Abstract
Adeno-associated virus (AAV) is a DNA virus with a small (~4.7kb) single-stranded genome. It is a naturally replication-defective parvovirus of the dependovirus group. Recombinant AAV (rAAV), for use as a gene transfer vector, is created by replacing the viral rep and cap genes with the transgene of interest along with promoter and polyadenylation sequences. Only the viral inverted terminal repeats (ITRs) are required in cis for replication and packaging during production. The ITRs are also necessary and sufficient for vector genome processing and persistence during transduction. The tissue tropism of the rAAV vector is determined by the AAV capsid. In this unit we will discuss several methods to deliver rAAV in order to transduce cardiac and/or skeletal muscle, including: intravenous delivery, intramuscular delivery, isolated limb infusion, intrapericardial injection in neonatal mice, and left ventricular wall injection in adult rats.
Keywords: Adeno-associated virus, gene therapy, heart, skeletal muscle, vector delivery
INTRODUCTION
Adeno-associated virus (AAV) is a small, (approximately 25nm) member of the Family Parvoviridae, Subfamily Parvovirinae, Group Dependovirus [International Committee on Taxonomy of Viruses http://ictvonline.org/virusTaxonomy.asp?version=2009&bhcp=1]. AAV has a single-stranded DNA genome of approximately 4.7kb. AAV is a replication deficient DNA virus, with the wild-type AAV genome containing rep and cap genes flanked by short inverted terminal repeat (ITR) sequences. No clinical disease has been associated with AAV, despite the fact that AAV infections are common in humans. Adeno-associated viruses have large capsid sequence variability with 6 clades of AAVs identified thus far (Gao, Vandenberghe et al. 2004). In both natural and experimental infections it is the AAV capsid (serotype) that determines the tissue tropism (Grimm, Pandey et al. 2006). In order to create recombinant AAV (rAAV) for use in gene transfer vectors the rep and cap genes are replaced with the transgene of interest along with enhancer/promoter and polyadenylation sequences. The ITRs remain intact in the recombinant genome because they are necessary for replication and packaging in production as well as vector genome processing and persistence in the transduction process. The rep and cap genes are provided in trans by a complementing plasmid, along with adenovirus or herpes simplex virus helper gene products, to successfully package the rAAVs. rAAV integrates into the transduced cell genome at extremely low efficiency, predominately persisting in non-dividing cells as an extra-chromosomal element (Flotte, Afione et al. 1994; Afione, Conrad et al. 1996).
Cardiac and skeletal muscles are both transduced well by several rAAV serotypes, with AAV9 showing the greatest tropism for both tissue types in most animal models (Bish, Morine et al. 2008). In this unit we will discuss several methods to deliver rAAV in order to transduce cardiac and/or skeletal muscle. Protocols include gene transfer to cardiac and skeletal muscle through intravenous delivery of rAAV in adult mice (Basic Protocol 1), gene transfer to skeletal muscle through direct intramuscular injection (Alternate Protocol 1) and direct intramuscular injection with skin incision (Alternate Protocol 2) in adult mice, gene transfer to skeletal muscle through isolated limb infusion in adult mice (Alternate Protocol 3), gene transfer to cardiac muscle through intrapericardial injection in neonatal mice (Alternate Protocol 4), and gene transfer to the rat myocardium via direct injection into the left ventricular wall (Alternate Protocol 5).
CAUTION: Adeno-associated virus is a Biosafety Level 1 (BSL-1) pathogen because both rAAV and wild-type AAVs are not known to cause disease in healthy human adults. BSL-1 status assumes that the rAAV construct does not encode and toxic or tumorigenic molecule and in produced without a helper virus. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms. Biosafety protocols should be approved by the Institutional Biosafety Committee at the institution where the research is being conducted.
CAUTION: This experiment requires Animal Biosafety Level 1 (ABSL-1) conditions. Biosafety protocols should be approved by the Institutional Biosafety Committee at the institution where the research is being conducted. Protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
BASIC PROTOCOL 1 Gene Transfer to Cardiac and Skeletal Muscle through Intravenous Delivery of rAAV in Adult Mice
Intravenous delivery allows transduction of both cardiac and skeletal muscles via a single injection. Because the vector is delivered systemically the skeletal muscle transduction is not limited to one injected muscle group or limb. The potential pitfall of intravenous injection is unwanted transgene expression in non-target organs such as the liver and the central nervous system. Intravenous delivery generally requires larger doses of vector when compared to local intra-muscular or intra-cardiac delivery. Recommended intra-venous vector doses in mice range from 1×1012 – 1×1014 vector genomes (vg)/kg, although doses as low as 1×1011 vg total have shown detectable luciferase expression in adult mice (Zincarelli, Soltys et al. 2008; Qiao, Koo et al. 2011; Shin, Nitahara-Kasahara et al. 2011). Vector titer quantitation should be carried out by the producer of the rAAV virus (either a University vector core or commercial manufacturer). If confirmation of vector titer is desired in your laboratory, please see Current Protocols in Microbiology UNIT 14D.1 for specifics on how this can be performed. The dose required will depend on the serotype selected, with AAV6-AAV9 showing significant luciferase expression in the hamstring, gastrocnemius, and quadriceps following IV injection (Zincarelli, Soltys et al. 2008). In this unit we will discuss the technique for intravenous delivery in adult mice. For a detailed description of intravenous delivery in neonatal mice, please see the protocol “AAV-mediated gene transfer to the mouse CNS” by Esteves-Sena and Gao, published recently in Current Protocols.
Materials
Mice – choose strain according to planned experiments
rAAV prepared as described in Support Protocol 1 – diluting the vector to a volume of 200ul in sterile PBS
Sterile phosphate buffered saline (PBS)
1ml syringe with 27 gauge 3/8 inch needle
Mouse restrainer
Heat lamp or warm water
Alcohol spray or wipes
Gauze sponges
Prepare the vector or PBS for injection as described in Support Protocol 1.
-
Warm the adult mouse for several minutes in its cage under a heat lamp or in the mouse restrainer by immersing the tail in warm water to dilate the tail vasculature.
Monitor the mice carefully while under the heat lamp to be sure that they don’t over heat. Young adult mice (2–3 months of age) are easiest to inject via the tail vein. Older mice (>6 months old) are more difficult due to the thicker skin of the tail at that age. -
Gently place the mouse in the restrainer.
The mouse should be restrained tightly enough that movement during injection is reduced but not so tightly that breathing is impaired. -
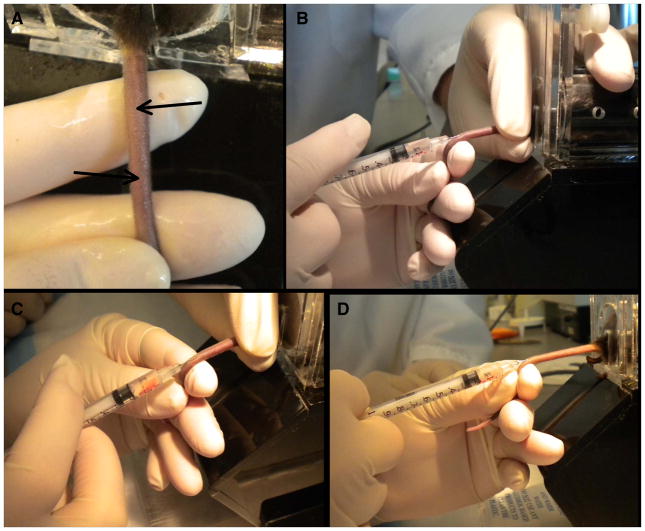
Spray or wipe the tail with 70% alcohol to better visualize the tail veins (Figure 1A).
The tail veins run along the sides of the tail (with the skin sometimes visibly raised over the vein), necessitating either rotating the tail or laying the restrainer on its side to better visualize them. The tail veins are much more easily visualized in white mice than darkly pigmented mice. We recommend that those new to this procedure have a second person hold the tail in the rotated position while they perform the injection; this also prevents the mouse from moving once the needle is positioned properly in the vein (Figure 1B). For more experienced injectors the injection can be performed solo (Figure 1D). Note that mice often have a pigmented line on the dorsal (top) and ventral (bottom) surface of the tail that can be mistaken for the vein. -
Grasp the distal end of the tail and insert the needle, bevel side up, about ½ way down the length of the tail once the vein is visualized (Figure 1B). The vein lies directly under the skin so the needle should be kept parallel to the skin and may need to be redirected to a more shallow location once the skin is pierced.
We recommend injecting ½ way down the tail for several reasons: it allows for good visualization of the vein, thinner skin in this region, easier manipulation of the needle within the tail, room to move up the tail if the injection fails at the first location, and room to visualize blanching of the vessel as the vector is injected. Note from Figure 1B that the tail is held parallel to the floor in the region that is being injected. Grasp the syringe such that minimal movement of the hand is necessary to check for a flash or blood or inject the vector. This will prevent inadvertent exiting of the vessel during these maneuvers. -
Once the needle is placed correct location in the vein can be determined by gently drawing back on the plunger and observing blood within the hub of the needle (Figure 1C).
Note that even when the needle is within the vein, blood is not always obtained. -
Slowly inject a small amount of vector, if the needle is correctly in the vein in vector will flow smoothly and the vein will blanch (turn white) as the vector injects. If the area around the vessels turns pale and the plunger does not advance easier you are likely not in the vein.
If the first attempt is not successful try further up on the same side or attempt to inject into the other tail vein. If both veins are lost during the initial attempts, the animals can be left for 24 hours and attempted again. Monitor the amount of vector that is lost during the failed attempt(s). We recommend diluting the amount of vector to be injected to a volume of 200ul. This allows for a small amount of loss without significantly affecting the titer delivered to the mouse. Once the vector has been injected, slowly remove the needle and place pressure on the tail using a gauze sponge until any bleeding has stopped. Return the mouse to its cage.
Figure 1. Intravenous Tail Vein Injection in Adult Mice.
A: Location of the tail veil along the side of the tail (arrows). Note that the tail has been rotated so that the side of the tail is facing upwards. B: Demonstration of the proper positioning of the tail, syringe, and injector’s hands to successful tail vein injection. C: Note blood in syringe seen when plunger is drawn back after proper placement of the needle within the vein. D: Demonstration of proper positioning when injecting the tail without a second person to hold and rotate the tail.
ALTERNATE PROTOCOL 1 Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection in Adult Mice
Direct intramuscular (IM) injection is a simple technique that allows delivery of vector to a targeted muscle group. Common sites for intramuscular injection in the mouse include the tibialis anterior (TA), gastrocnemius, and quadriceps muscle groups. IM injection allows for direct comparisons in transgene expression or function between injected and uninjected muscle groups. Downfalls of IM injection include variability in transduction due to missing or partially missing the target muscle group during the injection. Another limitation is the volume of vector that can be injected in each muscle group in the mouse due to their small size, this is particularly a problem if small or young mice are being injected. Recommended vector dosages for intra-muscular injection range from 5×108 to 5×1010 vg/site, with the muscle tropism of the serotype selected and the concentration of the vector determining the dosage deliverable (Isotani, Miyake et al. 2011; Qiao, Koo et al. 2011; Zhang and Duan 2012). Higher doses, up to 2.5×1012 have been described, but may be more likely to illicit an immune response (Zheng, Qiao et al. 2010).
Materials
Mice – choose strain according to planned experiments
rAAV prepared as described in Support Protocol 1
-
Xylazine (20mg/ml) and Ketamine (100mg/ml) or Isofluorane
Contact your institutional animal medicine department for assistance in procuring these medications. Injectable grade xylazine and ketamine at the concentrations listed should be used. -
Alternatively Isofluorane can be used
Webster Veterinary
137 Barnum Road
Devens, MA 01434
978-353-6000 (Phone)
800-225-7911 (Toll Free in the U.S.)
978-353-6016 (Fax)
Sterile phosphate buffered saline
Ophthalmic ointment (such as Puralube, Webster Item #: 07-888-2572)
Clippers with #40 blade or depilatory cream (such as Nair)
3/10 cc syringe with 31 gauge needle (8mm length needle) (such as: BD Ultra-Fine II Short Needle Insulin Syringes) or Hamilton Syringe
-
Prepare the vector to be injected (see Support Protocol 1). For smaller muscle groups such as the tibialis anterior or the gastrocnemius a maximum of 20–25ul can be injected per muscle. For the quadriceps up to 50–100ul can be injected (Isotani, Miyake et al. 2011).
If a larger vector dose per animal is desired then both hind limbs can be injected. -
Anesthetize the mouse to be injected with intraperitoneal xylazine plus ketamine (see Support Protocol 2 section for instructions on preparing and dosing) or 2.5% isofluorane through a nose cone or induction chamber. Apply ophthalmic ointment to eyes to prevent corneal drying.
Xylazine/ketamine will provide a longer period of anesthesia (15–20 minutes) which may be useful for the more inexperienced operator. If induction chamber isofluorane is used the animal will need to be left in the chamber for several minutes to allow a long enough period of anesthesia for the injection to be carried out. If a nose cone is used, the injection can be performed while the animal is still in the cone, preventing waking during injection. -
Remove the fur from the area of the limb to be injected, such as the TA or gastrocnemius, in order to better visualize the muscle group you are targeting (Figure 2A). Either clippers or depilatory cream can be used to remove the fur.
As the investigator becomes more experienced with targeting a specific muscle group, hair removal will no longer be necessary. Grasp the mouse such that the limb is held firmly on the limb to be dosed at a site above the area to be injected (Figure 2B).
-
Insert needle into the center of muscle group and slowly inject vector (Video 1).
If the vector is injected correctly into the tibialis anterior or the gastrocnemius the foot will flex upon injection. If no foot flexion is noted the muscle may not have been properly injected. It is important not to grasp the lower limb during delivery, this will prevent foot flexion. Return the animal to its cage and monitor until fully recovered. Normal ambulation (ability to walk) should be monitored the next day.
Figure 2. Muscle Locations and Positioning for Intramuscular Injections.
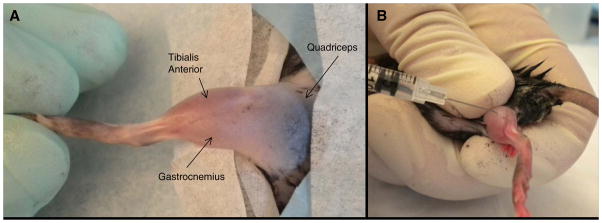
A: Hind limb of the mouse with fur removed to demonstration location of commonly injected muscle groups in the mouse. B: Proper positioning, restraint, and needle angle for proper injection of the gastrocnemius. The animal positioning and restraint would be identical for injecting the TA muscle, only the needle positioning would change.
ALTERNATE PROTOCOL 2 Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection with Skin Incision in Adult Mice
The background for this method of injection is nearly identical to Alternate Protocol 1. Because the muscle is visualized directly the chance of missing the target muscle group are minimized. This method is used in cases where certainty of the muscle group targeted is absolutely necessary, such a physiology of a specific muscle, vector genome isolation from a specific muscle, or retrograde targeting/tracking of virus from a specific muscle to the spinal cord. The common sites for intramuscular injection and vector dosing are identical to those listed for Alternate Protocol 1. Incisional complications are possible with this method.
Materials
Mice – choose strain according to planned experiments
rAAV prepared as described in Support Protocol 1
-
Xylazine (20mg/ml) and Ketamine (100mg/ml) or Isofluorane
Contact your institutional animal medicine department for assistance in procuring these medications. Injectable grade xylazine and ketamine at the concentrations listed should be used. -
Rodent Anesthesia Workstation, including rodent ventilator and isoflurane vaporizer (available fully assembled with all necessary accessories)
Systems Specialties
1800 Mearns Road, Building 3T
Warminster, PA 18974
215-443-9293
215-443-9640
(Manufactured by Hallowell EMC, Pittsfield, MA).
Sterile phosphate buffered saline
Ophthalmic ointment (such as Puralube, Webster Item #: 07-888-2572)
Clippers with #40 blade or depilatory cream (such as Nair)
Chlorhexidine 2% surgical scrub and 70% isopropyl alcohol wipes
#11 sterile scalpel blade with handle
5-0 monofilament suture on a cutting needle (such as polypropylene or nylon) or tissue glue (such as Dermabond)
Needle holder or forceps (if suturing skin)
3/10 cc syringe with 31 gauge needle (8mm length needle) (such as: BD Ultra-Fine II Short Needle Insulin Syringes) or Hamilton Syringe
Prepare the vector to be injected (see Support Protocol 1) as for Alternate Protocol 1.
-
Anesthetize the mouse to be injected with intraperitoneal xylazine plus ketamine or 2.5% isofluorane. Maintain anesthesia using 1.0% isofluorane using a nose cone. Apply ophthalmic ointment to eyes to prevent corneal drying.
Xylazine/ketamine may provide an adequate length of anesthesia for an operator experienced with murine surgical techniques. Animal must remain negative to toe pinch throughout the procedure. For more inexperienced operators maintenance isofluorane will likely be necessary to maintain anesthesia through the entire procedure or a partial dose of xylazine/ketamine can be re-administered if the level of anesthesia becomes too light (see Reagents and Solutions for dosage). Remove the fur from the area of the limb to be injected, such as the TA or gastrocnemius, in order to better visualize the muscle group you are targeting (Figure 2A). Either clippers or depilatory cream can be used to remove the fur.
Secure the mouse in lateral recumbency (on its side) with the leg to be injected extended and the foot taped in position to maintain extension.
Clean the surgical area with 3 scrubs using a Chlorhexidine 2% surgical scrub followed by a scrub with a 70% isopropyl alcohol wipe.
-
Make a 3–4mm skin incision using a #11 scalpel over the muscle group to be targeted.
Note that the skin on the mouse limb is very thin and great care should be taken to incise the skin without damaging the muscle underneath. Insert needle into the center of muscle group and slowly inject vector.
-
Suture skin using a simple continuous pattern or close using tissue glue.
Refer to your local animal care and use committee regarding their recommendation on using systemic or topical antibiotics with the procedure. Allow mice to recover on a heating pad until it is awake and ambulatory.
Return the animal to its cage and monitor until fully recovered. Normal ambulation (ability to walk) should be monitored the next day.
ALTERNATE PROTOCOL 3 Gene Transfer to Skeletal Muscle through Isolated Limb Infusion in Adult Mice
Isolated limb infusion allows vector delivery to the entire muscle mass of the hind limb with one injection. This technique was originally described to deliver non-viral vectors to the limbs of rats but has been subsequently described in mice and has been utilized in a mouse model of Pompe disease (Hagstrom, Hegge et al. 2004; Sun, Li et al. 2010; Phillips, Hegge et al. 2011). Dosage recommendations for this technique range from 1×109 to 1×1011 vg/animal, allowing similar vector doses as IM injection while targeting a larger number of muscle groups (Phillips, Hegge et al. 2011). Disadvantages include difficulty in catheterizing the vessel, the need to optimize the volume injected for the animal size being injected, and potential complications with the surgical site (infection, incisional dehiscence).
Materials
Mice – choose strain according to planned experiments
rAAV prepared as described in Support Protocol 1
-
Xylazine (20mg/ml) and Ketamine (100mg/ml) or Isofluorane
Contact your institutional animal medicine department for assistance in procuring these medications. Injectable grade xylazine and ketamine at the concentrations listed should be used. -
Rodent Anesthesia Workstation, including rodent ventilator and isoflurane vaporizer (available fully assembled with all necessary accessories)
Systems Specialties
1800 Mearns Road, Building 3T
Warminster, PA 18974
215-443-9293
215-443-9640
(Manufactured by Hallowell EMC, Pittsfield, MA).
Polyethylene PE-10 tubing
30 gauge needles
High-pressure syringe pump (Harvard Apparatus)
Heating pad
Clippers with #40 blade or depilatory cream (such as Nair)
Ophthalmic ointment
Chlorhexidine 2% surgical scrub and 70% isopropyl alcohol wipes
#11 sterile scalpel blade with handle
5-0 monofilament suture on a cutting needle (such as polypropylene or nylon) or tissue glue (such as Dermabond)
Needle holder or forceps (if suturing skin)
Small rubber-bands
-
Remove hub from a 30G needles and insert into the end of the PE-10 tubing. This is the needle that will be used for the injection. Use another 30G needle and insert the needle into the opposite end of the tubing (leaving hub intact for syringe attachment).
The length of tubing can be determined based on space between the animal and syringe pump. The tubing should be flushed with sterile saline or the vector solution prior to insertion of the needle in the vein in order to prevent an air embolus when the vector injection is initiated. Prepare vector for injection (see Supplemental Protocol 1). A total volume between 200–1000ul can be used with a vector dosing rage of 109 to 1012 vector genomes.
Anesthetize the mouse to be injected with intra-peritoneal xylazine plus ketamine or 2.5% isofluorane through a nose cone or induction chamber. Apply ophthalmic ointment to eyes to prevent corneal drying.
-
Maintain anesthesia during the procedure using 1% isofluorane through a nose cone.
If the procedure is performed quickly intra-peritoneal xylazine/ketamine may be sufficient to maintain anesthesia throughout the procedure without the need for 1% isofluorane. Animal must remain negative to toe pinch throughout the procedure. A partial dose of xylazine/ketamine can be re-administered if the level of anesthesia becomes too light (see Reagents and Solutions for dosage). Position the animal in dorsal recumbency (chest facing upward) with the foot of the limb to be dosed securely taped down. Shave or us depilatory cream to remove the hair from the medial (inside) aspect of the limb (Figure 3A).
Clean the surgical area with 3 scrubs using a Chlorhexidine 2% surgical scrub followed by a scrub with a 70% isopropyl alcohol wipe.
Apply a rubber band around the base of the hind limb to occlude blood flow (Figure 3A).
-
Make a 0.5–1 cm skin incision using a #11 scalpel along the medial saphenous vein (Figure 3B).
Note that the skin on the medial thigh is very thin and great care should be taken to incise the skin without damaging the vein or the muscle underneath. -
Insert needle, bevel side up, until bevel is fully inside of the vein. Start distally on the vein (toward the foot) in order to be able to move up slightly if the first attempt is unsuccessful.
An unsuccessful attempt at cannulating the vessel initially could cause the vessel to leak when the vector is injected subsequently. -
Attach the distal needle hub to the syringe within the syringe pump and begin infusion at a rate of 1 mL/minute.
The vessel should be monitored carefully to ensure that the needle remains in the vein and that no significant leakage is occurring during injection. It may be necessary to optimize the volume injected for the size mouse being utilized in order decrease vector leakage due to high pressure. After entire dose has been delivered, leave the needle in place for approximately an additional minute to allow the pressure to decrease in the limb.
Remove the catheter and apply pressure to the vessel until and bleeding has stopped.
-
Suture skin using a simple continuous pattern or close using tissue glue.
Refer to your local animal care and use committee regarding their recommendation on using systemic or topical antibiotics with the procedure. Allow mice to recover on a heating pad until it is awake and ambulatory.
Figure 3. Location of the Great (Medial) Saphenous Vein for Limb Infusion Needle Placement.
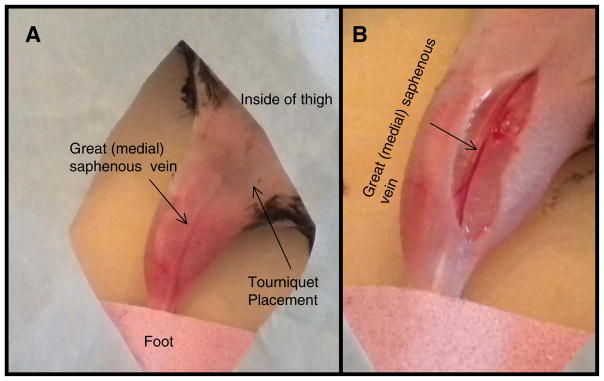
A: Proper restraint of the mouse to locate the great saphenous vein as it runs along the inside of the thigh prior to incision. B: Proper location of the skin incision to expose the great saphenous vein prior to cannulation.
ALTERNATE PROTOCOL 4 Gene Transfer to Cardiac Muscle through Intrapericardial Injection in Neonatal Mice
This method allows targeting of the neonatal mouse heart with a closed chest approach (Zhang, Woo et al. 1999; Bish, Sweeney et al. 2011). With a higher vector dose (2.5X1011 vg) this method allows simultaneous transduction of the heart and diaphragm, particularly with AAV8 and AAV9 (Bish, Morine et al. 2008). AAV9 has been shown to have superior cardiac gene transfer in both the rat and mouse heart over a range of doses (2.5X109 - 2.5X1011 vg) (Bish, Morine et al. 2008). Because this method targets the heart in neonates it offers a screening tool for potential therapeutic transgenes in available mouse models and offers a simple alternative to the creation of heart-specific knock-out (using shRNA) or transgenic animals (Bish, Sweeney et al. 2011).
Materials
Mice – choose strain according to planned experiments
rAAV prepared as described in Support Protocol 1
Hamilton Gastight 250ul glass syringe, series 1725TLL
Hamilton 33 gauge needle
25 gauge needle, 1.5 inch length
Normal saline (sterile)
Saint Gobain Tygon microbore tubing, Formula S-54-HL (inner diameter: 0.51mm, wall thickness: 0.51mm, outer diameter: 1.53mm)
-
On day of injection, prepare sufficient vector to allow for a 50uL injection per pup and an additional 100uL for losses during preparation and injection.
A vector dosage of 2.5 X 1011 vg/pup (50ul of 5 X 1012 vg/ml) results in high level transduction of the heart as well as the diaphragm without extensively targeting the liver. A vector dosage of 2.5 X 1010 vg/pup (50ul of 5 X 1011 vg/ml) results in high level transgene expression in the heart with minimal targeting of the diaphragm and liver. Keep diluted virus on ice until injection. -
Place the Tygon tubing over the 33 gauge Hamilton needle that will be used for injection. The tubing should be cut so that only 3mm of the needle is exposed.
The Tygon tubing will prevent inadvertent advancement of the needle beyond the pericardium into the myocardium or left ventricle allowing easier maintenance in the pericardial space during vector injections. If the needle is advanced too far global cardiac gene transfer will not be obtained. -
Immediately prior to injection, remove 4–5 day old mouse pups from their mother, and place on ice and water mix for approximately 2–3 minutes to induce cryoanesthesia. Mice should not come in direct contact with ice. They can either be placed in a latex glove finger and the glove immersed in ice up to the neck of the pup or alternatively they can be placed in a paper-lined tube which is then packed in ice.
The pups will become lethargic, but will not cease all activity. Prior to 6 days of age mouse pups have a non-fenestrated pericardium, limiting vector leakage from the pericardium into the thoracic cavity/mediastinum. -
While the pups are being anesthetized on ice, attach the 25 gauge needle to the Hamilton syringe and load with the appropriate volume of vector.
Multiple doses can be drawn up in the syringe at the same time. Remove the 25 gauge needle used for drawing up the vector, and attach the Hamilton 33 gauge needle covered with Tygon tubing (prepared in Step 2) to the Hamilton syringe. Remove any air from the syringe by carefully advancing the plunger.
To position the animal for injection, grasp the anesthetized pup by the skin at the back of the neck by pinching between the thumb and index finger of one’s non-dominant hand. Rotate one’s wrist so that the pup’s sternum, ribs, and xiphoid process (tip of the rib cage that extends over the abdomen), can be visualized.
-
Using one’s dominant hand, insert the Hamilton 33 gauge needle at the left costo-xiphoid angle of the pup, and advance the needle superiorly 3mm (until the Tygon tubing contacts the skin).
The left costoxiphoid angle is identified as the anatomic location where the last rib meets the xiphoid process. This is easy to visualize in mice of this age. When advancing the needle it is necessary to maintain and angle parallel to the left sternal border (left side of the sternum). This will allow the needle to remain within the pericardial sack due the pericardium’s attachment to the sternum. This will position the needle below the sternum but above the pericardium while the mouse is held in dorsal recumbency (with the sternum facing upward). -
Once the needle is in place the vector should be injected slowly in the pericardial space.
It is imperative to inject the vector slowly. The operator will experience delayed response time due to the small caliber and high resistance of the Hamilton 33-gauge needle, and rapid injection will result in inadvertent over-injection of vector. Volumes over 50ul are associated with increased pup mortality (Zhang, Woo et al. 1999). After injection, return the pup to the mother. It may be necessary to roll the pup in the dirty home-cage bedding or wipe the dam’s nose with an alcohol wipe to prevent rejection of the pup by the mother. The home cage can be placed on a heating pad to improve warming and the pup and speed recovery. The pup should not be placed directly on a heating pad or under a heating lamp as they may rapidly overheat.
ALTERNATE PROTOCOL 5 Gene Transfer to the Rat Myocardium via Direct Injection into the Left Ventricular Wall
This method allows delivery of vector to the entire left ventricular free wall of adult rats with minimal vector distribution to other organs (Bish, Morine et al. 2008). Use of rat models is important because, as larger animals, rats offer the opportunity to evaluate potentially therapeutic genes in a more clinically relevant model. For example, it is technically more feasible to create models of ischemic cardiomyopathy via coronary artery ligation or models of pressure overload cardiomyopathy via aortic banding in the rat than in the mouse, and these rat models are well-established in the literature (Pleger, Most et al. 2007; Sakata, Lebeche et al. 2007). The injection method described here has been demonstrated to be safe and is not associated with a significant alteration in fractional shortening, ejection fraction, or cardiac geometry in AAV8 and AAV9 injected animals compared to controls (Bish, Morine et al. 2008). The use of AAV9 results in superior transduction of the ventricular wall at doses of 5X1010 –5X1011 vg/rat compared to AAV1, AAV7 and AAV8 (Bish, Morine et al. 2008). One limitation of this technique of this technique is that it requires invasive thoracic surgery to perform.
Materials
Adult Rats – see Troubleshooting section for information on strain selection
rAAV prepared as described in Support Protocol 1
Buprenorphine
Cefazolin
Dobutamine (12.5 mg/ml)
-
Xylazine (20mg/ml) and Ketamine (100mg/ml)
Contact your institutional animal medicine department for assistance in procuring these medications. Injectable grade xylazine and ketamine at the concentrations listed should be used. Suture: 4-0 Maxon, 4-0 Vicryl, 5-0 Vicryl (taper and cutting needle), 7-0 Prolene
-
Rat intubation pack containing intubation speculum, endotracheal intubation tubes, an endotracheal tube guide wire, an incisor loop and a brief video tutorial on how to perform the intubations (catalog number RW-A37 46)
Braintree Scientific:
PO Box 361
Braintree, MA 02185
Phone: 781-348-0768
Fax: 781-843-7932
Rodent Work Stand for intubation (catalog number: RW A3467, Braintree Scientific)
Otoscope for intubation – rechargeable, Braintree Scientific.
18 gauge angiocath (1.88 inch length) (BD Angiocath Autoguard)
-
Rodent Anesthesia Workstation, including rodent ventilator and isoflurane vaporizer (available fully assembled with all necessary accessories)
Systems Specialties
1800 Mearns Road, Building 3T
Warminster, PA 18974
215-443-9293
215-443-9640
(Manufactured by Hallowell EMC, Pittsfield, MA).
-
Recovery ventilator (optional): Model 141
NEMI Scientific, Inc.
51 Main Street
Medway, MA 02053-0198
Phone: 508-533-2436
Email: sales@nemiscientific.com
Standard surgical instrument pack, sterile drapes, sterile gowns, sterile gloves.
Chlorhexidine 2% surgical scrub and alcohol wipes
0.5cc insulin syringe (U-100) with 28 gauge needle, 0.5 inch length
10ml syringe
Anesthetize the rat as described in Support Protocol 3. Administer buprenorphine (0.05 mg/kg) and cefazolin (20mg/kg) by subcutaneous injection prior to surgery. Apply ophthalmic ointment to eyes to prevent corneal drying.
After induction, place the rat in dorsal recumbency (on its back) on the rodent work stand using incisor loop and adjustable restraints.
Use forceps to extend tongue as necessary and visualize the vocal cords using the otoscope with speculum attached. Pass the guide wire through the vocal cords, and then remove the otoscope while keeping the guide wire in place. Pass the endotracheal tube over the guide wire and into position in the trachea; then remove the guide wire.
Use a 10ml syringe to confirm proper placement in the trachea by observing inflation of the chest. Pass a suture around the tube and through the cheek to secure the endotracheal tube in position.
-
Place the rat at the anesthesia work station in right lateral recumbency (left side facing up), and connect the endotracheal tube. The work station should be set with an oxygen flow rate of 2 liters/minute, a ventilation rate of 70 breaths/minute and the isoflurane at 1%.
Attaching the endotracheal tube to the ventilator may require an adaptor which can be acquired from the supplier of the Anesthesia Work Station. Shave the left chest wall and scrub the site three times with chlorhexidine solution followed by an alcohol wipe. Drape the chest with a sterile drape.
Make a 2 cm skin incision over the left fourth intercostal space from dorsal to ventral (top to bottom); gently dissect the subcutaneous tissue and underlying muscle using sterile forceps.
Enter the thorax through the 4th intercostals space. In order to avoid damaging the lung, gently make a small incision in the intercostal tissue (tissue between the ribs) and pleura (tissue lining the chest cavity) to allow a pneumothorax (air in the chest cavity) to form. This will collapse the lung and allow safe expansion of the incision into the thorax.
-
Once the thorax is entered, visualize the left phrenic nerve (it will appear as a thin white line within the pericardium) as it courses over the pericardium, and carefully open the pericardium without disrupting the nerve to expose the left ventricle.
If necessary a small self-retaining rib retractor can be used to mazimize heart exposure. Be careful not to injure the phrenic nerve as this will cause paralysis of the hemi-diaprhagm. -
Place a 7-0 Prolene suture through the apex (inferior most tip) of the left ventricle, and secure the end with a pair of hemostats.
This suture will allow manipulation of the heart during injection. -
Load an insulin syringe with 250 uL of vector. Grasp the 7-0 suture in order to manipulate the position of the heart and increase exposure of the left ventricle during injection.
Care must be taken that the heart is not placed in a position that will restrict blood inflow or outflow for more than 30 seconds at a time. If cardiac function is compromised 0.1ml of dobutamine can be injected into the left ventricular cavity. This can be repeated if necessary. -
The left ventricular myocardium should be injected in five equally spaced aliquots of 50ul.
Prior to injection of each aliquot, draw back on the needle to ensure that you are not in a blood vessel or in the ventricular cavity. If blood is drawn back in the syringe the needle should be repositioned prior to injection. A blanching of the ventricular wall can be seen with each injection.The needle should be manipulated during the injection to maximize the surface area covered by that injection. If the entire ventricular surface cannot be covered with 5 injections the number of injections can be increased, but not the total volume (250ul). Increasing the volume beyond 250ul can lead to cardiac impairment and death. -
After completing the injections, an 18 gauge angiocath should be placed through the skin into the thorax via the fifth or sixth intercostal space and the needle removed.
The catheter will allow decompression of the pneumothorax once the chest wall is closed in order to restore physiologic negative intrathoracic pressure. This will serve as a chest tube to restore negative intrathoracic pressure. Re-approximate the ribs with interrupted 4-0 Maxon suture. Close the muscle layer with continuous 4-0 Vicryl suture. The subcutaneous tissue should be closed with continuous 5-0 Vicryl suture (taper needle), gastrocnemious and the skin with continuous subcuticular 5-0 Vicryl suture (cutting needle).
-
Once suturing is complete, attach a 10cc syringe to the angiocath chest tube, and withdraw the plunger of the syringe until resistance is encountered. Withdraw the angiocath and syringe from the animal, maintaining resistance until it is completely removed. Give a manual sigh breath from the ventilator to re-inflate the lungs completely.
If multiple procedures are being performed the same day, the rat can be moved to a recovery ventilator until spontaneous respirations return. Give buprenorpine and cefazolin at the doses listed above 4 and 12 hours post surgery.
SUPPORT PROTOCOL 1 Preparing rAAV Vector for Delivery
The following are the steps we follow to prepare rAAV vectors for delivery to the mouse.
Materials
Recombinant Adeno-associated Virus (rAAV) – rAAV can be produced in-house (see Unit14D.1), through a local vector core or via a commercial manufacturer.
Pipette tips (200ul)
Pipette (20–200ul)
Parafilm or sterile petri dish
Ice
Delivery syringe or pipette (see particular delivery protocol)
Thaw rAAV vector on ice.
Calculate the volume to be delivered based on vector concentration and dose desired per mouse. If multiple groups are going to be injected (i.e. treatment and control) the same volume of vector should be delivered in each mouse, regardless of group. Therefore the most dilute vector will determine the volume and maximum dose possible. Vector titer quantitation should be carried out by the producer of the rAAV virus (either a university vector core or commercial manufacturer). If confirmation of vector titer is desired in your laboratory, please see Current Protocols in Microbiology UNIT 14D.1 for specifics on how this can be performed. See the dosing protocol that you are using (IM versus IV etc) above for appropriate volumes for each route.
If dilution of the vector is necessary it should be done using sterile saline.
Pipette amount of vector to be dosed per animal. Vector can be pipetted onto clean parafilm and drawn up into dosing syringe or pipetted directly into dosing syringe if using a syringe with a detachable needle. Carefully remove all air from syringe without ejecting vector.
Syringes should then be kept on ice until ready to dose mice.
Any unused, undiluted portion of thawed vector can be kept at 4 degrees Celsius. Refreezing rAAV vectors will result in reduction of infective titer.
SUPPORT PROTOCOL 2: Ketamine/Xylazine Anesthesia Mouse
| Xylazine (20mg/ml stock concentration) | 0.25 ml | (10 mg/kg dose to mouse) |
| Ketamine (100mg/ml stock concentration): | 0.5 ml | (100mg/kg dose to mouse) |
| Sterile Isotonic Saline: | 5 ml | |
|
|
||
| Total Cocktail: | 5.75 mls | |
Dose of cocktail to mouse: 0.10 ml/10 g, intraperitoneally using a 29 gauge needle with a 0.5ml syringe.
Weigh all animals to determine anesthetic dose. Place the animal in a quiet cage following anesthetic administration and allow at least 5 minutes to pass before checking anesthetic depth by toe pinch. If adequate anesthetic depth is not present allow the animal another 5 minutes in the quiet cage. If still not sufficiently anesthetized a 50 mg/kg dose of ketamine alone should be administered. If that does not result in sufficient anesthesia then a 0.05 ml/10 g dose of the xylazine/ketamine cocktail can be re-administered.
SUPPORT PROTOCOL 3 Ketamine/Xylazine Anesthesia Rat
| Xylazine (20mg/ml stock concentration) | 0.75 ml | |
| Ketamine (100mg/ml stock concentration): | 1.8 ml | |
| Sterile Isotonic Saline: | 0.45 ml | |
|
|
||
| Total Cocktail: | 3.0 mls | |
Initial dosage for a 300 gram rat is 0.15–0.20ml of this cocktail. An additional ½ dose can be given if the rat still responds to pain after 5 minutes.
COMMENTARY
Background Information
Adeno-associated virus vectors can efficiently transduce both cardiac and skeletal muscle through either local or systemic delivery. AAV1, AAV6, AAV7, AAV8 and AAV9 each have the ability to efficiently transduce muscle tissue, with multiple studies showing that cardiac transduction is most efficiently mediated by AAV9 (Inagaki, Fuess et al. 2006; Bish, Morine et al. 2008; Zincarelli, Soltys et al. 2008). AAV vectors are capable of targeting both dividing (myoblasts) and non-dividing (myofibers and cardiomyocytes) cells with long-term persistence, potentially for the life of the animal, possible in non-dividing cells.
Targeting cardiac and skeletal muscle tissue in mice and rats allows for the development of therapeutic vectors for diseases ranging from muscular dystrophies, to Pompe disease, to disorders of fatty acid oxidation that effect both the heart and skeletal muscle. Potential cardiac gene therapy targets include a wide range of models of cardiomyopathy, both acute and chronic. Skeletal muscle, because of its large mass, has also been targeted to express non-muscle secreted proteins such as alpha-1 anti-trypsin, coagulation factor IX, apolipoprotein E and erythropoietin. It is also possible to transduce both the peripheral and central nervous tissue innervating a specific muscle group by retrograde transport following intramuscular delivery (Zheng, Qiao et al. 2010).
While direct delivery to the heart or specific skeletal muscle groups allows for transgene expression in the desired tissue with minimal vector genomes reaching other organs, many times systemic delivery of a vector to target multiple or all skeletal muscle groups in addition to the heart is desired. In this case systemic intravascular delivery may be necessary, but this carries with it the risk of expressing the transgene of interest in off-target organs, such as the liver. Specificity for muscle and heart following intravenous delivery can be increased by including a muscle specific promoter and/or enhancer in the transgene cassette (Wang, Li et al. 2008; Prasad, Xu et al. 2011). Another potential option for limiting transgene expression in organs other than the muscle would be to include microRNA binding sites in the transgene cassette that will mediate the binding of tissue specific microRNAs in order to suppress expression of the transgene in those tissues. For example, inclusion of a microRNA binding site for MiR-122 will silence expression in the liver (Xie, Xie et al. 2011).
While all of the procedures described in this unit are scalable to large animal species such as dogs, pigs, and non-human primates, vector dosage becomes more of an issue because of the large amounts of vector required, especially for intravenous dosing. Local delivery such as intra-cardiac and limb vascular delivery can reduce the amount of vector required for adequately transducing the tissue in these larger species ((Rodino-Klapac, Janssen et al. 2007; Gao, Bish et al. 2011; Qiao, Koo et al. 2011). It should be noted that the vector tropism found even in non-human primates (other than chimpanzees) may not correlate to the vector tropism ultimately seen in humans when clinical trials are performed.
For a discussion of the potential immune responses that can be encountered with AAV delivery, please see the Current Protocol’s chapter on Gene Transfer in the Lung Using Recombinant Adeno-Associated Virus by Gruntman et al.
Critical Parameters and Troubleshooting
Gene Transfer to Cardiac and Skeletal Muscle through Intravenous Delivery of rAAV in Adult Mice
It is critical that the operator practice intravenous (IV) injections in multiple mice, until successful injection is possible the majority of the time, prior to attempting to inject vector. If efficient injections are not obtained consistently variable doses of vector will be delivered and the results will be difficult to interpret. The most common reason for IV injections to fail are inadequate vessel dilation prior to injection, inaccurate identification of vein (trying to inject into the pigmented line often present on the dorsal and ventral surface of the tail), and movement of the needle out of the vein as the plunger is either drawn back or advanced.
Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection in Adult Mice
The most common reason for intramuscular injection failure is missing the target muscle group when injecting the vector. Removing the fur from the area over the muscle group will aid in targeting the muscle. Observing the foot flex following injection of the TA and gastrocnemius will also insure that the muscle was injected. It is also imperative that the animal is properly anesthetized when the lower limb is being injected to allow the operator time to properly position and inject the animal. Anesthesia is not necessary when injecting the quadriceps and the back of the thigh (see internet reference for pictures demonstrating injections in the caudal thigh). Practicing several mice where a dye (such as methylene blue, 1%) is injected and then the muscle dissected will help the new operator ensure that they are using proper technique.
Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection with Skin Incision in Adult Mice
This method increases the likelihood of injecting the target muscle group. In order for the accuracy of this technique to be optimized the operator must ensure that they know the exact anatomic location of the muscle group to me targeted to ensure the incision is properly placed. Careful dissection of the muscles in the area on a single mouse prior to attempting the procedure will help ensure that the correct muscle is targeted.
Gene Transfer to Skeletal Muscle through Isolated Limb Infusion in Adult Mice
The most difficult aspect of this procedure is cannulation of the vessel with the needle. Practicing vessel cannulation on several animals until it can be successfully performed is necessary prior to vector dosing. Ensure that the syringe pump is set-up and working prior to anesthetizing the mouse to ensure that no complications are encountered with the equipment during the procedure. Titrating the vector volume delivered, allowing time for the delivered volume to disperse in the tissue prior to needle removal, and holding off of the vessel following catheter removal will decrease vector dose losses due to vector leakage.
Gene Transfer to Cardiac Muscle through Intrapericardial Injection in Neonatal Mice
The Tygon tubing will create a hub at 3mm, which will prevent advancement of the needle into the myocardium or left ventricular cavity and allow the investigator to maintain the needle easily in the pericardial space for injection. It is critical for global cardiac gene transfer that the vector be injected into the pericardial space and not the myocardium or left ventricular cavity.
It is critical to inject the vector solution slowly as we have frequently experienced a delayed response time when using a Hamilton 33 gauge needle. This will help to avoid over-injection and/or loss of vector. New investigators may consider injecting saline into several control pups to become familiar with the Hamilton needle and syringe prior to working with vector.
The injection volume of 50uL has been optimized previously. Larger volumes have been associated with increased mortality (Zhang, Woo et al. 1999). In our experience, mortality is extremely rare with the 50uL injection.
Gene Transfer to the Rat Myocardium via Direct Injection into the Left Ventricular Wall
An instructive video is supplied with the rat intubation pack that first-time users may find helpful. Intubation should proceed as quickly and as smoothly as possible. We have noted that loss of spontaneous respiration can be associated with prolonged and/or traumatic intubations. If this occurs, emergency tracheotomy can be performed.
We have found that the rate of re-intubation can be greatly reduced by extubating the rat only after spontaneous movement has been observed.
If rats are to be used in an ischemic cardiomyopathy model, we recommend the use of Lewis inbred rats. It has been previously reported that ligation of the left anterior descending artery in these rats results in a larger and more uniform infarct with lower mortality when compared to Sprague-Dawley rats (Liu, Yang et al. 1997).
Anticipated Results
All of the cardiac and skeletal muscle delivery techniques described above should result in widespread cardiac and/or skeletal muscle transduction, depending on serotype and promoter used. The duration to maximum expression of the delivered transgene will depend on multiple factors, including serotype, promoter, and whether vector is single stranded or self-complementary. Expression should be expected within 7 days with peak expression likely between 3–12 weeks. Because both cardiac and skeletal muscles have minimal cellular turnover, long-term transgene expression is expected.
Time Considerations
Gene Transfer to Cardiac and Skeletal Muscle through Intravenous Delivery of rAAV in Adult Mice
Intravenous delivery is relatively quick once the technique is practiced. It will take an average of 5–10 minutes per mouse plus 5 minutes to warm the mouse. Multiple mice can be warmed at once as long as they are monitored to be sure they don’t overheat. Efficiency can be increased if once person warms to mice and puts in the restrainer and holds the tail while a second person prepares the vector for injection and injects the mice.
Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection in Adult Mice
The injection itself takes less than 1 minute, especially once the technique is practiced. It takes several minutes for the mouse to become fully anesthetized following either intra-peritoneal xylazine/ketamine or isoflurane induction. Time per mouse can be decreased if multiple mice are anesthetized at once. It may take 30–45 minutes for the mice to fully recover from anesthesia.
Gene Transfer to Skeletal Muscle through Direct Intramuscular Injection with Skin Incision in Adult Mice
This method decreases the likelihood of misinjecting the target muscle group. Once the skin incision and closure are mastered the procedure should take less than 10 minutes to complete per animal plus time for induction and recovery of anesthesia (see direct intramuscular injection section above).
Gene Transfer to Skeletal Muscle through Isolated Limb Infusion in Adult Mice
It will take least 1 hour per mouse to perform this procedure and an additional hour for the mouse to recover from anesthesia. Additional mice can be dosed while the first mice are recovering. The most time consuming part for the novice operator will be needle placement and skin suturing.
Gene Transfer to Cardiac Muscle through Intrapericardial Injection in Neonatal Mice
This is a very quick procedure. It will take approximately 3–5 minutes to cool the mice in order to induce cryoanesthesia. Each single injection requires approximately 1 minute. Based on these estimates, a litter of 8–10 mice can easily be injected in 15 minutes.
Gene Transfer to the Rat Myocardium via Direct Injection into the Left Ventricular Wall
It will take approximately 20 minutes to sedate and intubate the rat. The surgical injection procedure requires an additional 20 minutes to perform. Recovery from anesthesia can be variable and can take up to 30 minutes per rat. If multiple surgeries are to be performed on the same day, it will be useful to purchase 1–2 ventilators that will be used solely for recovery. This will allow the next surgery to start immediately following skin closure of the previous surgery, without the need to wait for return of spontaneous respiration. These ventilators are less expensive than the Anesthesia Workstation since they are not equipped with isoflurane vaporizes.
Supplementary Material
Direct Intramuscular Injection. Video depicts proper grasping of the hind limb for gastrocnemius IM injection. Note that the foot twitches slightly as the PBS is injected, indicating that the injection is going intramuscular rather than perimuscular.
Acknowledgments
Special thanks to Cynthia Greer and Qiushi Tang for their assistance in taking the photographs for the figures. This work was supported in part by grants from the National Institutes of Health to L.H.S. (Wellstone Muscular Dystrophy Cooperative Center grant U54-AR-052646-06 and a grant from the Parent Project Muscular Dystrophy), to T.R.F. (P01 DK58327 and 2 R01 HL069877-10), and to G.G. (2P01 HL059407, 1P01AI100263-01 and 2R01NS076991-01).
Footnotes
Detailed protocols for rAAV vector production and titration/analysis.
Internet Resources
http://www.hallowell.com/index.php?doc=2&pr=Video_Presentations
Video demonstrating intubation using a speculum in the rat.
http://www.theodora.com/rodent_laboratory/injections.html
Photographs demonstrating intramuscular injection in the mouse caudal thigh.
Literature Cited
- Afione SA, Conrad CK, et al. In vivo model of adeno-associated virus vector persistence and rescue. Journal of Virology. 1996;70(5):3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Morine K, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19(12):1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Sweeney HL, et al. Adeno-associated virus vector delivery to the heart. Methods Mol Biol. 2011;807:219–237. doi: 10.1007/978-1-61779-370-7_9. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, et al. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. American Journal of Respiratory Cell and Molecular Biology. 1994;11(5):517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- Gao G, Bish LT, et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther. 2011;22(8):979–984. doi: 10.1089/hum.2011.042. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, et al. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. Journal of Virology. 2004;78(12):6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Pandey K, et al. Liver Transduction with Recombinant Adeno-Associated Virus Is Primarily Restricted by Capsid Serotype Not Vector Genotype. Journal of Virology. 2006;80(1):426–439. doi: 10.1128/JVI.80.1.426-439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom JE, Hegge J, et al. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10(2):386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isotani M, Miyake K, et al. Direct comparison of four adeno-associated virus serotypes in mediating the production of antiangiogenic proteins in mouse muscle. Cancer Invest. 2011;29(5):353–359. doi: 10.3109/07357907.2011.584585. [DOI] [PubMed] [Google Scholar]
- Liu YH, Yang XP, et al. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am J Physiol. 1997;272(2 Pt 2):H722–727. doi: 10.1152/ajpheart.1997.272.2.H722. [DOI] [PubMed] [Google Scholar]
- Phillips JL, Hegge J, et al. Systemic gene transfer to skeletal muscle using reengineered AAV vectors. Methods Mol Biol. 2011;709:141–151. doi: 10.1007/978-1-61737-982-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger ST, Most P, et al. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115(19):2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Xu Y, et al. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2011;18(1):43–52. doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C, Koo T, et al. Gene therapy in skeletal muscle mediated by adeno-associated virus vectors. Methods Mol Biol. 2011;807:119–140. doi: 10.1007/978-1-61779-370-7_5. [DOI] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Janssen PM, et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Lebeche D, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42(4):852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Nitahara-Kasahara Y, et al. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther. 2011;18(9):910–919. doi: 10.1038/gt.2011.36. [DOI] [PubMed] [Google Scholar]
- Sun B, Li S, et al. Hydrostatic isolated limb perfusion with adeno-associated virus vectors enhances correction of skeletal muscle in Pompe disease. Gene Ther. 2010;17(12):1500–1505. doi: 10.1038/gt.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, et al. Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther. 2008;15(22):1489–1499. doi: 10.1038/gt.2008.104. [DOI] [PubMed] [Google Scholar]
- Xie J, Xie Q, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19(3):526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Woo YJ, et al. Efficient transmural cardiac gene transfer by intrapericardial injection in neonatal mice. J Mol Cell Cardiol. 1999;31(4):721–732. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]
- Zhang JCL, Woo YJ, et al. Efficient Transmural Cardiac Gene Transfer by Intrapericardial Injection in Neonatal Mice. Journal of Molecular and Cellular Cardiology. 1999;31(4):721–732. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther. 2012;23(1):98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Qiao C, et al. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum Gene Ther. 2010;21(1):87–97. doi: 10.1089/hum.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, et al. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
Key Reference
- Mueller C, Zhong L, et al. Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol. 2012;Chapter 14(Unit14D):11. doi: 10.1002/9780471729259.mc14d01s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Direct Intramuscular Injection. Video depicts proper grasping of the hind limb for gastrocnemius IM injection. Note that the foot twitches slightly as the PBS is injected, indicating that the injection is going intramuscular rather than perimuscular.