only one study measured both brain function and cognitive performance longitudinally (19). Results of this study were mixed and cognitive changes were minimal, so we do not yet know how the brain may reorganize itself in people who maintain cognitive performance levels versus those that decline. The reviewed studies comparing brain-behavior associations among younger versus older samples generally supported the notion that the association strengthens and becomes more positive with age. Although this may sometimes have resulted from restricted variability in the younger sample leading to lower correlations, in some reports there were significant negative correlations in the young group and significant positive correlations in the old group. This suggests that a switch may occur at some point in adulthood so that more brain response is better instead of worse. This could perhaps be consistent with antagonistic pleiotropy in which effects of certain genes are detrimental at one point in life but beneficial at another (65). When and how such a switch may happen is currently unknown. Longitudinal functional neuroimaging, in combination with genetic studies, may be a particularly powerful combination for better understanding individual trajectories of neural aging (66).
The finding that additional brain response is generally positively associated with cognitive success in old age emphasizes the existence of continued functional plasticity which should lead to optimism about improving cognitive function in old age. Cognitive training is one type of intervention that is gaining in popularity and evidence for efficacy (67). Changes in brain function after cognitive training may result in a more youthful, unilateral pattern of brain response rather than a strengthening of the HAROLD-like response of untrained seniors (68), however. This raises the possibility that some interventions might be restorative for brain functioning, while others may result in patterns that more closely resemble the brain’s naturalistic compensatory changes.
There are several limitations to this review, including that, in spite of our best efforts, we might have missed some relevant papers on this topic. Also, as with any review, there may have been publication biases that influenced the type of results available to include. In addition, our review only focused on functional correlates of successful cognitive performance and did not address other aspects that may contribute to quality of life in old age, such as emotional well-being (69). Although functional neuroimaging has been applied to understanding emotional disorders in late life (70, 71), few studies have addressed brain correlates of individual differences in emotional outlook among healthy seniors (72).
The conclusions we drew could also be influenced by limitations of the reviewed papers. The studies’ sample sizes, although typical for neuroimaging investigations, were relatively small for examining correlations. Study participants were highly educated, which could limit generalizability of results. Also, studies varied greatly in terms of their selection criteria, cognitive tests administered, imaging techniques used, and analysis methods employed, making it difficult to summarize findings across studies. As discussed above, very few studies examined cognitive correlates of coordinated brain networks and many did not statistically compare correlations between young and old groups. Finally, few studies directly addressed the concern that observed brain response-behavior correlations may be accounted for partially or wholly by associations between cerebrovascular function and cognition (73). Future studies that measure blood flow, metabolic rate of oxygen, and blood oxygenation dependent response in both younger and older participants may help determine the relative contribution of vascular versus neuronal components to individual differences in cognitive performance, and how these may change with age.
In summary, results of this review offer support for the view that augmented brain response, particularly in frontal cortex, serves a compensatory role in older adults. In some cases, however, extraneous activation might be detrimental, such as when it occurs in posterior regions or during tasks that require inhibitory processes. The existing literature does not fully address the important question of how such relationships are built up or may change across adulthood, although there is some suggestion that positive brain function-cognition relationships strengthen with age. Whether there is eventually a point at which compensation fails and how the timing of such changes might relate to other genetic or environmental factors will be a productive area for further exploration.
Acknowledgements
This work was supported, in part, by the Sam and Rose Stein Institute for Research on Aging, by grants from the National Institute of Mental Health (P30-MH080002), and by the Department of Veterans Affairs.
AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Janssen donate medication for an NIMH funded research grant awarded to Dr. Jeste entitled “Metabolic Effects of Newer Antipsychotics in Older Patients.”
Footnotes
Financial Disclosures
The authors have no specific financial interests, relationships, or additional affiliations relevant to the subject of this manuscript.
References
- 1.Reichstadt J, Depp CA, Palinkas LA, Folsom DP, Jeste DV. Building blocks of successful aging: a focus group study of older adults' perceived contributors to successful aging. Am J Geriatr Psychiatry. 2007;15:194–201. doi: 10.1097/JGP.0b013e318030255f. [DOI] [PubMed] [Google Scholar]
- 2.Baltes MM, Wahl HW, Schmid-Furstoss U. The daily life of elderly Germans: activity patterns, personal control, and functional health. J Gerontol. 1990;45:P173–P179. doi: 10.1093/geronj/45.4.p173. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 4.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 5.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 7.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. doi: 10.1176/appi.neuropsych.23.1.6. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meltzer CC, Becker JT, Price JC, Moses-Kolko E. Positron emission tomography imaging of the aging brain. Neuroimaging Clin N Am. 2003;13:759–767. doi: 10.1016/s1052-5149(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 11.Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd ed. New York: Psychology Press; 2008. pp. 1–54. [Google Scholar]
- 12.Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 15.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 18.Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 19.Beason-Held LL, Kraut MA, Resnick SM. I. Longitudinal changes in aging brain function. Neurobiol Aging. 2008;29:483–496. doi: 10.1016/j.neurobiolaging.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- 21.Gron G, Bittner D, Schmitz B, Wunderlich AP, Tomczak R, Riepe MW. Variability in memory performance in aged healthy individuals: an fMRI study. Neurobiol Aging. 2003;24:453–462. doi: 10.1016/s0197-4580(02)00128-8. [DOI] [PubMed] [Google Scholar]
- 22.Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, et al. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. [DOI] [PubMed] [Google Scholar]
- 23.Hazlett EA, Buchsbaum MS, Mohs RC, Spiegel-Cohen J, Wei TC, Azueta R, et al. Age-related shift in brain region activity during successful memory performance. Neurobiol Aging. 1998;19:437–445. doi: 10.1016/s0197-4580(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 24.Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- 25.Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12 doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Bernard FA, Desgranges B, Eustache F, Baron JC. Neural correlates of age-related verbal episodic memory decline: a PET study with combined subtraction/correlation analysis. Neurobiol Aging. 2007;28:1568–1576. doi: 10.1016/j.neurobiolaging.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 29.Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- 30.Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- 31.Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- 33.Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE. Selective and divided visual attention: Age-related changes in regional cerebral blood flow measured by H2 15O PET. Hum Brain Mapp. 1997;5:389–409. doi: 10.1002/(SICI)1097-0193(1997)5:6<389::AID-HBM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Madden DJ, Gottlob LR, Denny LL, Turkington TG, Provenzale JM, Hawk TC, et al. Aging and recognition memory: changes in regional cerebral blood flow associated with components of reaction time distributions. J Cogn Neurosci. 1999;11:511–520. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- 35.Madden DJ, Langley LK, Denny LL, Turkington TG, Provenzale JM, Hawk TC, et al. Adult age differences in visual word identification: functional neuroanatomy by positron emission tomography. Brain Cogn. 2002;49:297–321. doi: 10.1006/brcg.2001.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, et al. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, et al. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, et al. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- 40.Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, et al. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- 41.van der Veen FM, Nijhuis FA, Tisserand DJ, Backes WH, Jolles J. Effects of aging on recognition of intentionally and incidentally stored words: an fMRI study. Neuropsychologia. 2006;44:2477–2486. doi: 10.1016/j.neuropsychologia.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Reuter-Lorenz PA, Marshuetz C, Jonides J, Smith EE. Neurocognitive ageing of storage and executive processes. European Journal of Cognitive Psychology. 2001;13:257–278. [Google Scholar]
- 43.Brassen S, Buchel C, Weber-Fahr W, Lehmbeck JT, Sommer T, Braus DF. Structure-function interactions of correct retrieval in healthy elderly women. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- 45.Langenecker SA, Briceno EM, Hamid NM, Nielson KA. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Res. 2007;1135:58–68. doi: 10.1016/j.brainres.2006.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Res. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Rehman HU, Masson EA. Neuroendocrinology of female aging. Gend Med. 2005;2:41–56. doi: 10.1016/s1550-8579(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 50.O'Hara R, Miller E, Liao CP, Way N, Lin X, Hallmayer J. COMT genotype, gender and cognition in community-dwelling, older adults. Neurosci Lett. 2006;409:205–209. doi: 10.1016/j.neulet.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- 52.Rypma B, Berger JS, Genova HM, Rebbechi D, D'Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- 53.Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- 54.Rajah MN, McIntosh AR. Age-related differences in brain activity during verbal recency memory. Brain Res. 2008;1199:111–125. doi: 10.1016/j.brainres.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 55.Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- 57.McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, et al. Recruitment of unique neural systems to support visual memory in normal aging. Curr Biol. 1999;9:1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- 58.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 59.Scarmeas N, Zarahn E, Anderson KE, Hilton J, Flynn J, Van Heertum RL, et al. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. Neuroimage. 2003;19:1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJ, et al. Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiol Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Gutchess AH, Hebrank A, Sutton BP, Leshikar E, Chee MW, Tan JC, et al. Contextual interference in recognition memory with age. Neuroimage. 2007;35:1338–1347. doi: 10.1016/j.neuroimage.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- 65.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 66.Mattay VS, Goldberg TE, Sambataro F, Weinberger DR. Neurobiology of cognitive aging: insights from imaging genetics. Biol Psychol. 2008;79:9–22. doi: 10.1016/j.biopsycho.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimers Dement. 2009;5:50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, et al. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Eyler LT, Kovacevic S. Neuroimaging of successful cognitive and emotional aging. In: Jeste DV, Depp C, editors. Successful Cognitive and Emotional Aging. Washington, DC: American Psychiatric Publishing, Inc; 2010. [Google Scholar]
- 70.Smith GS. Neuroimaging in geriatric psychiatry. Am J Geriatr Psychiatry. 2004;12:551–553. doi: 10.1176/appi.ajgp.12.6.551. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A, Aizenstein H, Ballmaier M. Multimodal neuroimaging in late-life mental disorders: entering a more mature phase of clinical neuroscience research. Am J Geriatr Psychiatry. 2008;16:251–254. doi: 10.1097/JGP.0b013e31816721e9. [DOI] [PubMed] [Google Scholar]
- 72.Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J Cogn Neurosci. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer's disease. Neuropsychol Rev. 2007;17:127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- 75.Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, et al. Neural mechanisms underlying probabilistic category learning in normal aging. J Neurosci. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, et al. Functional neuroimaging indicators of successful executive control in the oldest old. Neuroimage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]