Abstract
Background/Aim
Tumor progression is influenced by the microenvironment. We found stem cells are recruited to malignant mesothelioma spheroids. We aimed to determine if stem cell recruitment depends on the chemokine SDF1, and if inhibition of the cognate receptor CXCR4 affects tumor growth.
Materials and Methods
The kinetics of stem cell recruitment was determined using immunofluorescence staining, BrdU incorporation and eGFP transgenic mice. Chemokines were identified using PCR array. Inhibitors of CXCR4 were used to determine the effect on cell migration and tumor progression.
Results
The increasing number of stem cells found in tumor spheroids over time is attributed to cell recruitment. Stem cell migration in vitro was enhanced by exogenous SDF1 and abrogated by CXCR4 inhibition and. CXCR4 inhibition reduced tumor burden in vivo.
Conclusion
SDF1 is a candidate chemokine for recruitment of stem cells to malignant peritoneal mesothelioma and a potential target for therapy.
Keywords and phrases: malignant mesothelioma, animal tumor model, tumor spheroids, mesenchymal stem cell, chemokine
INTRODUCTION
Diffuse malignant mesothelioma is an aggressive tumor of the mesothelial lining surrounding pleural, peritoneal, and pericardial cavities and tunica vaginalis of the testis. Malignant mesothelioma is responsible for approximately 15,000–20,000 deaths annually worldwide (1), and most patients die within 18 months of diagnosis, for the tumor is highly resistant to current therapies. Proliferating mesothelioma cells can detach from extracellular matrix and proliferate within the pleural or peritoneal cavities as free-floating tumor spheroids (2). Formation and growth of tumor spheroids along with fluid accumulation in the peritoneal cavity result in ascitic tumors typical of ovarian cancer, colon cancer, gastric cancer and diffuse malignant mesothelioma. Tumor spheroids have been found to be more chemoresistant than primary tumors, potentially explaining tumor recurrence after chemotherapy (3). After tumor spheroids grow to a critical size, they attach to serosal surfaces and are capable of co-option of blood vasculature, tissue invasion, and formation of secondary solid tumors (4).
Stromal cells such as cancer-associated fibroblasts and endothelial cells also contribute to tumor progression (5). Using the stem cell marker stem cell antigen-1 (Sca-1), we identified a novel host stromal cell type, stem-like progenitor cells, in malignant peritoneal mesothelioma spheroids. Sca-1 is used as a marker to enrich for tissue-resident and cancer stem cells. Sca-1 expression occurs in embryonic and fetal cells, T lymphocytes, hematopoietic stem cells, mesenchymal stem cells, rare Sca-1-expressing tumor cells, and often overlaps with the side population (SP) phenotype found in tissues and cancers (6). Sca-1 expression has been correlated with a more malignant phenotype in tumors (7). A variety of murine cancers, including retinoblastoma, mammary tumors and prostate tumors, exhibit upregulation of Sca-1 (8).
We hypothesized that the Sca-1-expressing cells found in malignant mesothelioma spheroids contain mesenchymal stem cells, a stromal adult stem cell population. Mesenchymal stem cells are recruited to sites of injury as well as to growing tumors and are potential targets for chemotherapeutic therapy or drug delivery. The most commonly expressed factor responsible for mesenchymal stem cell recruitment is the chemokine SDF1/CXCL12. There is a growing list of tumors that overexpress SDF1, including ovarian carcinoma, glioblastoma, pancreatic cancer, prostate cancer and thyroid cancer (9).
SDF1 binds to CXCR4, a 7-transmembrane G-protein coupled receptor expressed by vascular endothelial cells, hematopoietic stem cells and mesenchymal stem cells (10–13). Clinically, high CXCR4 expression correlates with poorer prognosis in acute myelogenous leukemia and breast carcinoma (14). In epithelial ovarian carcinoma and gastric cancer, CXCR4 expression enhances peritoneal metastasis, a pathogenic feature similar to diffuse malignant mesothelioma (15,16). Therefore, the SDF1/CXCR4 chemotactic axis is a potential therapeutic target to prevent peritoneal tumor seeding and dissemination.
CXCR4 inhibition has been a focus of cancer therapeutics. Classes of drugs include small molecule inhibitors (AMD3100), antagonistic peptides (T22), antibodies, and small interfering RNA (17). Studies show that administration of these drugs decreased tumor growth, angiogenesis and metastasis; however, overall survival was frequently not improved (18). In cancer therapy, AMD3100 synergizes with cytotoxic chemotherapy in gliomas by decreasing activation of the Akt survival pathway (19).
We therefore hypothesize that mesenchymal stem cells home to transplanted malignant mesothelioma spheroids along a SDF1/CXCL12 chemotactic axis and that targeting this chemotactic axis can abrogate tumor growth and progression.
MATERIALS AND METHODS
Immunofluorescence staining for Sca-1
C57Bl/6 mice were injected intraperitoneally with 1×106 syngeneic malignant mesothelioma cells. The tumor cells aggregate and form spheroids that grow and eventually attach to serosal surfaces in the peritoneum, invade local organs and metastasize to distant organs over a 28-day period (20). Peritoneal lavage cells were collected, immunofluorescently stained for Sca-1 (antibody from BD Pharmingen) and visualized using a Zeiss Axiovert 100 confocal fluorescence microscope (Carl Zeiss, Thornwood NYC) or Nikon Eclipse E800 digital microscope.
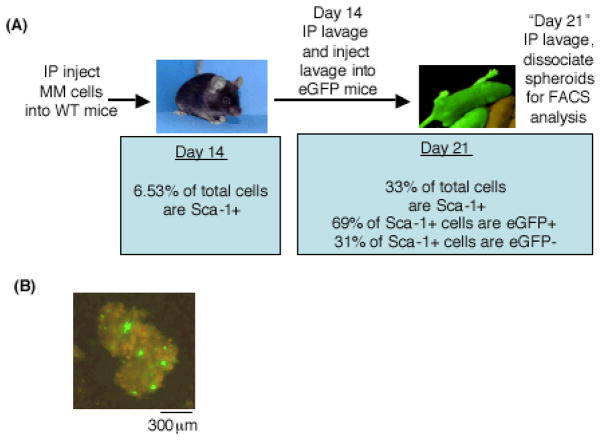
Flow cytometry studies to distinguish recruited from proliferating Sca-1-expressing cells
Peritoneal lavage cells from wild-type mice were collected 14 days after malignant mesothelioma cell injection and reinjected into eGFP transgenic mice. Seven days post-injection into eGFP transgenic mice, peritoneal lavage cells were collected, labeled (for eGFP, Sca-1 and pulse-labeled with BrdU) and analyzed on a BD FACS Calibur Flow Cytometer or imaged with confocal or epifluorescence microscopy. Lavage cells from five eGFP mice were evaluated.
Sorting of Sca-1 cells from peritoneal lavage fluid using flow cytometry
Sca-1-labeled peritoneal lavage cells collected 21 days after IP injection of malignant mesothelioma cells were sterilely sorted using a BD FACS Aria flow cytometer. The sorted cells were plated and allowed to adhere to the plastic overnight. Adherent and non-adherent cells were collected separately for immunofluorescence analysis using antibodies against antigens reported to be expressed by various Sca-1+ cells, including CD34, CD45, WT1, CD90, CD4 and CD8 (BD Pharmingen). Cells were pooled from ten C57Bl/6 mice.
RT2 Profiler™ PCR Arrays and ELISA for Mouse Cytokines and Receptors
Total RNA was isolated from peritoneal lavage at 7, 14, 21 and 28 days following intraperitoneal injection of mesothelioma cells or from mesothelioma cells grown in monolayer in vitro. Quantitative real-time PCR array was used to screen for expression of inflammatory cytokines, their receptors and angiogenic factors according to the manufacturer’s instructions (SuperArray Bioscience. Frederick, MD). A gene-wise, two sample, t-test was performed for each transcript to identify statistically significant differences in expression between solid tumors and the cell line in vitro. The results from 3 replicate arrays were analyzed. Quantitative ELISA (R&D Systems) assays in triplicate were used to determine the levels of SDF-1 protein in peritoneal lavage fluid collected from mice 7 and 21 days following injection of malignant mesothelioma cells.
Reverse transcription PCR
Total RNA was isolated using Tri-Reagent (Molecular Research Center Inc. Cincinnati, OH). Total RNA was reverse transcribed and PCR was performed using the following forward and reverse primers: CXCR4 (345bp product, 5′-TCTTCCTGCCCACCATCTAC, 3′-TCAGCAGCAGTTTCCTTGG); 18S (106bp product, 5′-AAACGGCTACCACATCCAAG, 3′-GGCCTCGAAAGAGTCCTGTA); β-actin (250bp product, 5′-GTGGGCCGCTCTAGGCACCA, 3′-TCACGGTTGGCCTTAGGGTTCAGGG). PCR products were separated on 3% agarose gels and visualized using a Bio-Rad Gel Doc Imaging Station (Bio-Rad, Hercules, CA).
Transwell migration assays
Mesenchymal stem cells (MSCs from Dr. Darwin Prockop of Tulane University) were plated on transwell filters and placed in wells containing lavage fluid collected 7 or 21 days after injection of malignant mesothelioma cells. After 24 hours, transmigrated mesenchymal stem cells were collected and counted using a Coulter® Cell Counter. In SDF1/CXCR4 studies, MSCs were pretreated for one hour with either T22 (3μM, Bachem) or AMD3100 (10μg/mL, Sigma), or 10ng/mL SDF1 recombinant protein (R&D Systems) was added to the lavage fluid. For transwell migration studies with conditioned media, spheroid cells were plated for five days before collection of conditioned media. Graphical representation shows standard deviation of the mean with p-values calculated using the student’s t-test.
In vivo studies of malignant mesothelioma progression
C57Bl/6 mice were intraperitoneally injected with syngeneic malignant mesothelioma cells followed by daily intraperitoneal injections of either 5mg/kg AMD3100 or 1mL PBS vehicle control. Fourteen and 21-days after tumor cell injection, tumor spheroids were harvested by peritoneal lavage and tissue was dissected for formalin-fixation and paraffin-embedding. The care and study of animals were in accordance with institutional guidelines. Organs harvested included: intestines with attached mesentery, kidneys, liver, spleen, pancreas, pelvic mesentery, diaphragm, lungs and heart. Formalin-fixed tissues were sectioned at 5μm, and two sections from each tissue were selected for staining with hematoxylin and eosin (H&E; Richard Allan Scientific, Kalamazoo, MI). Tumor number and area were calculated from brightfield images. Statistics were calculated from 6 mice per group. Graphical representation shows standard deviation from the mean with p-values calculated using the student’s t-test.
RESULTS
Spatial and temporal distribution of Sca-1-positive cells in mesothelioma spheroids
Z-stack images illustrate that Sca-1-positive cells are located towards the center of the tumor spheroids (Figure 1a). The kinetics of Sca-1-positive cell recruitment to tumor spheroids after 14–21 days show an increasing number of Sca-1-positive cells (Figure 1b). The single cell population not incorporated into the tumor spheroids consists of a low percentage of Sca-1-positive cells throughout this time course, while tumor spheroids showed an increasing percentage of Sca-1-positive cells over time (Figure 1c).
Figure 1.
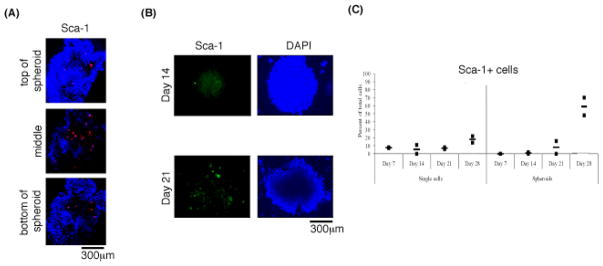
Spatial and temporal distribution of Sca-1+ cells. (A) Confocal Z-stack (60μm) spheroid showing Sca-1+ cells (red) in the center. (B) Increasing number of Sca-1+ cells (green) in spheroids over time. (C) Flow cytometry data showing no increase in percentage of Sca-1+ cells amongst single cells but an increasing percentage in spheroids over time.
Ongoing cell recruitment rather than cell proliferation accounts for the increasing number of Sca-1-positive cells
The percentage and total number of Sca-1-positive cells in tumor spheroids increase from 14 to 21 days after injection of malignant mesothelioma cells (Figure 2a). We aimed to determine whether the increasing number and percentage was due to cell recruitment or proliferation. Double positive (eGFP+/Sca-1+) cells are indicative of Sca-1-positive host cells recruited to the tumor spheroids. In contrast, eGFP-/Sca-1+ cells are the original Sca-1-positive cells injected into the transgenic mice. Flow cytometry results show the majority of Sca-1-positive cells are recruited (69% eGFP+) versus proliferating (31% eGFP−) (Figure 2a).
Figure 2.
Sca1-positive cells are recruited to tumor spheroids and are not proliferating. (A) Schematic of experimental design. Flow cytometry shows that 69% of Sca-1-positive cells are recruited (eGFP+) while 31% are proliferative (eGFP−). (B) Day 21 tumor spheroids were pulse-labeled with BrdU and immunostained for BrdU incorporation and Sca-1. BrdU+ cells (green) were not Sca-1-positive (red).
Further, BrdU-positive cells visualized in the tumor spheroids did not co-localize with Sca-1-positive cells (Figure 2b). Therefore Sca-1-positive cells in tumor spheroids are not proliferating in S phase of the cell cycle at day 21.
Sca-1-positive cells sorted from tumor spheroids are a heterogenous cell population
Cells expressing T lymphocyte markers comprise the majority of the Sca-1-positive cell population (94%), followed by mesenchymal stem cells (4.5%), malignant mesothelioma cells (1%) and hematopoietic stem cells (0.5%) (Table 1). T lymphocytes and hematopoietic stem cells are nonadherent in cell culture and together comprise 94.5% of the Sca-1-positive cell population. The adherent cells, mesenchymal stem cells and mesothelioma cells, comprise the other 5.5% of the Sca-1-positive cell population.
Table I.
The Sca-1+ cell population contains multiple cell types.
| Antigen expression (putative cell type) | % of total Sca-1+ cells | Adherence after 48 hr |
|---|---|---|
| CD34+CD45+ (hematopoietic stem cell) | 0.5% | Non-adherent |
| CD34+ WT1+ (mesothelioma cell) | 1% | Adherent |
| CD34− CD45−CD90+ (mesenchymal stem cell) | 4.5% | Adherent |
| CD34−CD45−/CD4+ or CD8+ (T lymphocytes) | 94% | Non-adherent |
Gene and protein expression of the SDF1/CXCR4 chemotactic axis in the tumor spheroid microenvironment
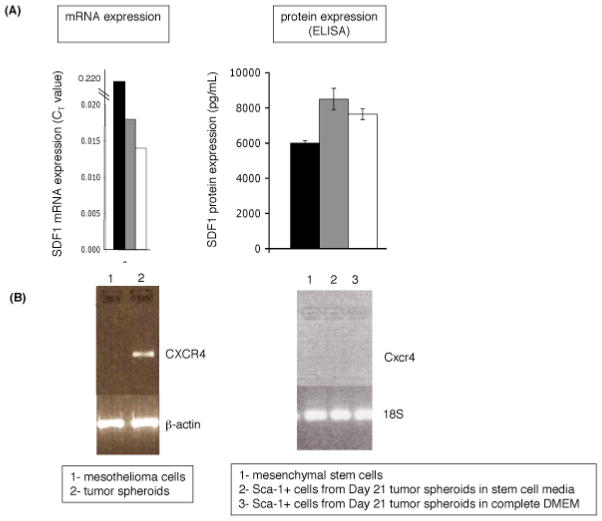
Previously reported factors for mesenchymal stem cell recruitment to tumors include the chemokines stromal derived factor 1 (SDF1/CXCL12) and macrophage inhibitory protein 3β (MIP3β/CCL19); and the growth factors platelet derived growth factor (PDGF), insulin-like growth factor (IGF) and hepatocyte growth factor (HGF) (21). Transplanted malignant mesothelioma cells and tumor spheroids express all of these factors, as determined using real-time PCR array analysis in our laboratory. Of note is significant and stable expression of the chemokine SDF1/CXCL12 at both the RNA and protein levels at 7, 14 and 21 days following injection (Figure 3a).
Figure 3.
SDF1 and CXCR4 expression in tumor spheroids. (A) SDF1 expression at the mRNA and protein levels. (B) left- CXCR4 expression by tumor spheroids but not by mesothelioma cells grown in vitro. β-actin is the loading control. right- No CXCR4 expression by mesenchymal stem cells grown in monolayer or by Sca-1-positive cells collected from day 21 lavage. 18S is the loading control.
The cognate chemokine receptor for SDF1/CXCL12 is CXCR4. Mesenchymal stem cells have been reported to express CXCR4 (10,11,12). Using RT-PCR, CXCR4 expression was absent in mesothelioma tumor cells and mesenchymal stem cells cultured in vitro, but present in multicellular tumor spheroids isolated ex vivo.
In vitro migration of mesenchymal stem cells is dependent on the CXCR4/SDF1 chemotactic axis
A significantly greater number of mesenchymal stem cells transmigrated to the lower chamber containing lavage fluid from tumor cell-injected mice than from PBS-injected mice. There was also more robust transmigration to lavage fluid collected 21 days after injection of malignant mesothelioma cells than to lavage fluid collected after 7 days (Figure 4a).
Figure 4.
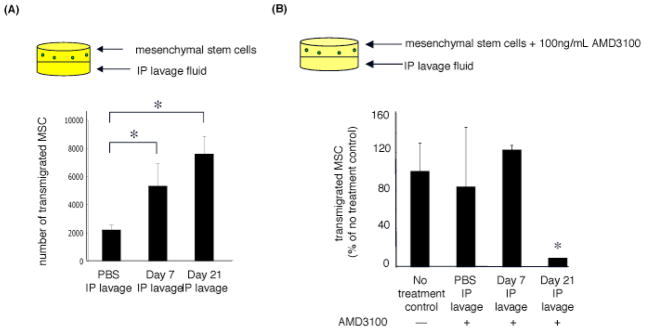
(A) Migration of mesenchymal stem cells to lavage fluid collected from PBS-injected mice and tumor-bearing mice 7 and 21 days following injection of mesothelioma cells. (B) AMD3100 inhibits transmigration of mesenchymal stem cells to lavage fluid. (*p value<0.05 compared to no treatment control unless otherwise noted, student’s t-test)
CXCR4 inhibition with AMD3100 resulted in no significant abrogation of transmigration of mesenchymal stem cells to PBS lavage fluid or to lavage fluid collected 7 days after injection of malignant mesothelioma cells; but significant abrogation of transmigration occurred to lavage fluid collected 21 days after injection (Figure 4b). These findings suggest that migration of mesenchymal stem cells to malignant mesothelioma spheroids is not dependent on the SDF1/CXCR4 chemotactic axis early after tumor cell injection, but is increasingly dependent at 21 days.
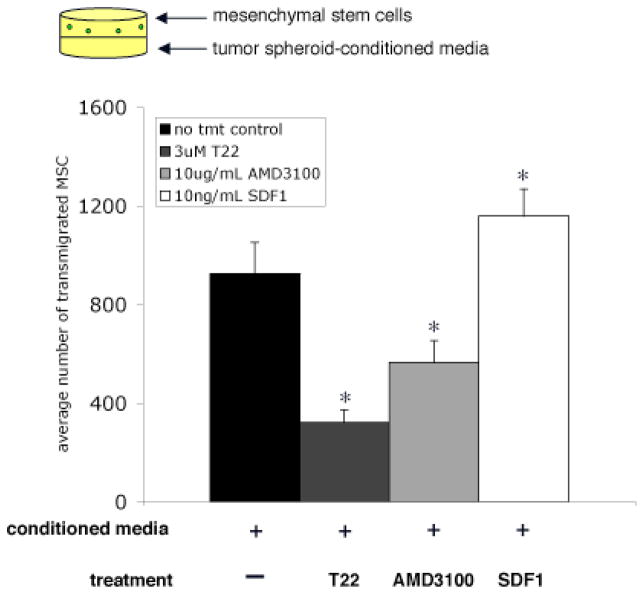
We hypothesized that tumor spheroids are a significant source of SDF1 in the malignant mesothelioma microenvironment. There was significant abrogation of transmigration of MSC pretreated with CXCR4 inhibitors to conditioned media from tumor spheroids (Figure 5). Also, addition of SDF1 recombinant protein to the lavage fluid significantly increased MSC transmigration (Figure 5).
Figure 5.
Transmigration of mesenchymal stem cells to conditioned media from tumor spheroids is dependent on the SDF1/CXCR4 chemotactic axis. MSC transmigration to conditioned media is abrogated after CXCR4 inhibition with T22 or AMD3100. Transmigration is enhanced by addition of exogenous SDF1. (*p value < 0.05 compared to no treatment control, student’s t-test)
CXCR4 inhibition and tumor growth and progression in vivo
From studies using CXCR4 inhibition in vivo, the total number of tumors in PBS-injected control mice significantly increases from 14 days to 21 days post-injection of malignant mesothelioma cells (Figure 6a). The total number of tumors in AMD3100-treated mice also increases up to 14 days, but then the number significantly decreases in the treatment group. These results are consistent with the kinetics of the in vitro findings that AMD3100 treatment abrogates stem cell migration to lavage fluid collected from mice 21 days, but not 14 days, after injection of malignant mesothelioma cells.
Figure 6.
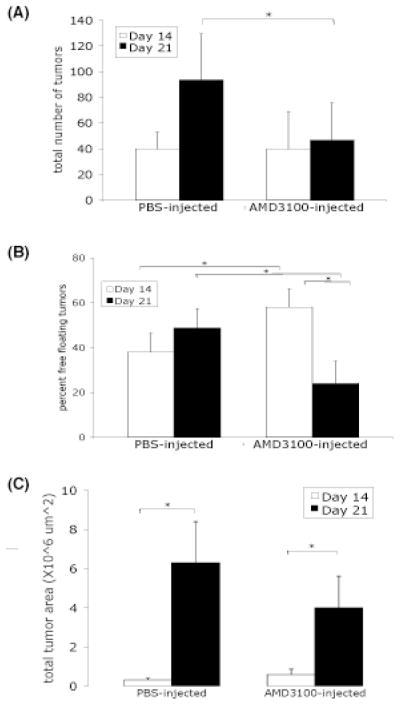
Tumor burden in mice after mesothelioma cell injection followed by daily injection of PBS or AMD3100. (A) Total tumor number significantly increased in PBS-injected but not in AMD3100-injected mice. (B) Initial higher percentage of tumor spheroids in AMD3100-injected mice compared to control. (C) Greater increasing trend in total tumor area in control than in AMD3100-injected mice. (*p-value ≤ 0.05, student’s t-test)
The percentage of tumor spheroids in both treatment groups was determined after 14 and 21 days (Figure 6b). The percentage was consistently around 40% in PBS-injected mice at both time points. In AMD3100-treated mice, the percentage significantly decreased from 60% at 14 days following injection to an average of 20% at 21 days (Figure 6b). At 14 days after injection of malignant mesothelioma cells, AMD3100-treated mice had a significantly higher percentage of tumor spheroids than PBS-injected control mice.
Total tumor area was also assessed as a measure of tumor burden. In both PBS-injected mice and AMD3100-injected mice, the total tumor area increased from 14 days to 21 days post-tumor cell injection (Figure 6c). However, the increase in total tumor area was greater in the PBS-injected mice. In summary, CXCR4 inhibition with the small molecule inhibitor AMD3100 in vivo decreases tumor burden in this orthotopic murine model of malignant mesothelioma, especially between 14 and 21 days after injection of tumor cells.
DISCUSSION
The work presented here describes the recruitment of a putative stem cell population to malignant mesothelioma spheroids in a transplantable murine tumor model. Stem cells are slowly proliferating cells whose proliferation status is niche-dependent (22). We found that the increasing number of Sca-1-positive cells found in tumor spheroids over time is due to cell recruitment rather than cell proliferation, also confirmed by BrdU incorporation studies It is possible there is a number of proliferative Sca1-positive cells cycling but not in S-phase during this BrdU pulse labeling. Alternatively, recruited Sca-1-positive cells may undergo cell proliferation at a slow rate.
The Sca-1-positive cells consist of adherent mesenchymal stem cells. Recruitment of mesenchymal stem cells to malignant mesothelioma tumors is a novel finding. Mesenchymal stem cells comprised 82% of the total adherent Sca-1-positive cell population. We hypothesized that the malignant mesothelioma tumor spheroid microenvironment is conducive to recruitment of mesenchymal stem cells. While many chemokines and growth factors are reported to be involved in recruitment of mesenchymal stem cells (11,12) the highest expression levels were found for the chemokine SDF1/CXCL12, at both the mRNA and protein levels. Trends in mRNA expression were not paralleled by protein expression in these studies; this could be explained by the fact that mRNA expression was measured using tumor spheroid cells alone while protein expression was measured using cell culture media samples containing secreted SDF1 protein. However, there is slightly higher expression of SDF1 after 7 days compared to 21 days after injection of malignant mesothelioma cells. This supports the hypothesis that the malignant mesothelioma microenvironment is conducive to ongoing recruitment of mesenchymal stem cells.
RT-PCR studies showed no expression of CXCR4 by malignant mesothelioma cells grown in monolayer in vitro, but significant CXCR4 expression was found in ex vivo malignant mesothelioma spheroids. These findings suggest that malignant mesothelioma cells do not express CXCR4, rather, malignant mesothelioma cells injected in vivo recruit CXCR4-expressing host cells that are integrated into tumor spheroids. In addition to a CXCR4-expressing mesenchymal stem population, there may be recruitment of CXCR4+ lymphocyte population similar to the lymphocyte population found in human malignant pleural and peritoneal mesothelioma (23).
CXCR4 expression could be dependent on cell culture conditions. Cells grown in monolayer in culture have different gene expression profiles than cells grown as spheroids in culture (24). It is possible that the mesenchymal stem cells and adherent Sca-1-positive cells used in our studies would upregulate CXCR4 expression if grown as spheroids in vitro or immediately used ex vivo.
Transwell migration studies showed increased mesenchymal stem cell recruitment to lavage fluid collected from mice 21 days after malignant mesothelioma cell injection compared to lavage fluid from mice after 7 days, and there was greater mesenchymal stem cell recruitment at both time points compared to lavage fluid from PBS-injected mice. This trend of increasing Sca-1-positive cell recruitment over time is consistent with the ex vivo experiments described earlier.
Mesenchymal stem cell migration to lavage fluid from PBS-injected mice and to lavage fluid collected 7 days after injection of malignant mesothelioma cells was unaffected by AMD3100 pretreatment. It is possible that other chemotactic factors play a more significant role for mesenchymal stem cell recruitment at this early time point in tumor growth and progression. However, at 21 days following injection of mesothelioma cells, there is significant inhibition of mesenchymal stem cell recruitment. This result suggests that the SDF1/CXCR4 chemotactic axis plays a significant role in recruitment of mesenchymal stem cells at the later stages of malignant mesothelioma growth and progression.
The peritoneal fluid can be a depot for secreted molecules from multiple cell types found in the peritoneal cavity, including tumor cells, recruited host cells, and resident cells of the intestinal mesentery including milky spots. In experiments where the only source of SDF1 would be from cells of the tumor spheroids, pretreatment of mesenchymal stem cells with CXCR4 inhibitors significantly abrogated mesenchymal stem cell recruitment. Taken together, these findings demonstrate the dependence of MSC transmigration on the SDF1/CXCR4 chemotactic axis in this murine tumor model, and that tumor spheroids are a significant source of SDF1.
CXCR4 inhibition in vivo decreased tumor burden in our murine tumor model. Interestingly, the percent of total tumor consisting of free-floating tumor spheroids was significantly different between the two treatment groups. By 21 days after tumor cell injection, the percentage of tumor spheroids significantly decreased in AMD3100-injected mice but not in PBS-injected mice. This decrease may be attributed to tumor cell death due to apoptosis in AMD3100-injected mice during this time frame resulting in a decreased percentage of tumor spheroids. When total tumor area is divided into solid tumor area versus tumor spheroid area, it is clear that AMD3100-injected mice have less tumor area than PBS-injected mice, and that tumor area in AMD3100-injected mice is comprised mostly of tumor spheroids and not solid tumor. Taken together, there is a trend towards decreased tumor burden in mice treated with AMD3100. This may correlate with decreased Sca1-positive cell recruitment and incorporation into tumor spheroids. It is also possible that AMD3100 inhibits other stages in malignant mesothelioma progression, such as angiogenesis.
In conclusion, tumor spheroids from this orthotopic murine mesothelioma model represent a dynamic state of mesenchymal stem cell recruitment during tumor growth and progression. The SDF1/CXCR4 chemotactic axis plays a significant role in mesenchymal stem cell recruitment to malignant mesothelioma spheroids and could be a novel therapeutic target for this aggressive tumor, especially as part of a multimodal therapeutic approach following surgical debulking.
Acknowledgments
We thank Dr. Darwin Prockop of the Center of Gene Therapy at Tulane University for the mesenchymal stem cells used these studies. We also thank Paula Weston from Brown University’s Molecular Pathology Core Facility for her contributions to the histology work.
This research was supported by:
The National Institutes of Health grants RO1 ES03721 and F30 ES013639 with use of a core facility funded by the Superfund Research Program P42 ES013660.
Footnotes
Author Contribution:
Bonnie Lau, PhD: conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing
Agnes Kane, MD, PhD: conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript
References
- 1.Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma. Current Opinion in Pulmonary Medicine. 2008;14:303–309. doi: 10.1097/MCP.0b013e328302851d. [DOI] [PubMed] [Google Scholar]
- 2.Nagy JA, Herzberg KT, Dvorak JM, et al. Pathogenesis of malignant ascites formation: initiating events that lead to fluid accumulation. Cancer Research. 1993;53:2631–2643. [PubMed] [Google Scholar]
- 3.Shield K, Acklan ML, Ahmed N, et al. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecologic Oncology. 2008 doi: 10.1016/j.ygyno.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 4.Gerber SA, Rybalko VY, Bigelow CE, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathology. 2006;169(5):1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangai T, Ishii G, Kodama K, et al. Effect of differences in cancer cells and tumor growth sites on recruiting bone marrow-derived endothelial cells and myofibroblasts in cancer-induced stroma. Int J Cancer. 2005;115(6):885–892. doi: 10.1002/ijc.20969. [DOI] [PubMed] [Google Scholar]
- 6.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 7.Witz IP. Differential expression of genes by tumor cells of a low or a high malignancy phenotype: the case of murine and human Ly-6 proteins. J Cell Biochem Suppl. 2000;34:61–66. [PubMed] [Google Scholar]
- 8.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 9.Kryczek I, Wei S, Keller E, et al. Stroma-derived factor (SDF1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 10.Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 11.Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–528. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 12.Lopez Ponte A, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 13.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasumoto K, Koizumi K, Kawashima A, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Research. 2006;66(4):2181–2187. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- 15.Kajiyma H, Shibata K, Terauchi M, et al. Involvement of SDF1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 16.Yan T, Sugarbaker P. Rectus abdominus muscle resection for abdominal wall recurrence of mucinous adenocarcinoma and peritoneal mesothelioma. Tumorigenesis. 2008;94(3):309–313. doi: 10.1177/030089160809400304. [DOI] [PubMed] [Google Scholar]
- 17.Wong D, Korz W. Translating an antagonist of chemokine receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14(24):7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 18.Redjal N, Chan JA, Segal RA, et al. CXCR4 inhibition synergizes with cytotox chemotherapy in gliomas. Clin Cancer Res. 2006;12(22):6765–6771. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 19.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 20.Miselis NR, Wu ZJ, Van Rooijen N, et al. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Therap. 2008;7(4):788–799. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 21.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis & Rheumatism. 2009;60(3):813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 22.Levi BP, Morrison SJ. Stem cells use distinct self renewal programs at different ages. Cold Spring Harbor Sym Quant Biol. 2009 doi: 10.1101/sqb.2008.73.049. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu Y, Dobashi K, Imai H, et al. CXCR4+FOXP3+CD25+ lymphocytes accumulate in CXCL12-expressing malignant pleural mesothelioma. Int J Immunopath and Pharmacology. 2009;22(1):43–51. doi: 10.1177/039463200902200106. [DOI] [PubMed] [Google Scholar]
- 24.Zietarska M, Maugard CM, Filali-Mouhim A, et al. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC) Mol Carcinogenesis. 2007;46(10):872–885. doi: 10.1002/mc.20315. [DOI] [PubMed] [Google Scholar]