Dear Editors:
Epigenetic modifications resulting in decreased gene expression is a proposed cause of schizophrenia (Petronis, 2004; Costa et al., 2002). The deacetylation of histone 3, catalyzed by the histone deacetylase (HDAC) family of enzymes, is an example of a change in chromatin structure that leads to a restrictive chromatin state and a consequent reduction in gene transcription both globally as well as at individual gene promoters (Berger, 2002).
We recently reported a clinical study in which we treated schizophrenia and bipolar subjects with the HDAC inhibitor Depakote ER® (VPA) (Göttlicher et al., 2001) for four weeks. We found that after four weeks of VPA treatment schizophrenia patients had significantly smaller increases in acetylated lysine 9 and 14 of histone 3 (H3K9,K14ac), compared with bipolar subjects, implying that schizophrenia is associated with more ‘rigid’ chromatin (Sharma et al., 2006).
In the current study we sought to further these results by testing an in vitro assay using cultured lymphocytes from a clinical population. Based on our previous findings our hypotheses were that H3K9, K14ac levels at baseline would be lower, and that the highly potent HDAC inhibitor, Trichostatin A (TSA), would induce smaller increases in H3K9, K14ac levels in lymphocytes from schizophrenia subjects compared to normal controls. We also expected TSA to induce smaller increases in the expression of the epigenetically regulated schizophrenia candidate gene, GAD1, among schizophrenia subjects.
Patients of the University of Illinois Medical Center who fulfilled DSM-IV criteria for schizophrenia or schizoaffective disorder and had not used valproic acid, carbamazepine, or oxcarbazepine in the past 30 days were referred by the screening clinical psychiatrist for the study. Healthy comparison subjects who volunteered for the study, were recruited from among hospital staff and their associates, and were excluded if they reported a history of major mental illness or treatment with valproic acid. The study was approved by the Institutional Review Board at the University Of Illinois College Of Medicine.
Cells were incubated with either DMSO (control) or 100nM TSA in media for 24 hours based on previous dose response experiments. Extraction of basic nuclear histone proteins and Western blot analysis procedures of H3K9, K14ac (Millipore #06-599) and total histone 1 (H1) (Millipore #05-457) were performed with H3K9,K14 being normalized to H1, using published procedures, (Sharma et al., 2006). GAD1 was measured using qRT-PCR, analyzed using a Stratagene Mx3005P™ QPCR System, and normalized to the housekeeping gene GAPDH.
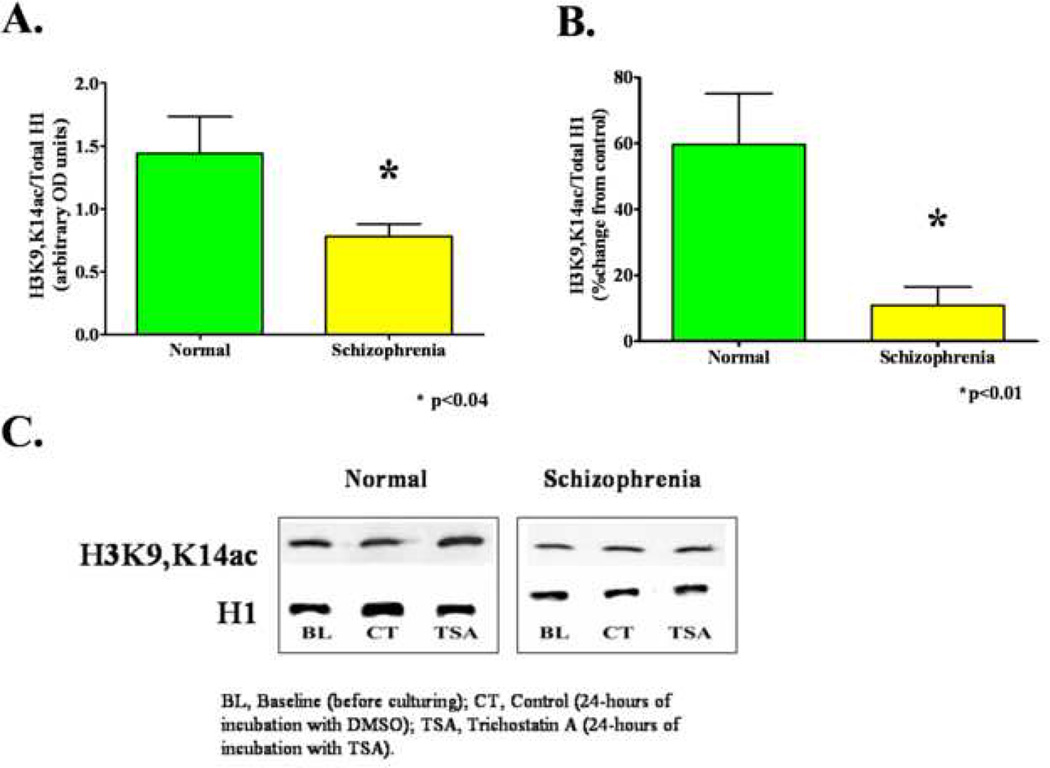
Schizophrenia subjects had significantly lower baseline H3K9, K14ac levels than normal controls (mean=0.78 [SD=0.47] vs. mean=1.44 [SD=1.34]; p<0.04) (Fig 1A and 1C). In addition, after 24-hours of incubation with TSA, the percent change in H3K9,K14ac levels significantly differed between schizophrenia and normal control subjects in cultured lymphocytes, (mean=11% [SD=16] vs. mean=60% [SD=54]; p<0.01) (Figure 1B and 1C).
Fig. 1.
Differences in acetylated histone 3 (H3K9, K14) between schizophrenia and normal control subjects. H3K9, K14ac was normalized to total histone 1 (H1). Panel A: Baseline levels of H3K9, K14ac are lower in schizophrenia compared with normal control subjects. Panel B. After 24 hours of incubation with the histone deacetylase inhibitor, Trichostatin A (TSA) H3K9, K14ac levels increase significantly less in schizophrenia compared with normal control subjects. Panel C. Representative Western blot showing reduced baseline H3K9, K14ac and blunted TSA-induced increase in H3K9, K14ac in a schizophrenia compared with normal subject-derived sample.
There was a substantial though not significant difference between the ability of TSA to induce GAD1 expression in schizophrenia and normal subjects (mean percent change=−18% [SD=38] vs. mean percent change=134% [SD=339]; p<0.08). There was a significant correlation between TSA-induced increases in GAD1 expression and H3K9, K14ac among normal subjects (Spearman’s ρ=0.692; n=12; p<0.01), but not among schizophrenia subjects (Spearman’s ρ=−0.476; n=8; p<0.2).
We found no significant differences within groups based on race, age, gender, medication use, duration of illness, or number of previous hospitalizations. We did find female subjects to have non-significantly higher baseline H3K9, K14ac levels and greater increases in GAD1 expression following TSA treatment.
There exist some limitations to the current study. Presented here are global levels of H3K9, K14ac measured using Western blot analysis. We did not use a chromatin immunoprecipitation (ChIP) analysis, which would have been informative as to the H3K9, K14 levels specifically at the GAD1 promoter. We have recently performed a ChIP assay, and have been able to show that TSA treatment does increase H3K9, K14ac levels at the GAD1 promoter in cultured lymphocytes (unpublished data). Additionally, the current study utilized lymphocytes, which has limitations when used as a proxy to brain tissue. However, supporting its use for the study of epigenetic gene regulation is the fact that epigenetic parameters have been shown to be similar and reliably measurable in a variety of tissues including lymphocytes (Fraga et al., 2005), lymphocytes are exposed to much the same environment as neurons in terms of neurohormones, neuropeptides, chemo/cytokines, metabolites, and medication blood levels, GAD1 expression is similarly repressed in both tissues (Sullivan et al., 2006), and using lymphocytes allows one to measure changes in real clinical time.
In the future it may be possible to isolate those schizophrenia patients characterized by ‘rigid’ chromatin, then using HDAC inhibitors to release the chromatin restraints on a global-basis on gene expression thereby allowing for more efficient gene regulation. Once the genome has been ‘relaxed’ patients may be more likely to benefit from conventional pharmacological treatment (Sharma et al., 2005). Alternatively, it may be possible to design chromatin remodeling agents which directly target disease candidate gene expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger SL. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002;12(2):142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Costa E, Chen Y, Davis J, Dong E, Noh JS, Tremolizzo L, Veldic M, Grayson DR, Guidotti A. REELIN and schizophrenia: a disease at the interface of the genome and the epigenome. Mol. Interv. 2002;2(1):47–57. doi: 10.1124/mi.2.1.47. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suñer D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS ONE. 2007;2(8):e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20(24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol. Psychiatry. 2004;55(10):965–970. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Sharma RP. Schizophrenia, epigenetics and ligand-activated nuclear receptors: a framework for chromatin therapeutics. Schizophr. Res. 2005;72(2–3):79–90. doi: 10.1016/j.schres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Rosen C, Kartan S, Guidotti A, Costa E, Grayson DR, Chase K. Valproic acid and chromatin remodeling in schizophrenia and bipolar disorder: preliminary results from a clinical population. Schizophr. Res. 2006;88(1–3):227–231. doi: 10.1016/j.schres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. American Journal of Medical Genetics B Neuropsychiatry Genetics. 2006;141:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]