Abstract
Aim
To estimate the proportional contribution of influenza viruses (IV), parainfluenza viruses (PIV), adenoviruses (AV), and coronaviruses (CV) to the burden of severe acute lower respiratory infections (ALRI).
Methods
The review of the literature followed PRISMA guidelines. We included studies of hospitalized children aged 0-4 years with confirmed ALRI published between 1995 and 2011. A total of 51 studies were included in the final review, comprising 56 091 hospitalized ALRI episodes.
Results
IV was detected in 3.0% (2.2%-4.0%) of all hospitalized ALRI cases, PIV in 2.7% (1.9%-3.7%), and AV in 5.8% (3.4%-9.1%). CV are technically difficult to culture, and they were detected in 4.8% of all hospitalized ALRI patients in one study. When respiratory syncytial virus (RSV) and less common viruses were included, at least one virus was detected in 50.4% (40.0%-60.7%) of all hospitalized severe ALRI episodes. Moreover, 21.9% (17.7%-26.4%) of these viral ALRI were mixed, including more than one viral pathogen. Among all severe ALRI with confirmed viral etiology, IV accounted for 7.0% (5.5%-8.7%), PIV for 5.8% (4.1%-7.7%), and AV for 8.8% (5.3%-13.0%). CV was found in 10.6% of virus-positive pneumonia patients in one study.
Conclusions
This article provides the most comprehensive analysis of the contribution of four viral causes to severe ALRI to date. Our results can be used in further cost-effectiveness analyses of vaccine development and implementation for a number of respiratory viruses.
Acute lower respiratory tract infections (ALRI) are the leading cause of global mortality in children under five years of age (1,2). Studies of pre-school children from developed and developing countries alike suggest that the majority of respiratory infections generally have viral etiology (2-4). Clinically, ALRIs can be divided into pneumonias and bronchiolitis (5,6). Differentiating those two conditions can be particularly difficult in younger children, who typically exhibit less specific clinical symptoms (3,7-9). In high-income countries (HIC), pneumonia rarely causes deaths in children (10), although it continues to be a major cause of morbidity and poses a significant economic burden (11). Bronchiolitis is characterized by a distressing pattern of symptoms: low-grade/absent fever progressing to cough, coryza, tachypnoea, hyperinflation, chest retraction, and widespread crackles or wheezes (12). Bronchiolitis deaths are very rare in HIC (13,14), but children are at increased risk of recurrent wheezing and the data on mortality in low and middle income countries (LMIC) are scarce (15).
Etiology of severe ALRI episodes is not well understood: limited contribution of the three major pathogens (S. pneumoniae, H. influenza, and respiratory syncytial virus) is established, but the role of other viruses has not been explored. The importance of viruses as major causes of ALRI is becoming increasingly apparent because the sensitivity of detection techniques has greatly improved and new molecular tests increasingly replace conventional methods. The use of polymerase-chain reaction (PCR) now allows identification of viruses that have previously been difficult or impossible to culture. In the past decade, numerous novel respiratory viruses that can cause ALRI have been discovered, and new diagnostic methods for the use in high and low-resource settings alike are continuously evolving (3,16-20). It seems that the conventional diagnostic methods have systematically underestimated the role of viruses as causal pathogens in ALRI (3), and also that viruses are capable of causing severe, life-threatening ALRI (3). The emergence of the severe acute respiratory syndrome (SARS), caused by a novel coronavirus, and the avian influenza type A (H5N1) outbreak are good recent examples (16,20).
Impressive progress has been made in the last decade in increasing the global availability of vaccines against the main bacterial causes of ALRI – S. pneumoniae and H. influenzae type B – leading to marked reductions in both hospitalizations and deaths (21,22). This will lead to increased focus on viral causes and their prevention and management. Strains of influenza type A and B viruses can be life threatening (3), although infection in the majority of young children is vaccine-preventable (23,24). Parainfluenza viruses (PIV) are the most common cause of croup in young children, with PIV1 and PIV3 also being the causes of severe bronchiolitis and pneumonia (3,4,25), but there are currently no licensed PIV vaccines. Adenoviruses (AV) have long been recognized as pathogens of the lower respiratory tract that can be associated with severe or lethal lower respiratory tract infection (3,26,27) or bronchiolitis obliterans (28-31). Coronaviruses (CV) cause common cold and have been historically thought to be a very rare cause of ALRI (32), despite the fact that they sporadically caused catastrophic disease in livestock (33). The SARS-CV outbreak in 2003, which was a highly virulent zoonosis capable of human-to-human transmission, renewed the interest in CV as human pathogens (32). This led to a discovery of two previously unrecognized CVs as causes of ALRI (16,17).
This study analyzed the available information on the role of four viruses (IV, PIV, AV, and CV), all of which have been historically considered to be relatively uncommon causes of severe ALRI in hospitalized cases. Our study did not assess the role of common causes – RSV, S. pneumonia, and H. influenzae – because their roles have already been systematically characterized and well-established (34). We are not aware of any systematic analyses of the global prevalence of viruses in severe childhood ALRI. We aimed to assess the proportion of cases of severe ALRI with a viral etiology and explore the contribution of mixed viral infections and separate contributions of IV, PIV, AV, and CV to severe ALRI in children under five years of age.
Methods
This systematic review was carried out using the PRISMA and MOOSE protocols (35-37). These protocols have been developed to ensure standardized and replicable approach to systematic review of the available evidence on the burden of specific health problems, the role of risk factors, or the effectiveness of available health interventions, and the unified reporting of the findings.
Literature search and inclusion criteria
A systematic literature review was performed using the search terms detailed in Supplementary online material(supplementary material). This was supplemented by hand searching of key online journals and reference lists of selected papers. The search included the following databases: Medline, EMBASE, CINAHL, Global Health Library, WHOLIS, LILACS, IndMed, AIM, SciELO, IMEMR, IMSEAR, WPRIM, and SIGLE (gray literature).
All studies included in the analysis reported on inpatients aged 0-4 years with a clinical diagnosis of community-acquired ALRI, bronchiolitis, or pneumonia. Investigation of viral etiology was a requirement and the participants needed to be free of co-morbid conditions. Children admitted to emergency departments were excluded, and so were intensive care patients wherever data was not reported for all other inpatients in the hospital, to avoid potential bias. We included studies conducted between 1995 and 2011 with a continuous study period of one year (or multiples of one year), to avoid effects of seasonality. Studies that relied solely on serology for diagnosis were excluded, because this method could not reliably differentiate acute from past infections (38,39). Studies that were conducted during an epidemic or pandemic outbreak were also excluded.
Study selection and data extraction
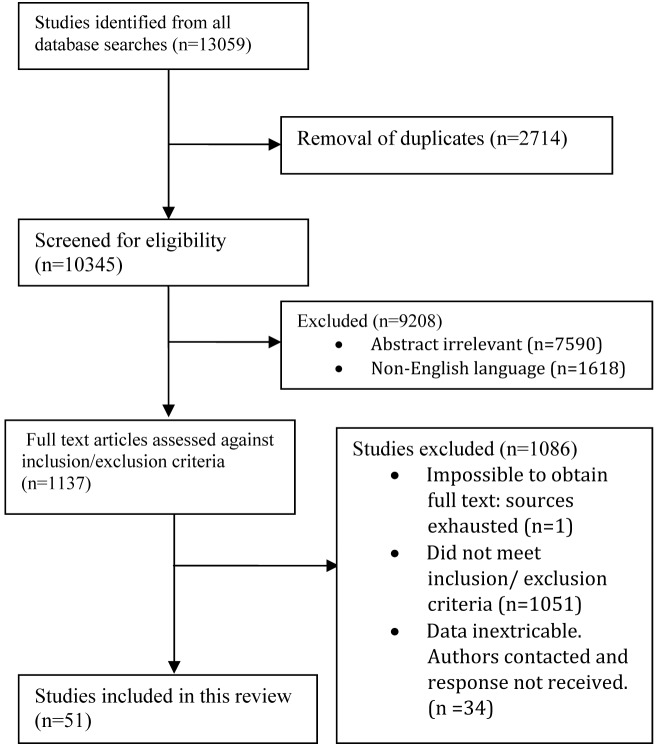
Study selection was performed following the removal of duplicates. Authors were contacted by email in cases when study data were not published in an extractable form, to collect further details. Data were extracted for study location, period of study, sample, diagnostic assay, clinical diagnosis, age range and median age of study population, potential etiological agents investigated, proportion of patients in whom no etiological diagnosis was found, viruses and bacteria detected, and age breakdown of patients by diagnosis where available (Figure 1).
Figure 1.
Details of the systematic review and study selection process.
Assessment of bias within studies
During the process of data extraction, information was drawn from each study on possible sources of bias that could affect the results, such as:
• Respiratory sample used (as there is no “gold standard;” samples from lower respiratory tract are preferable, but they require invasive procedures and are difficult to obtain without contamination from the upper airway; because of this, most studies consider nasopharyngeal aspirates for viral detection as acceptable, although acknowledging limitations. Viruses detected in the upper airway of a patient with ALRI are not necessarily pathogens of the lower respiratory tract);
• HIV co-infection (as this is known to increase the susceptibility to ALRI and the rate of atypical infection) (40);
• Viral detection technique and timing (as there is large variability in the sensitivity of different techniques; viral culture can only reliably be used within 2 days of onset of acute rhinorrhea, when viable virus shedding is at its peak (41); PCR can be used much later, because it does not require viable viruses in the sample, offering much improved sensitivity, but also greatly increased rates of detection of benign co-infections).
Summary measures
Proportional contributions of IV, PIV, AV, and CV to severe ALRI and associated confidence intervals were derived through meta-analysis using StatsDirect software package (StatsDirect Ltd, Academic version 2.7.9., Cheshire, UK). Due to large variation in methodology and patient demographics between studies, random effects models were used in all analyses, as proposed by DerSimonian and Laird (42). Heterogeneity and bias analyses were also performed for all meta-analyses. All presented results were shown to be free of publication bias, as demonstrated using funnel plots and analysis methods proposed by Begg (43), Egger (44), and Harbold (45). This triple approach represents robust protection from the sources of bias.
Results
Fifty one studies meeting the inclusion criteria were included in this review, including 56 091 episodes of severe hospitalized ALRI. Figure 2 presents geographical distribution of the retained studies, Figure 3 shows proportion of studies investigating different viruses (any virus, RSV, IV, PIV, AV, CV), while Table 1 presents their basic characteristics in terms of case definition, sample size, period of study, and diagnostic methods used (15,40,46-94). Only four studies investigated children hospitalized with ALRI for both bacterial and viral etiology (59,64,69,74) and only six studies reported HIV co-infection as their exclusion criteria (15,58,59,63,64,86). A total of 19 studies were from high-income countries (95), investigating on average 6.5 viruses per study, while studies in LMIC investigated 2.7 viruses (unpaired t test: P = 0.002). It seems likely that this difference reflects the fact that more tests are typically used in establishing diagnosis in high-income settings, without certainty over the causal role of all identified viral pathogens, and this may introduce systematic bias and heterogeneity between studies in HIC and LMIC.
Figure 2.
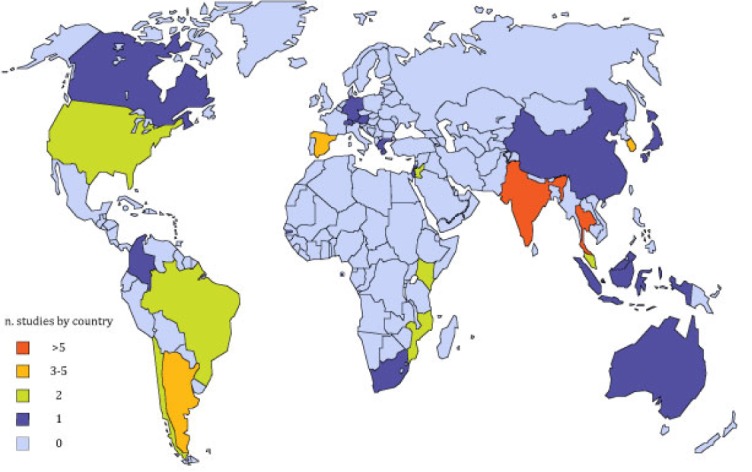
Geographic distribution of studies included in this review (N = 51).
Figure 3.
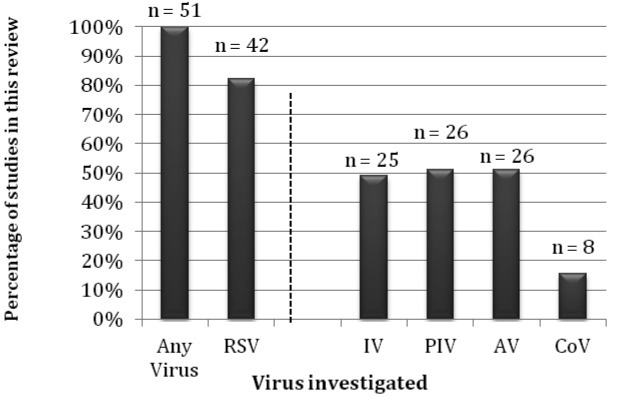
Proportion of studies retained for the final analyses that investigated individual viruses: approximately half investigated influenza virus (IV), parainfluenza virus (PIV), and/or adenovirus (AV), with no studies on coronavirus (CV) before the 2003 SARS-CV outbreak. Twenty studies only described one viral agent (11 of these respiratory syncytial virus, RSV).
Table 1.
| Author and reference number | Year | Country | Case def. | Cases (n) | Period of study | Age range (months) | Sample | Diagnostic assay | Viruses tested (n) |
|---|---|---|---|---|---|---|---|---|---|
|
Aberle, J.H., et al. (46) |
2005 |
Austria† |
LRTI‡ |
772 |
Oct 2000 - July 2004 |
<12 |
NPA |
PCR |
5 |
|
Al-Toum, R., et al. (47) |
2009 |
Jordan |
LRTI |
141 |
Sep 2002 - Mar 2004 |
<24 |
NPA |
Culture |
1 |
|
Avendano, L.F., et al. (48) |
2003 |
Chile |
LRTI |
4618 |
Jan 1989 - Dec 2000 |
<24 |
NPA |
IFA |
1 |
|
Banerji, A., et al. (49) |
2009 |
Canada† |
LRTI |
121 |
Jan 2002 - Mar 2003 |
<24 |
NPA |
IFA and PCR |
20 |
|
Bdour, S., et al. (50) |
2001 |
Jordan |
LRTI |
271 |
Jan 1997 - May 1999 |
<24 |
NPW |
IFA |
1 |
|
Bedoya, V.I., et al. (51) |
1996 |
Colombia |
LRTI |
103 |
Apr 1994 - Apr 1995 |
<12 |
NPW |
IFA |
1 |
|
Bharaj, P., et al. (52) |
2010 |
India |
LRTI |
181 |
Apr 2005 - Mar 2007 |
<62 |
NPA |
PCR |
1 |
|
Bharaj, P., et al. (53) |
2009 |
India |
LRTI |
135 |
Apr 2005 - Mar 2007 |
<72 |
NPA |
PCR |
20 |
|
Bolisetty, S., et al. (54) |
2005 |
Australia† |
AB |
167 |
Jan 2000 - Dec 2000 |
<24 |
NPA |
EIA and Culture |
4 |
|
Calvo, C., et al. (55) |
2010 |
Spain† |
AB |
318 |
Sep 2005 - Aug 2008 |
<12 |
NPA |
PCR |
16 |
|
Canducci, F., et al. (56) |
2008 |
Spain† |
LRTI |
230 |
Oct 2004 - Sep 2006 |
<24 |
NPA |
PCR |
4 |
|
Carballal, G., et al. (57) |
2000 |
Argentina |
LRTI |
1304 |
Jan 1990 - Dec 1996 |
<24 |
NPA |
IFA |
1 |
|
Carballal, G., et al. (58) |
2001 |
Argentina |
LRTI |
1234 |
Apr 1993 - Dec 1994 |
<60 |
NPA |
IFA |
4 |
|
Cevey-Macherel, M., et al. * (59) |
2009 |
Switzerland† |
CAP |
99 |
Mar 2003 - Dec 2005 |
<60 |
NPA |
PCR |
7 |
|
Chakravarti, A., et al. (60) |
1995 |
India |
LRTI |
45 |
Jul 1990 - Jun 1991 |
<24 |
NPA |
EIA |
1 |
|
Chan, K.B., et al. (61) |
1999 |
Malaysia |
LRTI |
5691 |
Jan 1982 - Dec 1997 |
<24 |
NPA |
IFA and Culture |
4 |
|
Charanjit, K., et al. (62) |
2010 |
India |
AB |
245 |
Jan 2007 - Dec 2007 |
<12 |
NPA/NPW |
PCR, EIA and Culture |
6 |
|
Choi, E., et al. (63) |
2006 |
Rep. Korea† |
LRTI |
515 |
Sep 2000 - Aug 2005 |
<60 |
NPA |
PCR |
11 |
|
Chong, C.Y., et al. * (64) |
1997 |
Singapore† |
LRTI |
333 |
May 1994 - Apr 1995 |
<60 |
NPA |
Not declared |
4 |
|
Chung, T., et al. (65) |
2007 |
Rep. Korea† |
LRTI |
233 |
Jul 2004 - Jan 2006 |
<60 |
NPA |
PCR + IFA |
7 |
|
Cifuentes, L., et al. (15) |
2003 |
Chile |
AB |
36 |
May 1999 - Aug 2000 |
<24 |
NPA |
EIA |
1 |
|
Cilla, G., et al. (66) |
2009 |
Spain † |
LRTI |
533 |
Jul 2004 - Jun 2007 |
<35 |
NPA |
PCR |
1 |
|
Dare, R.K., et al. (67) |
2007 |
Thailand |
CAP |
510 |
Sep 2003-Aug 2005 |
<60 |
NPS |
PCR and Culture |
1 |
|
Djelantik, I.G., et al. (68) |
2003 |
Indonesia |
LRTI |
2677 |
Jan 2000 - Dec 2001 |
<24 |
NPW |
EIA |
1 |
|
Ekalaksananan, T., et al. * (69) |
2001 |
Thailand |
LRTI |
62 |
Aug 1992 - Nov 1994 |
<60 |
NPA |
EIA and Culture |
2 |
|
Fry, A.M., et al. (70) |
2011 |
Thailand |
CAP |
352 |
Sept 2003-Aug 2005 |
<60 |
NPS |
PCR and Culture |
1 |
|
Fry, A.M., et al. (71) |
2007 |
Thailand |
CAP |
369 |
Sep 2004 - Aug 2005 |
<60 |
NPS |
PCR |
1 |
|
Garcia, C.G., et al. (72) |
2010 |
USA† |
AB |
4285 |
Jan 2002 - Dec 2007 |
<23 |
NPA |
IFA and Culture |
5 |
|
Kabra, S.K., et al. * (73) |
2003 |
India |
LRTI |
95 |
Mar 1995 - Feb 1997 |
<60 |
NPA |
Culture |
4 |
|
Kabra, S.K., et al. (74) |
2004 |
India |
LRTI |
200 |
Mar 1995 - Feb 1997 |
<60 |
NPA |
Culture |
4 |
|
Kim, Y.K., et al. (75) |
2005 |
Rep. Korea† |
LRTI |
166 |
Aug 1997 - Mar 2000 |
<60 |
NPA |
PCR |
5 |
|
Loscertales, M.P., et al. (76) |
2002 |
Mozambique |
LRTI |
1001 |
Oct 1998 - May 2000 |
<60 |
NPA |
EIA |
1 |
|
Moodley, T., et al. (40) |
2010 |
S. Africa |
AB |
106 |
Jan 2006 - Dec 2007 |
<24 |
NPA |
IFA |
6 |
|
Moriyama, Y., et al. (77) |
2010 |
Japan† |
LRTI |
402 |
April 2007-July 2009 |
<24 |
NPS |
PCR |
6 |
|
Nascimento-Carvalho, C.M., et al. (78) |
2011 |
Brazil |
CAP |
268 |
Sep 2003 - May 2005 |
<60 |
NPA |
PCR |
1 |
|
Nokes, J., et al. (79) |
2008 |
Kenya |
LRTI |
223 |
Jan 2002 - Feb 2005 |
<30 |
NPA/NPW |
IFA |
1 |
|
Nokes, J., et al. (80) |
2009 |
Kenya |
CAP |
6026 |
Jan 2002 - Dec 2007 |
<60 |
NPA/NPW |
IFA |
1 |
|
O'Callaghan-Gordo, C., et al. (81) |
2011 |
Mozambique |
CAP |
807 |
Sep 2006 - Sep 2007 |
<60 |
NPA |
PCR |
7 |
|
Oliveira, D.B., et al. (82) |
2009 |
Brazil |
LRTI |
226 |
Jan 2003 -Dec 2003 |
<60 |
NPA/NPS |
PCR |
2 |
|
Samransamruajkit, R., et al. (83) |
2008 |
Thailand |
CAP |
239 |
Mar 2006-Feb 2007 |
<60 |
NPA |
PCR |
1 |
|
Singleton, R.J., et al. (84) |
2010 |
USA† |
LRTI |
424 |
Oct 2005 - Sep 2007 |
<36 |
NPS/NPW |
PCR |
4 |
|
Teeratakulpisarn, J., et al. (85) |
2007 |
Thailand |
AB |
170 |
Apr 2002 - Aug 2004 |
<24 |
NPA |
PCR |
2 |
|
Videla, C., et al. (86) |
1998 |
Argentina |
LRTI |
158 |
May 1991-Dec 1992 |
<60 |
NPA |
IFA |
2 |
|
Viegas, M., et al. (87) |
2004 |
Argentina |
LRTI |
18561 |
Jan 1998 - Dec 2002 |
<24 |
NPA |
IFA |
4 |
|
Weber, M.W., et al. (88) |
2002 |
Gambia |
LRTI |
2252 |
Oct 1993 - Dec 1997 |
<60 |
NPA |
IFA |
1 |
|
Weigl, J.A., et al. (89) |
2005 |
Germany† |
CAP |
187 |
Jul 1996 - Jun 2000 |
<60 |
NPA |
PCR |
5 |
|
Wolf, D.G., et al. (90) |
2006 |
Israel† |
CAP |
88 |
Nov 2001 - Oct 2002 |
<60 |
NPW |
IFA and PCR |
3 |
|
Xepapadaki, et al. (91) |
2004 |
Greece † |
AB |
56 |
Oct 1999- Sep 2000 |
<24 |
NPW |
PCR |
5 |
|
Xiang, Z., et al. (92) |
2010 |
China |
CAP |
384 |
Apr 2007-Mar 2008 |
<60 |
NPA |
PCR |
1 |
|
Yin, C.C., et al. (93) |
2003 |
Singapore† |
LRTI |
1011 |
Aug 1998-Jul 1999 |
<60 |
NPA |
IFA |
4 |
| Yoo, S.J., et al. (94) | 2007 | Rep. Korea† | LRTI | 158 | Jan 2004 - Dec 2004 | <60 | NPA | PCR and IFA | 7 |
*Studies that investigate viral and non-viral etiological agents.
†High income country, as classified by the global burden of disease (GBD21), excluding studies of indigenous groups.
‡Abbreviations: CAP – Community acquired pneumonia; AB – acute bronchiolitis; LRTI – lower respiratory tract infection (or equivalent); NPA – nasopharyngeal aspirate; NPS – nasopharyngeal swab; NPW – nasopharyngeal wash; PCR – polymerase chain reaction; IFA – immunofluorescent assay; EIA – enzyme immuno-assay.
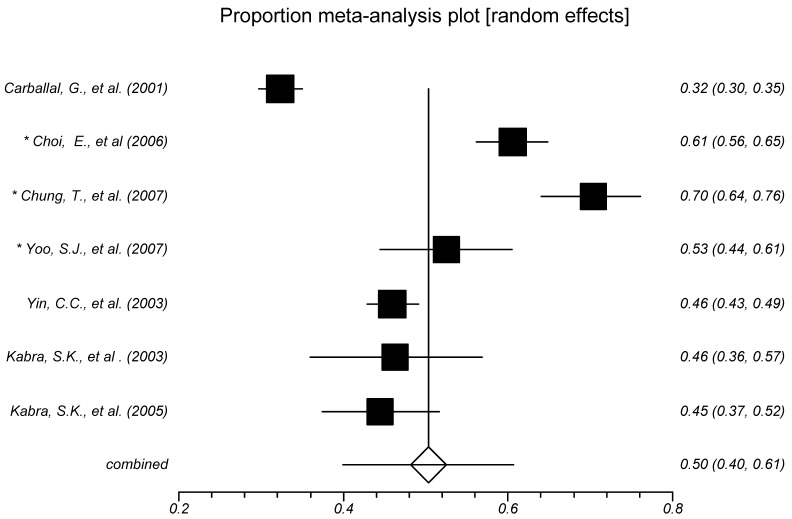
Figure 4 presents the results of meta-analysis of the proportion of children with severe ALRI aged 0-4 years in whom at least one virus was detected (including RSV). Only studies that investigated three or more viruses were included in this analysis – 7 studies in total. This is an arbitrary cut off: these studies were deemed sufficiently active in their approach to detect viral infection, although the final result is likely to under-estimate the true burden. Pooled proportion was 50.4% (95% confidence interval [CI], 40.0% to 60.7%), with I˛ (inconsistency) parameter estimate of 97.0% (95% CI, 96.0% to 97.7%) (Table 2).
Figure 4.
Meta-analysis of the proportion of patients aged 0-4 years with severe acute lower respiratory infections (ALRI) in whom a viral infection was detected (asterisk denotes investigation by polymerase-chain reaction).
Table 2.
Summary of estimated proportions of influenza virus (IV), parainfluenza virus (PIV), and adenovirus (AV) in all hospitalized acute lower respiratory infections (ALRI) and viral hospitalized ALRI using meta-analysis of eligible studies.
| Proportion (%) | 95% confidence interval (%) | |
|---|---|---|
| Proportion of all severe ALRI in children 0-4 y detecting: |
||
| influenza |
3.0 |
2.2-4.0 |
| parainfluenza |
2.7 |
1.9-3.7 |
| adenovirus |
5.9 |
3.4-9.1 |
| Proportion of children with severe ALRI aged 0-4 y in whom at least one virus was detected (including respiratory syncytial virus, RSV): |
50.4 |
40.0-60.7 |
| Proportion of children with severe bronchiolitis aged 0-4 y in whom at least one virus was detected (including RSV): |
66.3 |
56.2-75.6 |
| Proportion of children with severe pneumonia aged 0-4 y in whom at least one virus was detected (including RSV): |
48.7 |
38.0-59.4 |
| Proportion of viral severe ALRI in children 0-4 y detecting: |
||
| influenza |
7.0 |
5.5-8.7 |
| parainfluenza |
5.8 |
4.1-7.7 |
| adenovirus | 8.8 | 5.4-13.0 |
Pneumonia and bronchiolitis
Bronchiolitis as a clinical diagnosis is useful in identifying children who can be presumed unlikely to benefit from antibiotics. Pneumonia (especially focal) is known to have a different spectrum of etiological agents and antibiotics are usually warranted. Six studies that differentiate between these conditions were analyzed separately. The proportion of viruses detected in the bronchiolitis analysis was 66.3% (95% CI, 56.2% to 75.6%) and in the pneumonia analysis 48.7% (95% CI, 38.0% to 59.4%) (Table 2).
Mixed viral ALRI
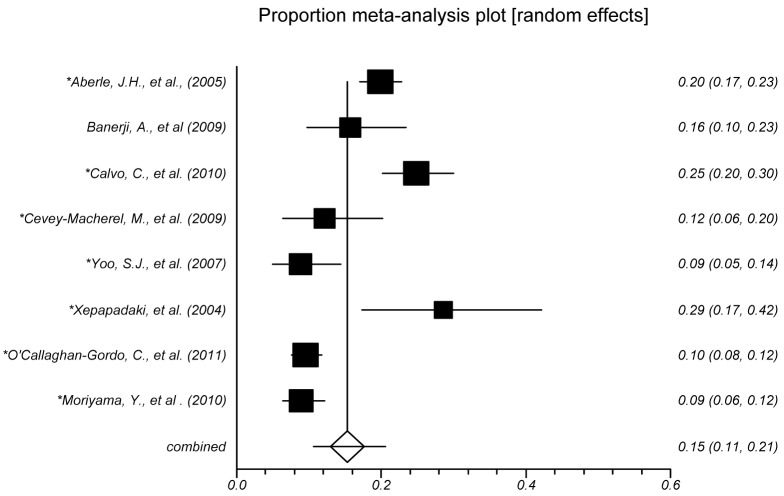
Seven studies investigated three or more viruses using PCR method and reported the proportion of hospitalized childhood ALRI where mixed viral infections were detected (ie, more than one viral pathogen was confirmed). A meta-analysis of those studies showed that pooled proportion was 15.3% (95% CI, 10.6%-20.5%), with I˛ parameter estimate of 91.3% (95% CI, 85.7%-94.0%). Further analysis, which only included virus-positive ALRI cases, estimated that at least 21.9% (95% CI, 17.7%-26.4%) of the viral ALRI were mixed (Figure 5).
Figure 5.
Meta-analysis of the proportion of patients aged 0-4 years with severe acute lower respiratory infections (ALRI) in whom multiple viral infections were detected (asterisk denotes investigation by polymerase-chain reaction).
Proportion of hospitalized ALRI due to influenza viruses
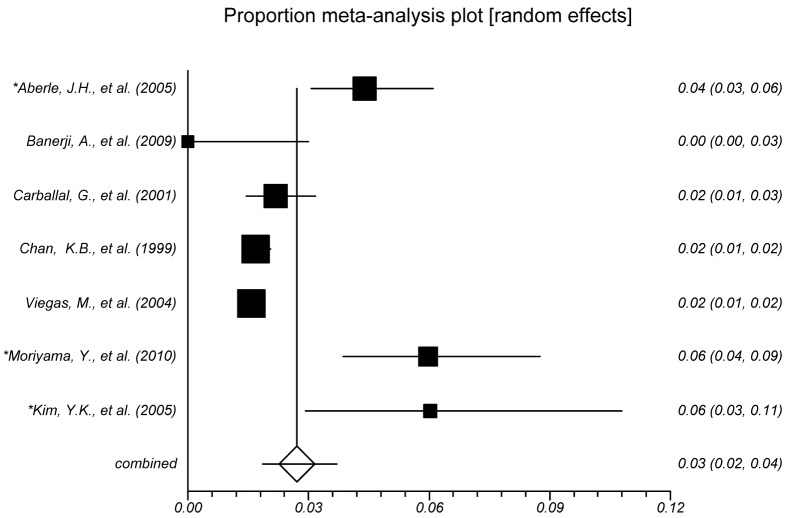
Meta-analysis included 9 studies in which IV infection was laboratory confirmed (Figure 6). We used only the 9 studies in which study population diagnosis was ALRI, rather than pneumonia or bronchiolitis separately. We estimated that 3.0% (95% CI, 2.2%-4.0%) of hospitalized ALRI in children were due to IV, with I˛ parameter estimate of 89.1% (95% CI, 81.7%-92.6%). Further analysis was performed to quantify IV infection as a proportion of all viral ALRI; IV accounted for 7.0% (95% CI, 5.5%-8.7%), with I˛ parameter estimate of 77.3% (95% CI, 47.2%-87.0%) (Table 2).
Figure 6.
Meta-analysis of the proportion of patients aged 0-4 years with severe acute lower respiratory infections (ALRI) in whom a laboratory confirmed influenza infection was detected (asterisk denotes investigation by polymerase-chain reaction).
Proportion of hospitalized ALRI due to parainfluenza viruses
Meta-analysis included 7 studies in which PIV infection was confirmed (Figure 7). Similarly to IV infection, we only included those 7 studies where diagnosis was ALRI, rather than pneumonia or bronchiolitis separately. This analysis also excluded 3 studies where croup was a suspected diagnosis, because PIV are the major cause of croup. We estimated that 2.7% (95% CI, 1.9%-3.7%) of hospitalized ALRI in children were due to PIV, with I˛ parameter estimate of 90% (95% CI, 82.0%-93.5%). Among all virus-positive hospitalized ALRI cases, PIV accounted for 5.8% (95% CI, 4.1%-7.7%), with I˛ parameter estimate of 85.4% (95% CI, 67.1%-91.5%) (Table 2).
Figure 7.
Meta-analysis of the proportion of patients aged 0-4 years with severe acute lower respiratory infections (ALRI) in whom parainfluenza infection was confirmed (asterisk denotes investigation by polymerase-chain reaction).
Proportion of hospitalized ALRI due to adenoviruses
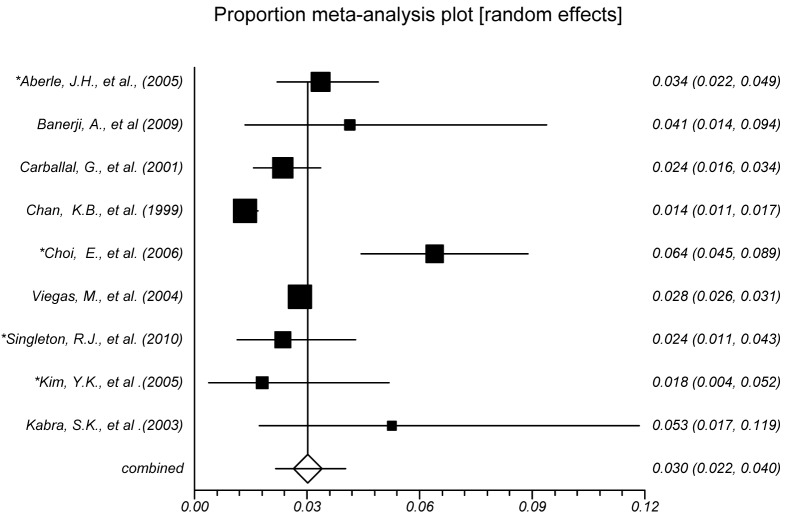
Meta-analysis included 9 studies in which AV infection was confirmed (Figure 8). Similarly to IV infection, we only included those 9 studies in which diagnosis was ALRI, rather than pneumonia or bronchiolitis separately. We estimated that 5.8% (95% CI, 3.4%-9.1%) of hospitalized ALRI in children were due to AV, with I˛ parameter estimate of 98.2% (95% CI, 97.8%-98.5%). Among all virus-positive ALRI cases, AV accounted for 8.8% (95% CI, 5.3%-13.0%), with I˛ parameter estimate of 96.3% (95% CI, 94.9%-97.1%) (Table 2).
Figure 8.
Meta-analysis of the proportion of patients aged 0-4 years with severe acute lower respiratory infections (ALRI) in whom adenovirus infection was confirmed (asterisk denotes investigation by polymerase-chain reaction).
Proportion of hospitalized ALRI due to coronaviruses
The number of available studies on human CV was much smaller than on the other 3 viruses: 8 studies were retained after the initial review, but they did not provide sufficient epidemiological information on the role of CV to perform the meta-analysis and develop reliable estimates. CV are technically difficult to culture, and they were the sole virus detected in 4.8% of patients in one ALRI study (94) and were detected in 10.6% of virus-positive pneumonia patients in another study (59) (supplementary Figure S1(supplementary Figure 1)).
Discussion
This review estimated that 50% of all hospitalized ALRI in pre-school children, 66% of hospitalized bronciolitis episodes, and 49% of hospitalized pneumonia episodes showed viral involvement. All these estimates were derived using very heterogeneous sets of studies (I2>90% in many analyses). This heterogeneity is not surprising, given the large differences in study methods used, participants’ ethnicity, climate and viral endemicity, to name a few. For this reason, random effects models were used, sacrificing statistical power to ensure estimates that would be as valid as realistically possible with available information.
In one third of the episodes of bronchiolitis in hospitalized patients, no virus could be detected, although bronchiolitis is expected to be almost exclusively of viral etiology. We could hypothesize that this lack of sensitivity could be attributed both to imperfections of the tests and the timing of obtaining the sample. It is also possible that, in some studies, the diagnostic process for bronchiolitis can include some asthmatic (non-viral) patients (12). If we assume that the etiological estimates for pneumonia are subject to the same lack of sensitivity, this would mean that our estimates are likely to present a lower bound of the true role of viruses in all ALRI, and that the likely direction of bias is toward under-estimation of the true burden of viruses.
Detection of viral etiologies in hospitalized ALRI has been markedly increased by the use of PCR. We estimated that multiple viruses were involved in at least 15.3% of all cases of ALRI and 21.9% of virus positive ALRI cases. Both of those figures are likely to be underestimates, because they were based on studies that only tested for a limited number of viruses, and not for all known viruses (96).
The analyzed studies might have been affected by differences in regional practice: there were subtle differences in defining criteria for bronchiolitis in different areas (12) and inter-observer reliability in assessing some clinical signs has been low (97). Furthermore, some regions are prone to classifying tracheobronchitis and croup as LRTIs. According to several textbooks of respiratory and pediatric medicine these should be considered upper respiratory tract infections (URTIs) (5,6), because the vocal cords are not the division between upper and lower airway. Surprisingly, no study included in this review detailed the study populations’ past vaccinations; in areas where vaccination against bacterial causes has been implemented, higher proportion of viral etiology would be expected. It also seems likely that a higher proportion of viral ALRI cases that are complicated by bacterial infection would be hospitalized than of those that are pure viral infections. It was beyond the scope of this study to consider virus seasonality or age breakdown for specific viral infections. In contrast to the other main respiratory viruses, PIV has been suggested to cause ALRI more frequently in summer months, while IV is thought to affect older children than RSV (3). Further work is necessary to elucidate these issues.
The delay between sample collection and establishing a diagnosis makes identifying causative agents of little practical use in the majority of acute cases of ALRI. Thus, good etiological epidemiology is important to guide management. There is an argument, both economic and humanitarian, for prevention of viral ALRI over cure. Antiviral agents (with the possible exception of neuraminidase inhibitors for IV) have been shown largely ineffective. Following the successes of vaccination programs for bacterial ALRI, this review makes the case for the growing importance of viral agents and provides the first comprehensive estimates for the burden of viral etiology other than RSV.
Our study conveys some very broad and general messages for the further development of global health policy. First, there seems to be a viral etiological component to at least half of all severe ALRI that require hospitalization, and this number is probably an underestimate for the reasons discussed in this study. This is somewhat unexpected, because it establishes a larger role for viruses in severe ALRI than generally presumed in international health community. Second, although RSV is a dominant viral cause, the role of influenza, parainfluenza, adenoviruses, and coronaviruses should not be neglected: they seem to be jointly responsible for at least a third of all viral severe ALRI and one in six of all severe ALRI. Given that respiratory viruses are amenable to prevention through vaccination, and that their role at the community level is likely to be larger than at the hospital level, our study should allow for modeling of cost-effectiveness of developing such vaccines. With a global roll-out of the existing vaccines against S. pneumoniae and H. influenzae, viral etiology of ALRI will come under increased focus, and understanding the burden associated with particular viral pathogens should help plan global prevention and save further lives.
Acknowledgments
Funding received from Bill and Melinda Gates Foundation.
Ethical approval: Not required.
Declaration of authorship The study was initiated by HN and HC. PKK and FS collected and coded the data and performed data analysis, under supervision by HN and HC. PKK and FS made the first draft. The manuscript was conceptualized by IL and IR, who wrote and edited the final version and prepared it for submission. All authors read and approved the final manuscript.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Additional Material
References
- 1.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters D, Theodoratou E, Campbell H, Rudan I, Chopra M. Optimizing community case management strategies to achieve equitable reduction of childhood pneumonia mortality: An application of Equitable Impact Sensitive Tool (EQUIST) in five low- and middle-income countries. J Glob Health. 2012;2:20402. doi: 10.7189/jogh.02.020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–75. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl 4):S284–9. doi: 10.1093/cid/cir043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaton A, Seaton D, Leitch AG, editors. Crofton and Douglas's Respiratory Diseases. 5th ed. Oxford (UK): Blackwell Science Ltd; 2000. [Google Scholar]
- 6.McIntosh N, Helms PJ, Smyth RL, Logan S, editors. Forfar and Arneil's textbook of pediatrics. 7th ed. Edinburgh (UK): Elsevier; 2008. [Google Scholar]
- 7.Bordley WC, Viswanathan M, King VJ, Sutton SF, Jackman AM, Sterling L, et al. Diagnosis and testing in bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2004;158:119–26. doi: 10.1001/archpedi.158.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Don M, Valent F, Korppi M, Canciani M. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int. 2009;51:91–6. doi: 10.1111/j.1442-200X.2008.02678.x. [DOI] [PubMed] [Google Scholar]
- 9.Korppi M. Community-acquired pneumonia in children: issues in optimizing antibacterial treatment. Paediatr Drugs. 2003;5:821–32. doi: 10.2165/00148581-200305120-00005. [DOI] [PubMed] [Google Scholar]
- 10.De Wals P, Robin E, Fortin E, Thibeault R, Ouakki M, Douville-Fradet M. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2008;27:963–8. doi: 10.1097/INF.0b013e31817cf76f. [DOI] [PubMed] [Google Scholar]
- 11.File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–41. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 12.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 13.Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis-associated deaths among infants in the United States. Pediatr Infect Dis J. 2003;22:483–90. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 14.Panickar JR, Dodd SR, Smyth RL, Couriel JM. Trends in deaths from respiratory illness in children in England and Wales from 1968 to 2000. Thorax. 2005;60:1035–8. doi: 10.1136/thx.2005.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cifuentes L, Caussade S, Villagrán C, Darrigrande P, Bedregal P, Valdivia G, et al. Risk factors for recurrent wheezing following acute bronchiolitis: a 12-month follow-up. Pediatr Pulmonol. 2003;36:316–21. doi: 10.1002/ppul.10365. [DOI] [PubMed] [Google Scholar]
- 16.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner TA, Gravett CA, Healy S, Soma V, Patterson JC, Gravett MG, et al. Emerging biomarkers for the diagnosis of severe neonatal infections applicable to low resource settings. J Glob Health. 2011;1:210–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Meem M, Modak JK, Mortuza R, Morshed M, Islam MS, Saha SK. Biomarkers for diagnosis of neonatal infections: A systematic analysis of their potential as a point-of-care diagnostics. J Glob Health. 2011;1:201–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–6. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC Progress Toward Elimination of Haemophilus influenzae type b Disease Among Infants and Children–United States, 1987-1993. MMWR Morb Mortal Wkly Rep. 1994;43:144–8. [PubMed] [Google Scholar]
- 22.Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998;17:S117–22. doi: 10.1097/00006454-199809001-00005. [DOI] [PubMed] [Google Scholar]
- 23.Harris KM, Maurer J, Kellermann AL. Influenza vaccine – safe, effective, and mistrusted. N Engl J Med. 2010;363:2183–5. doi: 10.1056/NEJMp1012333. [DOI] [PubMed] [Google Scholar]
- 24.Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–16. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 25.Johnson D. Croup. Clin Evid (Online) 2009;2009:0321. [Google Scholar]
- 26.Gray GC, Goswami PR, Malasig MD, Hawksworth AW, Trump DH, Ryan MA, et al. Adult adenovirus infections: loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin Infect Dis. 2000;31:663–70. doi: 10.1086/313999. [DOI] [PubMed] [Google Scholar]
- 27.Gray GC. Adenovirus transmission–worthy of our attention. J Infect Dis. 2006;194:871–3. doi: 10.1086/507435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirschheimer M, Silva PS, Giudici R, Carrilho M, Mauad T, Ishida M. Simultaneous viral infection and childhood bronchiolitis obliterans. Braz J Infect Dis. 2002;6:146–8. doi: 10.1590/S1413-86702002000300009. [DOI] [PubMed] [Google Scholar]
- 29.Massie R, Armstrong D. Bronchiectasis and bronchiolitis obliterans post respiratory syncytial virus infection: think again. J Paediatr Child Health. 1999;35:497–8. doi: 10.1046/j.1440-1754.1999.355369.x. [DOI] [PubMed] [Google Scholar]
- 30.Tristram DA, Miller RW, McMillan JA, Weiner LB. Simultaneous infection with respiratory syncytial virus and other respiratory pathogens. Am J Dis Child. 1988;142:834–6. doi: 10.1001/archpedi.1988.02150080040017. [DOI] [PubMed] [Google Scholar]
- 31.Smith KJ, Fan LL. Insights into post-infectious bronchiolitis obliterans in children. Thorax. 2006;61:462–3. doi: 10.1136/thx.2005.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes KV. SARS-associated coronavirus. N Engl J Med. 2003;348:1948–51. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- 33.Decaro N, Mari V, Desario C, Campolo M, Elia G, Martella V, et al. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet Microbiol. 2008;126:30–9. doi: 10.1016/j.vetmic.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 36.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 39.Glezen WP. The changing epidemiology of respiratory syncytial virus and influenza: impetus for new control measures. Pediatr Infect Dis J. 2004;23:S202–6. doi: 10.1097/01.inf.0000144662.86396.07. [DOI] [PubMed] [Google Scholar]
- 40.Moodley T, Masekela R, Kitchin O, Risenga S, Green RJ. Acute viral bronchiolitis: aetiology and treatment implications in a population that may be HIV co-infected. South African Journal of Epidemiology and Infection. 2010;25:6–8. [Google Scholar]
- 41.Cheng PK, Wong KK, Mak GC, Wong AH, Ng AY, Chow SY, et al. Performance of laboratory diagnostics for the detection of influenza A(H1N1)v virus as correlated with the time after symptom onset and viral load. J Clin Virol. 2010;47:182–5. doi: 10.1016/j.jcv.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 43.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 44.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 46.Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–10. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 47.AlToum R, Bdour S, Ayyash H. Adenovirus infections in Jordanian hospitalized pediatric patients: prevalence and clinical features. Jordan Medical Journal. 2009;43:171–9. [Google Scholar]
- 48.Avendano LF, Palomino MA, Larranaga C. Surveillance for respiratory syncytial virus in infants hospitalized for acute lower respiratory infection in Chile (1989 to 2000). J Clin Microbiol. 2003;41:4879–82. doi: 10.1128/JCM.41.10.4879-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banerji A, Greenberg D, White LF, Macdonald WA, Saxton A, Thomas E, et al. Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children: a case-control study. Pediatr Infect Dis J. 2009;28:697–701. doi: 10.1097/INF.0b013e31819f1f89. [DOI] [PubMed] [Google Scholar]
- 50.Bdour S. Respiratory syncytial virus subgroup A in hospitalized children in Zarqa, Jordan. Ann Trop Paediatr. 2001;21:253–61. doi: 10.1080/02724930120077844. [DOI] [PubMed] [Google Scholar]
- 51.Bedoya VI, Abad V, Trujillo H. Frequency of respiratory syncytial virus in hospitalized infants with lower acute respiratory tract infection in Colombia. Pediatr Infect Dis J. 1996;15:1123–4. doi: 10.1097/00006454-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Bharaj P, Sullender WM, Kabra SK, Broor S. Human bocavirus infection in children with acute respiratory tract infection in India. J Med Virol. 2010;82:812–6. doi: 10.1002/jmv.21637. [DOI] [PubMed] [Google Scholar]
- 53.Bharaj P, Sullender WM, Kabra SK, Mani K, Cherian J, Tyagi V, et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89. doi: 10.1186/1743-422X-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolisetty S, Wheaton G, Chang AB. Respiratory syncytial virus infection and immunoprophylaxis for selected high-risk children in Central Australia. Aust J Rural Health. 2005;13:265–70. doi: 10.1111/j.1440-1584.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 55.Calvo C, Pozo F, García-García ML, Sanchez M, Lopez-Valero M, Pérez-Breńa P, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99:883–7. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canducci F, Debiaggi M, Sampaolo M, Marinozzi MC, Berrč S, Terulla C, et al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol. 2008;80:716–23. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carballal G, Videla C, Sequeira MD, Mistchenko A, Requeijo PV, Arbiza J. Respiratory syncytial virus: changes in prevalence of subgroups A and B among Argentinian children, 1990-1996. J Med Virol. 2000;61:275–9. doi: 10.1002/(SICI)1096-9071(200006)61:2<275::AID-JMV15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 58.Carballal G, Videla CM, Espinosa MA, Savy V, Uez O, Sequeira MD, et al. Multicentered study of viral acute lower respiratory infections in children from four cities of Argentina, 1993-1994. J Med Virol. 2001;64:167–74. doi: 10.1002/jmv.1032. [DOI] [PubMed] [Google Scholar]
- 59.Cevey-Macherel M, Galetto-Lacour A, Gervaix A, Siegrist CA, Bille J, Bescher-Ninet B, et al. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168:1429–36. doi: 10.1007/s00431-009-0943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakravarti A, Chopra K, Setty S. Respiratory syncytial virus in lower respiratory tract infections. Indian Pediatr. 1995;32:1303–5. [PubMed] [Google Scholar]
- 61.Chan PW, Goh AY, Chua KB, Kharullah NS, Hooi PS. Viral aetiology of lower respiratory tract infection in young Malaysian children. J Paediatr Child Health. 1999;35:287–90. doi: 10.1046/j.1440-1754.1999.00359.x. [DOI] [PubMed] [Google Scholar]
- 62.Kaur C, Chohan S, Khare S, Puliyel JM. Respiratory viruses in acute bronchiolitis in Delhi. Indian Pediatr. 2010;47:342–3. doi: 10.1007/s13312-010-0058-6. [DOI] [PubMed] [Google Scholar]
- 63.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis. 2006;43:585–92. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chong CY, Lim WH, Heng JT, Chay OM. The changing trend in the pattern of infective etiologies in childhood acute lower respiratory tract infection. Acta Paediatr Jpn. 1997;39:317–21. doi: 10.1111/j.1442-200X.1997.tb03744.x. [DOI] [PubMed] [Google Scholar]
- 65.Chung JY, Han TH, Kim SW, Hwang ES. Respiratory picornavirus infections in Korean children with lower respiratory tract infections. Scand J Infect Dis. 2007;39:250–4. doi: 10.1080/00365540600999126. [DOI] [PubMed] [Google Scholar]
- 66.Cilla G, Ońate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E. Hospitalization rates for human metapneumovirus infection among 0- to 3-year-olds in Gipuzkoa (Basque Country), Spain. Epidemiol Infect. 2009;137:66–72. doi: 10.1017/S0950268808000666. [DOI] [PubMed] [Google Scholar]
- 67.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis. 2007;196:1321–8. doi: 10.1086/521308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Djelantik IG, Gessner BD, Soewignjo S, Steinhoff M, Sutanto A, Widjaya A, et al. Incidence and clinical features of hospitalization because of respiratory syncytial virus lower respiratory illness among children less than two years of age in a rural Asian setting. Pediatr Infect Dis J. 2003;22:150–7. doi: 10.1097/01.inf.0000048908.43063.c6. [DOI] [PubMed] [Google Scholar]
- 69.Ekalaksananan T, Pientong C, Kongyingyoes B, Pairojkul S, Teeratakulpisarn J, Heng S. Etiology of acute lower respiratory tract infection in children at Srinagarind Hospital, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. 2001;32:513–9. [PubMed] [Google Scholar]
- 70.Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, et al. Human bocavirus: A novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–45. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fry AM, Lu X, Olsen SJ, Chittaganpitch M, Sawatwong P, Chantra S, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.García CG, Bhore R, Soriano-Fallas A, Trost M, Chason R, Ramilo O, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–60. doi: 10.1542/peds.2010-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kabra SK, Broor S, Lodha R, Maitreyi RS, Ghosh M. Can we identify acute severe viral lower respiratory tract infection clinically? Indian Pediatr. 2004;41:245–9. [PubMed] [Google Scholar]
- 74.Kabra SK, Lodha R, Broor S, Chaudhary R, Ghosh M, Maitreyi RS. Etiology of acute lower respiratory tract infection. Indian J Pediatr. 2003;70:33–6. doi: 10.1007/BF02722742. [DOI] [PubMed] [Google Scholar]
- 75.Kim YK, Lee HJ. Human metapneumovirus-associated lower respiratory tract infections in korean infants and young children. Pediatr Infect Dis J. 2005;24:1111–2. doi: 10.1097/01.inf.0000190042.65120.23. [DOI] [PubMed] [Google Scholar]
- 76.Loscertales MP, Roca A, Ventura PJ, Abacassamo F, Dos Santos F, Sitaube M, et al. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr Infect Dis J. 2002;21:148–55. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 77.Moriyama Y, Hamada H, Okada M, Tsuchiya N, Maru H, Shirato Y, et al. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur J Pediatr. 2010;169:1087–92. doi: 10.1007/s00431-010-1183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nascimento-Carvalho CM, Cardoso MR, Ruuskanen O, Lappalainen M. Sole infection by human metapneumovirus among children with radiographically diagnosed community-acquired pneumonia in a tropical region. Influenza Other Respi Viruses. 2011;5:285–7. doi: 10.1111/j.1750-2659.2011.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49:1341–9. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nokes DJ, Okiro EA, Ngama M, Ochola R, White LJ, Scott PD, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi district, Kenya. Clin Infect Dis. 2008;46:50–7. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Callaghan-Gordo C, Bassat Q, Morais L, Díez-Padrisa N, Machevo S, Nhampossa T, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural mozambique: A malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J. 2011;30:39–44. doi: 10.1097/INF.0b013e3181f232fe. [DOI] [PubMed] [Google Scholar]
- 82.Oliveira DB, Durigon EL, Carvalho AC, Leal AL, Souza TS, Thomazelli LM, et al. Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in southeastern Brazil. J Med Virol. 2009;81:915–21. doi: 10.1002/jmv.21436. [DOI] [PubMed] [Google Scholar]
- 83.Samransamruajkit R, Hiranrat T, Chieochansin T, Sritippayawan S, Decrojanawong J, Prapphal N, et al. Prevalence, clinical presentations and complications among hospitalized children with influenza pneumonia. Jpn J Infect Dis. 2008;61:446–9. [PubMed] [Google Scholar]
- 84.Singleton RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–90. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teeratakulpisarn J, Ekalaksananan T, Pientong C, Limwattananon C. Human metapneumovirus and respiratory syncytial virus detection in young children with acute bronchiolitis. Asian Pac J Allergy Immunol. 2007;25:139–45. [PubMed] [Google Scholar]
- 86.Videla C, Carballal G, Misirlian A, Aguilar M. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clin Diagn Virol. 1998;10:17–23. doi: 10.1016/S0928-0197(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 87.Viegas M, Barrero PR, Maffey AF, Mistchenko AS. Respiratory viruses seasonality in children under five years of age in Buenos Aires, Argentina. A five-year analysis. J Infect. 2004;49:222–8. doi: 10.1016/j.jinf.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Weber MW, Milligan P, Sanneh M, Awemoyi A, Dakour R, Schneider G, et al. An epidemiological study of RSV infection in the Gambia. Bull World Health Organ. 2002;80:562–8. [PMC free article] [PubMed] [Google Scholar]
- 89.Weigl JA, Puppe W, Belke O, Neusüss J, Bagci F, Schmitt HJ. Population-based incidence of severe pneumonia in children in Kiel, Germany. Klin Padiatr. 2005;217:211–9. doi: 10.1055/s-2004-822699. [DOI] [PubMed] [Google Scholar]
- 90.Wolf DG, Greenberg D, Kalkstein D, Shemer-Avni Y, Givon-Lavi N, Saleh N, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–4. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 91.Xepapadaki P, Psarras S, Bossios A, Tsolia M, Gourgiotis D, Liapi-Adamidou G, et al. Human Metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–70. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiang Z, Gonzalez R, Xie Z, Xiao Y, Liu J, Chen L, et al. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J Clin Virol. 2010;49:94–9. doi: 10.1016/j.jcv.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin CC, Huah LW, Lin JT, Goh A, Ling H, Moh CO. Lower respiratory tract infection in hospitalized children. Respirology. 2003;8:83–9. doi: 10.1046/j.1440-1843.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- 94.Yoo SJ, Kuak EY, Shin BM. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system. Korean J Lab Med. 2007;27:420–7. doi: 10.3343/kjlm.2007.27.6.420. [DOI] [PubMed] [Google Scholar]
- 95.Global Burden of Disease Study2009. Available from: http://www.globalburden.org/GBD_Study_Operations_Manual_Jan_20_2009.pdf Accessed: April 16, 2013.
- 96.Rudan I, Theodoratou E, Zgaga L, Nair H, Chan KY, Tomlinson M, et al. Setting priorities for development of emerging interventions against childhood pneumonia, meningitis and influenza. J Glob Health. 2012;2:10304. doi: 10.7189/jogh.01.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elphick HE, Lancaster GA, Solis A, Majumdar A, Gupta R, Smyth RL. Validity and reliability of acoustic analysis of respiratory sounds in infants. Arch Dis Child. 2004;89:1059–63. doi: 10.1136/adc.2003.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.