Abstract
Rotator cuff tears are common conditions that can alter shoulder mechanics and may lead to damage of intact joint tissues. These injuries are of particular concern in populations who perform tasks requiring repetitive overhead activity (e.g., athletes and laborers) and who are likely to return to aggressive pre-injury activity levels despite limited understanding of the potentially damaging effects on the remaining tissues. Therefore, we investigated the effect of returning to overuse activity following a supraspinatus tear on shoulder function and the mechanical properties of the remaining intact tendons and glenoid cartilage. Forty rats underwent 4 weeks of overuse activity to create a tendinopathic condition followed by detachment of the supraspinatus tendon and were then randomized into two groups: continued overuse or cage activity. Ambulatory measurements were performed throughout the 8 weeks prior to euthaniasia, and properties of the adjacent tendons and cartilage were evaluated. Results demonstrated that shoulder function was not compromised in the return to overuse group. However, alterations of the glenoid cartilage and biceps tendon properties occurred. Our results help define the contributory roles of common mechanical injury mechanisms and provide a framework by which physicians could better prescribe long-term treatment strategies for patients.
Keywords: rotator cuff, animal model, overuse injury
Rotator cuff tendon tears are common and are of particular concern in populations who perform repeated overhead activities (e.g., laborers and athletes). If left untreated, rotator cuff tears may lead to secondary damage in the surrounding tissues of the shoulder, particularly to adjacent tendons1,2 and articular cartilage (termed cuff tear arthropathy).3 Two mechanical mechanisms, altered loading (due to force imbalance) and excessive loading (due to overload or overuse), have both been implicated as causative factors for the initiation and progression of tendon and cartilage damage.1,4,5 However, the mechanism by which rotator cuff tears lead to joint damage is poorly understood, and clinicians often rely on anecdotal evidence to recommend treatment options without understanding the potentially damaging effects on the remaining shoulder joint tissues.
Rotator cuff tears are commonly isolated to the supraspinatus tendon, resulting in increased superior translation of the humeral head and abnormal glenohumeral joint kinematics. This may place patients at higher risk for the development of secondary joint damage due to compensation from the remaining tendons (i.e., infraspinatus, long head of the biceps, and subscapularis),2,6 and over time, isolated supraspinatus tears may progress to these posterior or anterior portions of the shoulder (infraspinatus and subscapularis tendons, respectively). Clinical investigations showed a correlation between supraspinatus tears and secondary joint pathology, such as impaired joint function7 and long head of the biceps pain.8 Basic science studies support these findings; in the presence of supraspinatus tears, joint function is altered, and the biceps, infraspinatus, and subscapularis tendons have decreased mechanical properties.1,2,9,10
Overuse activity has also been implicated as an etiologic factor in many tendon injuries (e.g., tendinitis, tendinosis, and partial tendon rupture)11 and cartilage degeneration.12,13 Previous basic science studies showed that overuse activity in the uninjured supraspinatus tendon leads to decreased mechanical properties and fiber organization, and increased cellularity and cross-sectional area.5 Additionally, excessive running induces cartilage degeneration in the knee joint.12 However, no scientific evidence exists on overuse activity following tears of the rotator cuff tendons. This is of particular concern for overhead athletes and manual laborers, who are at high risk for developing cuff tears, and due to the nature of their sport or occupation, will return to such “overuse” activity if they are physically able, despite their torn rotator cuff tendons. Currently, only anecdotal evidence exists to determine if returning to activity is recommended or may lead to further damage of the surrounding joint structures (due to overload or overuse). Therefore, we investigated the effect of returning to overuse activity following a supraspinatus rotator cuff tear on shoulder function and the mechanical properties of the remaining intact tendons and glenoid cartilage. We hypothesized that overuse activity following a tendon tear would not alter shoulder function, but would decrease mechanical properties among the remaining intact tendons (including the biceps, infraspinatus, and upper and lower subscapularis) and the superior glenoid cartilage.
METHODS
Following a 2-week training period, 40 adult male Sprague–Dawley rats (400–450 g) underwent 4 weeks of overuse activity (downhill (10°) treadmill running at 17 m/min for 1 h/day, 5 days/week)5 to induce a tendinopathic condition in the supraspinatus tendon. Overuse activity was performed prior to tendon detachment to replicate the human condition by which tendons first degenerate over time and then ultimately fail and tear. The animals then underwent unilateral detachment of the left supraspinatus tendon (IACUC approved) as described previously.14 Briefly, with the arm in external rotation, a 2 cm skin incision was made followed by blunt dissection down to the rotator cuff musculature. The rotator cuff was exposed, and the tendons were visualized at their humeral insertions. The supraspinatus tendon was then separated from the other rotator cuff tendons before sharp detachment at its insertion on the greater tuberosity using a scalpel blade. Any remaining fibrocartilage at the insertion was left intact, and the detached tendon was allowed to freely retract. The overlying muscle and skin were closed, and the animals were randomized into cage activity or continued overuse groups. The overuse group returned to 1 week of cage activity and was gradually returned to the overuse protocol over a period of 2 weeks, followed by an additional 5 weeks of overuse activity. All animals were euthanized 8 weeks following surgical tendon detachment and frozen (−20°C) until mechanical testing.
Quantitative Ambulatory Assessment
Forelimb gait and ground reaction forces were quantified using an instrumented walkway.15 Data was collected 1 day prior to rotator cuff detachment surgery to obtain baseline ambulatory values and then collected at Days 3, 7, 14, 28, 42, and 56 postdetachment surgery. Ground reaction force data, including medial/lateral, braking, propulsion, and vertical forces were collected for each walk. Paw print analysis allowed for measurement of several temporal and spatial parameters, including stride length, step width, and speed. At each time, at least two walks were recorded/animal, as well as body weight. For each animal, parameters were averaged across walks and normalized to body weight for each day.
Tendon Mechanical Testing
The animals were thawed, and the scapula and humerus were dissected with the biceps, infraspinatus, and subscapularis tendons intact. Tendon testing was performed as previously described.1,16 Briefly, stain lines, for local optical strain measurement (at insertion and mid-tendon), were placed on the biceps, infraspinatus, and upper and lower bands of the subscapularis tendons. Cross-sectional area was measured using a custom laser device.17 The scapula and humerus were embedded in a holding fixture using PMMA, gripped with cyanocrylate annealed sand paper in custom grips, and immersed in PBS at 37°C. Tensile testing was performed as follows: preload to 0.08 N, preconditioning (10 cycles of 0.1–0.5 N at a rate of 1% strain/s), stress relaxation to 4% (biceps) or 5% (infraspinatus and subscapularis) strain at a rate of 5% strain/s for 600 s, and ramp to failure at 0.3% strain/s. Stress was calculated as force divided by initial area, and 2D Lagrangian strain was determined from stain line displacements that were measured from images using custom texture tracking software.2
Cartilage Mechanical Testing
Following tendon testing, the glenoid was prepared for testing by sharply detaching the biceps tendon at its insertion at the superior rim of the glenoid using a scalpel blade. The glenoid was then preserved by wrapping in soft tissue and refrozen (−20°C).
For the cartilage thickness measurement, each scapula was thawed and immersed in PBS containing a protease inhibitor cocktail (5 mM Benz–HCl, 1 mM PMSF, 1 M NEM) at room temperature. Specimens were scanned at 0.25 mm increments using a 55 MHz ultrasound probe (Visualsonics, Inc., Toronto, Ontario, Canada) in plane with the scapula. Captured B-mode images of each scan were manually segmented (three times and averaged) by selecting the cartilage and bony surfaces of the glenoid. The 3D positions of these surfaces were reconstructed with a custom program (MATLAB) and used to determine cartilage thickness maps. Each map was divided into six regions (center (C), posterior–superior (PS), posterior–inferior (PI), anterior–superior (AS), anterior–inferior (AI), and superior (S)), and an average thickness was computed for each region.9 Following scanning, specimens were again preserved by wrapping in soft tissue and refreezing (−20°C) until testing.
For cartilage mechanical testing, each scapula was thawed and immersed in PBS containing the protease inhibitor cocktail at room temperature. Utilizing a 0.5 mm diam., non-porous spherical indentor tip, cartilage indentation testing was performed9: preload (0.005 N), eight step-wise stress relaxation tests (8 μm ramp at 2 μm/s followed by a 300 s hold). The scapula was repositioned for each localized region using angular, rotational, and linear stages such that the indenter tip was perpendicular to the cartilage surface in each region. Cartilage thickness for indentation testing was determined by identifying the indentation location on each cartilage thickness map. Equilibrium elastic modulus was calculated, as described previously,18 at 20% indentation and assuming Poisson’s ratio (v = 0.30).
Histology
Separate samples for histology were left intact with muscle and processed using standard paraffin procedures. The intact rotator cuff muscles were pinned to prevent tissue contracture during paraffin processing, and the humeral head was maintained in neutral rotation. For the scapula, the bony glenoid origin of the biceps was resected, and the bone–tendon–muscle units of the biceps tendon were pinned and processed separately from the remaining glenoid cartilage. Sagittal sections (7 μm) were collected, and tendon samples were stained with hematoxylin–eosin (H&E) while cartilage samples were stained with Safranin O, Fast Green, and Iron Hematoxylin. H&E stained sections were imaged at the insertion site and mid-substance of each tendon using a microscope (Leica DM LB 100T, Buffalo Grove, IL) at 200× magnification and were graded on a scale from one to three for cellularity and cell shape (with a value of three corresponding to the largest cell number and the most rounded cell shape, respectively).19 Due to the unique anatomy of the biceps tendon, the biceps mid-substance region was further subdivided into regions (intra-articular space, proximal groove, and distal groove).1 Cartilage sections were imaged at 200× magnification in five regions (C, PS, PI, AS, and AI) corresponding to the indentation locations and graded using a Modified Mankin Score.20 All histology scoring (for tendon and cartilage) was performed by three blinded investigators, and the mode of these three values was taken as the score for each specimen.21
Statistics
For ambulation data, to determine the effect of group and time, significance was assessed using a two-way ANOVA with repeated measures on time. If a significant interaction effect was observed, follow-up paired t-tests were conducted to determine where the significant interaction occurred. Due to the nature of measuring rat ambulation, data points were occasionally absent (~7%) for a specific animal on a specific day. Therefore, multiple imputations were conducted on the ambulation data to allow for a repeated measures analysis. For tendon and cartilage mechanics, significance was assessed using an unpaired one-tailed t-test. For cartilage thickness data, the normality of the data was assessed, and due to the non-parametric distribution, significance was assessed using a Mann–Whitney test. For tendon and cartilage histology, median grades were compared between groups within each region using a Mann–Whitney test. For all comparisons, significance was set at p < 0.05 and trends at p < 0.1.
RESULTS
Ambulatory Data
Overuse activity following supraspinatus tendon detachment did not have a significant main effect for group (p < 0.05) on the ambulatory measurements (including ground reaction forces, spatial, and temporal parameters) compared to the cage activity group (S-Table I). However, there was a significant interaction effect (p = 0.03) for propulsion force. Post hoc paired t-tests demonstrated the overuse group had a significantly larger propulsion force at 42 days compared to cage activity.
Tendon Properties
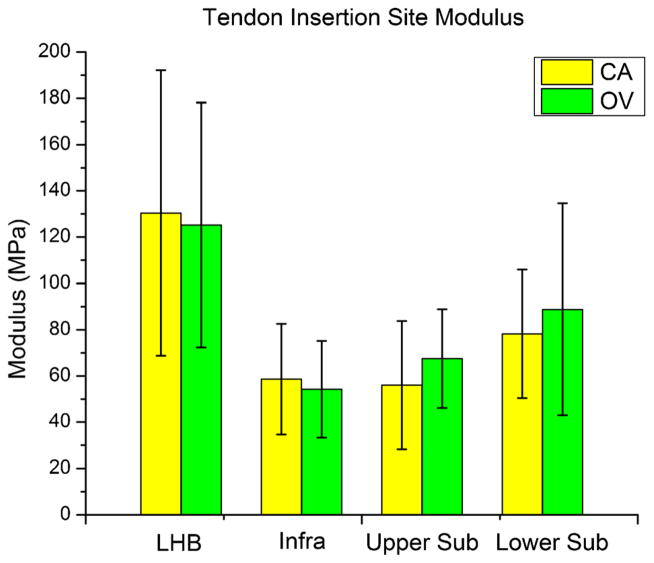
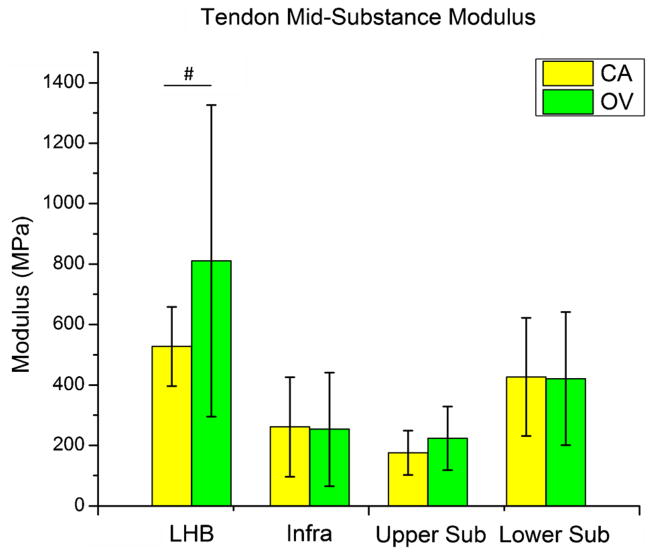
Overuse activity following supraspinatus tendon detachment did not increase (p = 0.43) long head of the biceps tendon elastic modulus at the insertion site (Fig. 1). However, a trend (p = 0.08) of increased long head of the biceps tendon elastic modulus at the mid-substance was observed (Fig. 2). Also, no differences were observed in the remaining intact tendons (infraspinatus, upper subscapularis, or lower subscapularis) with overuse activity compared to cage activity (Figs. 1 and 2). Similarly, no differences in cross-sectional area were observed in any tendon (S-Table II). Histology results demonstrated a significant decrease in cellularity in the long head of the biceps tendon distal groove (Fig. 3 and S-Fig. I) and a trend towards increased cellularity at the insertion site of the lower subscapularis tendon with overuse (S-Table III). No differences were observed in any other tendon in either the insertion or mid-substance regions.
Figure 1.
Tendon insertion modulus for the adjacent intact tendons (LHB, long head of the biceps; infra, infraspinatus; upper sub, upper subscapularis; and lower sub, lower subscapularis) of both the cage activity (CA) and overuse (OV) groups. No significant group differences were observed in any of the intact tendons (p < 0.05).
Figure 2.
Tendon mid-substance modulus for the adjacent intact tendons (LHB, long head of the biceps; infra, infraspinatus; upper sub, upper subscapularis; and lower sub, lower subscapularis) of both the cage activity (CA) and overuse (OV) groups. A trend toward increased modulus with overuse activity was observed at the biceps tendon mid-substance. No other changes were observed in any tendon (#p < 0.1).
Figure 3.
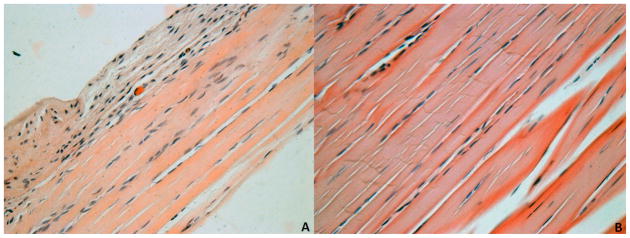
Biceps tendon histology was blindly graded for cellularity and cell shape at four locations along the length of the tendon (insertion, intra-articular space, proximal groove, and distal groove). Cellularity was significantly decreased (*p < 0.05) in the return to overuse group (B) at the distal groove location compared to the return to cage activity group (A). Note: Grading was performed on original images (S-Fig. I). For publication, filters were applied to images (A and B): auto-tone, auto-contrast, auto-color (all three to each individually).
Cartilage Properties
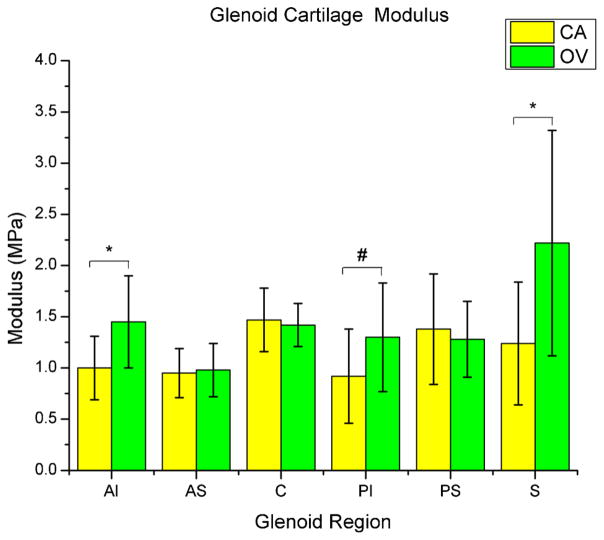
The overuse group had a significantly greater equilibrium elastic modulus in the superior region (p = 0.02) of the glenoid cartilage (Fig. 4). Also, there was a significantly greater equilibrium elastic modulus in the AI region (p = 0.01), and a trend toward increased modulus in the PI region (p = 0.06). There was also a trend toward decreased cartilage thickness in the center (p = 0.08) and both inferior regions (anterior and posterior, p = 0.09 and 0.08, respectively; S-Table IV). Histology results demonstrated no significant differences between groups in any region (S-Table V).
Figure 4.
Glenoid cartilage modulus at six regions (center (C), anterior–superior (AS), anterior–inferior (AI), posterior–superior (PS), posterior–inferior (PI), and superior(S)) of both the cage activity (CA) and overuse (OV) groups. Cartilage elastic modulus was significantly greater (*p < 0.05) in the overuse group at the superior (S) and anterior–inferior (AI) regions, with a similar trend (#p < 0.1) in the posterior–inferior (PI) region.
DISCUSSION
This study demonstrates that 8 weeks of overuse activity following detachment of the supraspinatus tendon leads to altered properties of the surrounding joint structures. Specifically, alterations occurred in the mechanical properties and histology of the long head of the biceps tendon (mid-substance) and mechanical properties of the glenoid articular cartilage (in the superior and inferior regions) without alteration of joint function or adjacent (intact) rotator cuff tendons.
Both clinical and animal studies showed that joint function is altered following periods of excessive loading (overuse) and fatigue in the shoulder and knee.6,12 Independently, altered glenohumeral function was also observed in the presence of supraspinatus tears clinically7 and in this animal model.10 However, the effect of overuse in combination with a supraspinatus tendon tear has not been examined. Therefore, we hypothesized that additional overuse in combination with a supraspinatus tendon tear would not further diminish shoulder function when compared to reducing activity following tendon tear. The results demonstrated that returning to overuse activity following an isolated supraspinatus tear did not alter shoulder joint function, except for one parameter (propulsion force) at one time point (42 days). Due to the small difference in propulsion force observed (~2% bodyweight), the clinical significance of this difference is likely negligible. It has been suggested that patients with rotator cuff tendon tears isolated to the supraspinatus may be able to achieve normal pain-free function due to the presence of an intact force balance (often referred to as a “force couple”).22 Specifically, glenohumeral joint stability can be maintained by the anterior–posterior force balance of the subscapularis and infraspinatus in the transverse plane.22,23 These muscles function to provide concavity compression and concentric rotation of the humeral head on the glenoid and resist superior translation, despite the superior pull of the deltoid. Our results suggest that an intact anterior–posterior force balance in the shoulder is sufficient to achieve normal function, independent of activity level.
Despite similarities in joint function, overuse activity did alter the long head of the biceps tendon mechanical properties, but not the adjacent (intact) rotator cuff tendons. The intact anterior and posterior rotator cuff (subscapularis and infraspinatus) muscles successfully compensated and maintained equilibrium, despite the increased activity level. However, the biceps tendon was affected; in particular, there was a trend toward increased modulus in the mid-substance. Decreased cellularity was also observed in the biceps mid-substance, providing further evidence to support the observed mechanical tissue changes. Previous research from our laboratory found decreases in the mechanical properties of the biceps tendon following a supraspinatus tear.1 However, this is the first study to examine the effect of returning to overuse activity following such a tear. Clinically, the role of the biceps tendon is controversial; however, it has been suggested by some to function as a humeral head depressor, resisting superior migration, especially in patients with a torn supraspinatus tendon.24 In our study, with a return to overuse activity following a supraspinatus tear, the absence of the supraspinatus abduction torque may require an increase in force generation by the deltoid muscle. This may cause superior humeral head translation (while anterior–posterior loading is preserved), resulting in mechanical alterations localized to the mid-substance of the biceps tendon.
Mechanical alterations were also observed in the superior and inferior regions of the glenoid articular cartilage. Specifically, modulus increased in the superior and both inferior regions (anterior and posterior), while the center and both inferior regions had trends toward decreased thickness. Previous studies showed a correlation between rotator cuff tears and glenohumeral arthritis.3,25 This may be a result of altered contact patterns between the humeral head and the glenoid in the presence of rotator cuff tears. Our results suggest that alterations in superior–inferior glenoid cartilage loading following a supraspinatus tear were magnified with repetitive overuse and are consistent with the observed biceps changes.
Clinically, our functional and mechanical results provide evidence that will lead to further clinical investigation of the recommendations for activity level modifications following full thickness supraspinatus tendon tears. Our results suggest that long term implications of returning to overuse activity following a full thickness supraspinatus tear may exist. Specifically, patients returning to a high level of overhead use following an isolated supraspinatus tear may place the joint at a higher risk for cartilage and long head of the biceps injury despite having normal joint function.
This study has several limitations. Although the rat shoulder has similar bony and soft tissue anatomy to the human and has been widely used as a model of rotator cuff injury, the use of a quadruped animal does not replicate the human condition. However, during quadruped walking, the rat shoulder produces large amounts of glenohumeral forward flexion that, combined with supraspinatus acromial impingement, replicates repetitive overhead activity observed in the human.26 Also, for our histologic assessment, the superior region of the glenoid cartilage was removed (along with the biceps tendon) and therefore a modified Mankin score for this region does not exist. However, this superior region is more anatomically pronounced in the rat than in the human making clinical translation of findings in this region difficult. Despite these limitations, our results demonstrate that mechanical consequences are localized to the biceps tendon and cartilage after returning to overuse following a supraspinatus tear. Future studies will examine additional time points to determine the progression of joint changes and the other consequences associated with disrupting the anterior–posterior force balance (with a combined supraspinatus and infraspinatus tear) to further understand the mechanisms by which rotator cuff tears lead to joint damage.
In conclusion, our results demonstrate that returning to overuse activity following an isolated supraspinatus tear alters biceps tendon and glenoid cartilage properties but does not alter joint function or adjacent intact rotator cuff tendon properties. These results are consistent with an intact force balance and the preservation of anterior–posterior glenohumeral stability in the human. This study helps define the contributory roles of common mechanical injury mechanisms (rotator cuff tendon tear and overuse) using an established in vivo animal model that mimics the human condition. The results provide a framework and model system by which physicians could better advise patients on outcomes and in which targeted treatment modalities could be evaluated in a controlled manner to guide physicians on an optimal long-term treatment strategy for these common injuries.
Supplementary Material
Acknowledgments
Grant sponsor: NIH/NIAMS; Grant number: R01AR056658.
The authors acknowledge Lena Edelstein and Sarah Ilkhani-Pour.
Footnotes
Additional supporting information may be found in the online version of this article.
References
- 1.Peltz CD, Perry SM, Getz CL, et al. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry SM, Getz CL, Soslowsky LJ. After rotator cuff tears, the remaining (intact) tendons are mechanically altered. J Shoulder Elbow Surg. 2009;18:52–57. doi: 10.1016/j.jse.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neer CS, II, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 4.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 5.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 6.Chen SK, Simonian PT, Wickiewicz TL, et al. Radiographic evaluation of glenohumeral kinematics: a muscle fatigue model. J Shoulder Elbow Surg. 1999;8:49–52. doi: 10.1016/s1058-2746(99)90055-1. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi K, Sher JS, Andersen WK, et al. Glenohumeral motion in patients with rotator cuff tears: a comparison of asymptomatic and symptomatic shoulders. J Shoulder Elbow Surg. 2000;9:6–11. doi: 10.1016/s1058-2746(00)90002-8. [DOI] [PubMed] [Google Scholar]
- 8.Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc. 2008;16:180–186. doi: 10.1097/JSA.0b013e3181824f1e. [DOI] [PubMed] [Google Scholar]
- 9.Reuther KE, Sarver JJ, Schultz SM, et al. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res. 2012;30:1435–1439. doi: 10.1002/jor.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry SM, Getz CL, Soslowsky LJ. Alterations in function after rotator cuff tears in an animal model. J Shoulder Elbow Surg. 2009;18:296–304. doi: 10.1016/j.jse.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbe MF, Barr AE, Gorzelany I, et al. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckett J, Jin W, Schultz M, et al. Excessive running induces cartilage degeneration in knee joints and alters gait of rats. J Orthop Res. 2012;30:1604–1610. doi: 10.1002/jor.22124. [DOI] [PubMed] [Google Scholar]
- 13.Driban JB, Barr AE, Amin M, et al. Joint inflammation and early degeneration induced by high-force reaching are attenuated by ibuprofen in an animal model of work-related musculoskeletal disorder. J Biomed Biotechnol. 2011;2011:691412. doi: 10.1155/2011/691412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 15.Sarver JJ, Dishowitz MI, Kim SY, et al. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SJ, Miller KS, Soslowsky LJ. The upper band of the subscapularis tendon in the rat has altered mechanical and histologic properties. J Shoulder Elbow Surg. 21:1687–1693. doi: 10.1016/j.jse.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favata M, Beredjiklian PK, Zgonis MH, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–2132. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- 18.Hayes WC, Keer LM, Herrmann G, et al. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JE, Flanagan CL, Thomopoulos S, et al. The effects of overuse combined with intrinsic or extrinsic alterations in an animal model of rotator cuff tendinosis. Am J Sports Med. 1998;26:801–807. doi: 10.1177/03635465980260061101. [DOI] [PubMed] [Google Scholar]
- 20.Salo PT, Hogervorst T, Seerattan RA, et al. Selective joint denervation promotes knee osteoarthritis in the aging rat. J Orthop Res. 2002;20:1256–1264. doi: 10.1016/S0736-0266(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 21.Dourte LM, Perry SM, Getz CL, et al. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–1492. doi: 10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop Relat Res. 1991;267:45–56. [PubMed] [Google Scholar]
- 23.Hsu JE, Reuther KE, Sarver JJ, et al. Restoration of anterior–posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J Orthop Res. 2011;29:1028–1033. doi: 10.1002/jor.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su WR, Budoff JE, Luo ZP. The effect of posterosuperior rotator cuff tears and biceps loading on glenohumeral translation. Arthroscopy. 2010;26:578–586. doi: 10.1016/j.arthro.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Hsu HC, Luo ZP, Stone JJ, et al. Correlation between rotator cuff tear and glenohumeral degeneration. Acta Orthop Scand. 2003;74:89–94. doi: 10.1080/00016470310013725. [DOI] [PubMed] [Google Scholar]
- 26.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.