Abstract
Rationale
Little is known about the smoking topography characteristics of heavy cannabis users. Such measures may be able to predict cannabis use-related outcomes and could be used to validate self-reported measures of cannabis use.
Objectives
The current study was conducted to measure cannabis smoking topography characteristics during periods of ad libitum use and to correlate topography assessments with measures of self-reported cannabis use, withdrawal and craving during abstinence, and cognitive task performance.
Methods
Participants (N=20) completed an inpatient study in which they alternated between periods of ad libitum cannabis use and abstinence. Measures of self-reported cannabis use, smoking topography, craving, withdrawal, and sleep measures were collected.
Results
Participants smoked with greater intensity (e.g., greater volume, longer duration) on initial cigarette puffs with a steady decline on subsequent puffs. Smoking characteristics were significantly correlated with severity of withdrawal, notably sleep quality and architecture, and craving during abstinence, suggesting dose-related effects of cannabis use on these outcomes. Smoking characteristics generally were not significantly associated with cognitive performance. Smoking topography measures were significantly correlated with self-reported measures of cannabis use, indicating validity of these assessments, but topography measures were more sensitive than self-report in predicting cannabis-related outcomes.
Conclusions
A dose–effect relationship between cannabis consumption and outcomes believed to be clinically important was observed. With additional research, smoking topography assessments may become a useful clinical tool.
Keywords: Smoking topography, Cannabis use disorders, Cannabis, Withdrawal, Craving, Sleep
Introduction
Cannabis is the most widely used illicit substance, with an estimated 160 million users worldwide (UNODC 2010). A preponderance of research has been conducted to characterize the effects of cannabis in humans. This includes research conducted to characterize the neurobiology and subjective effects of acute intoxication, the cognitive and physiological health consequences of acute and chronic use, and the epidemiology and epigenetics of users (Grotenhermen 2003; Budney et al. 2008a, 2011; Vandrey and Mintzer 2009). However, relatively little research has been conducted to examine the methods and characteristics of cannabis consumption and how these may relate to cannabis use-related outcomes.
Cannabis is primarily smoked or inhaled by users. However, there are a variety of methods in which this is done, including cigarettes (joints), pipes, bongs, blunts (cannabis rolled in hollowed out cigars), and more recently, vaporizers. Interestingly, a recent study suggests that the type of preparation used to consume cannabis may be a marker for risk of developing cannabis dependence. Timberlake (2009) reported that, among frequent cannabis users, those who primarily smoked cannabis in blunts were more likely to meet DSM-IV criteria for cannabis and tobacco dependence compared with cannabis users who typically used other methods of administration. This highlights the potential utility of cannabis use characteristics as a clinical tool for identifying at-risk cannabis users.
Another factor likely to contribute to dependence risk is the amount of cannabis a user typically consumes. However, accurate measures of cannabis consumption (dose) are difficult to ascertain in clinical assessments due to the differences in route of administration, the variability in cannabis potency (tetrahydrocannabinol [THC] concentration), and the tendency for cannabis to be shared by multiple individuals during an episode of use (Gray et al. 2009; Mariani et al. 2011). Thus, reliance on self-reported assessments of cannabis use (e.g., timeline follow-back [TLFB]) may not yield valid data for cannabis consumption, but this has not yet been empirically tested. Laboratory studies in which cannabis consumption is measured precisely provide an opportunity to assess specific aspects of smoking that may correspond to individual patterns of use outside of the laboratory.
Smoking topography devices have been developed to study the characteristics of smoking behavior among tobacco users. These devices provide precise measures of puffing behavior, including the exact volume of smoke that is inhaled for each puff, puff duration, puff velocity (rate of air flow during inhalation), and interpuff interval. Studies assessing the smoking topography characteristics of tobacco cigarette smokers have shown that puff volume decreases steadily throughout the course of smoking a cigarette in adults (Gust et al. 1983; Guyatt et al. 1989; Kolonen et al. 1992) and adolescents (Collins et al. 2010; Veilleux et al. 2011), even when the length of the cigarette was not visible (adults: Nemeth-Coslett and Griffiths 1985). This pattern indicates that smokers titrate their nicotine intake throughout the course of the cigarette by taking smaller puffs. Smokers also regulate their intake by smoking low-yield cigarettes more intensely than high-yield cigarettes (Hammond et al. 2005; Strasser et al. 2007; Woodward and Tunstall-Pedoe 1993).
These studies have been important in understanding the variables controlling puffing behavior in tobacco smokers, and some preliminary studies have found that assessment of smoking topography may also hold clinical relevance as an indicator of dependence risk and predictor of cessation success. For example, a study of adolescent smokers showed that those participants who sustained or increased puff volume over the course of smoking a cigarette in the laboratory had heightened dependence escalation over the subsequent 2 years compared with those whose puff volume decreased over the course of smoking a cigarette, which is a more typical use pattern (Veilleux et al. 2011). Strasser et al. (2004) found that adults who smoked with a high puff velocity, small puff volume, and long interpuff interval prior to a quit attempt were more likely to achieve sustained abstinence compared to adults with opposing smoking characteristics. In a similar study with adolescents, smaller puff volumes prior to quitting were also associated with greater abstinence during treatment (Franken et al. 2006). These reports from the tobacco literature suggest the potential utility of studying smoking topography with other inhaled drugs of abuse, namely, cannabis.
Analysis of cannabis smoking topography characteristics has been limited and largely focused on comparisons with tobacco smoking topography. Compared with tobacco smoking, cannabis is typically inhaled in greater volume and held in the lungs for a longer duration (Perez-Reyes et al. 1981, 1982; Wu et al. 1988a). Interestingly, cannabis users who also smoke tobacco do not differ in their tobacco smoking topography characteristics compared with tobacco users who do not use cannabis (Aung et al. 2004; Nemeth-Coslett et al. 1986; Simmons and Tashkin 1995).
Studies have also shown that, similar to tobacco users, cannabis users titrate their smoking mechanics based on dose. Specifically, studies of cannabis smoking topography have shown an inverse relationship between cannabis potency (percent THC concentration) and the quantity, volume, and duration of puffs taken during self-administration of smoked cannabis (Heishman et al. 1989; Kelly et al. 1993). Studies have also demonstrated a positive relationship between puff volume and the subjective effects of smoked cannabis (Azorlosa et al. 1995). Increased breath hold durations have also been associated with increased tar deposition in the lung (Tashkin et al. 1991a; Wu et al. 1988a).
The present study was conducted to extend previous research by assessing smoking topography characteristics of experienced cannabis users participating in a residential research study evaluating the effects of cannabis withdrawal on sleep (Vandrey et al. 2011).
This study was unique in that participants were allowed to self-administer smoked cannabis ad libitum on four study days. This allowed for collection of a substantial amount of naturalistic smoking topography data. We expected that the volume of cannabis self-administered would be positively correlated with cannabis withdrawal and craving measured during a supervised abstinence period and negatively correlated with cognitive performance assessments conducted when participants were not acutely intoxicated. Exploratory analyses were also conducted to assess agreement between self-reported measures of prestudy cannabis use severity and the amount of cannabis consumed in the laboratory under ad libitum conditions.
Methods
Participants
Heavy cannabis users, aged 18–55 years, were recruited through newspaper advertisements. Participants were generally healthy and used cannabis multiple times daily. Study completers (N=20) had an average (standard deviation [SD]) age of 29 (8) years and were 85% male, 65% African American, 25% Caucasian, and 10% Asian or of mixed ethnicity. Participants on average first used cannabis at 14 (2) years of age, had been using cannabis regularly for 14 (8) years, and currently used cannabis 4 (3) times per day. Half of the participants met DSM-IV criteria for cannabis abuse, and the other half met criteria for cannabis dependence. Though participants reported a history of using various preparations of cannabis, blunts were the preferred method of cannabis administration among the majority of study participants. Sixteen of the 20 participants were current tobacco users, and all participants had some history of tobacco use in their lifetime. The participants’ tobacco use was not restricted during the course of the study. Other detailed participant demographic characteristics and recruitment procedures are described in a previous paper (Vandrey et al. 2011). Written informed consent was obtained prior to study participation. The study was approved by the Johns Hopkins Medicine IRB and conducted in accordance with the ethical standards of the Helsinki Declaration.
Procedures
This study was conducted within the framework of a within-subject, crossover study conducted at the Johns Hopkins Bayview Medical Center (Vandrey et al. 2011). Following an initial training and acclimation day, study participants completed two inpatient study phases, each of which consisted of two consecutive days when participants had ad libitum access to cannabis for 9 h (between 1200 and 2100 each day), followed by three consecutive days of supervised cannabis abstinence. During one abstinence period, participants were administered placebo medication each night at bedtime, and in the other, they were administered extended-release zolpidem. The two inpatient study phases were separated by a 1-week outpatient period during which participants used cannabis in their home environment in their typical manner. Subjective and physiological assessments, cognitive performance tasks, and sleep polysomnography (PSG) assessments were conducted each day. Smoking topography measures were obtained for all cannabis use that occurred during the inpatient study phases, but were not assessed during the outpatient period.
Cannabis self-administration and smoking topography assessment
Cannabis was obtained from the National Institute on Drug Abuse. Cannabis cigarettes (joints) contained approximately 0.8 g of cannabis each and had a THC concentration of approximately 3%. During ad libitum cannabis self-administration periods, participants were able to request cannabis cigarettes from research nurses. Cannabis smoking occurred in a specially ventilated room. Cannabis cigarettes were smoked through portable smoking topography devices (Borgwaldt KC, Richmond, VA). The device included a holder for the cannabis cigarette. Research staff placed the cigarette in the holder for the participant, and the participant lit the cigarette with the first puff registering immediately upon inhalation. Smoking topography output data were compared with logs maintained by research staff documenting each cannabis cigarette smoked, ensuring an accurate count of cannabis cigarettes smoked. Data from smoking topography devices were downloaded electronically to a desktop computer and included the following assessments: number of cigarettes, number of puffs, puff volume (in milliliters), puff duration (in milliseconds), average puff velocity (in milliliters per second), and time to peak puff velocity (in milliseconds).
Self-report, physiological and cognitive performance assessments
On study admission, locally constructed questionnaires were used to obtain participant demographic information and general drug use history. The TLFB method (Sobell and Sobell 1992) was used to obtain self-reported amount and frequency of cannabis use in the past 30 days, and diagnosis of current cannabis use disorders was conducted using the DSM checklist (Hudziak et al. 1993). During the study, several assessments were collected daily. The Marijuana Withdrawal Checklist (MWC; Budney et al. 1999), Marijuana Craving Questionnaire (MCQ; Heishman et al. 2009), and Pittsburgh Sleep Quality Index (PSQI; Buysse et al. 1989) were administered each morning at 1000. A battery of cognitive performance tasks were completed from 1030 to 1145 (prior to cannabis self-administration access). Tasks included: N-Back, a measure of working memory (Gevins and Cutillo 1993); a computerized Digit Symbol Substitution Test (DSST), a measure of psychomotor ability and working memory (McLeod et al. 1982); a divided attention task (Kleykamp et al. 2010); Word Memory, a measure of episodic memory and metamemory (Mintzer et al. 1997) in which measures included proportion of old responses to old words (hit rate), proportion of old responses to new words (false alarm rate), and signal detection measures of sensitivity in distinguishing between old and new words (d′) and response bias (C); Flicker Fusion, a sensory perception task (Simonson and Brozek 1952); and Tower of London (TOL), a measure of executive function and planning (Ramaekers et al. 2006; Shallice 1982). Sleep continuity and architecture was measured nightly via Embla N-7000 digital PSG data recorder.
Data analysis
Data obtained on the training and acclimation day (day 1) were not included in data analyses. Smoking topography data were assessed for outliers. Specifically, individual cigarettes smoked in which topography measures were more than 2 SDs from the mean of other cigarettes smoked by the same participant on the same day were deemed out of range and were imputed with the mean values obtained from other cigarettes that day. Mean values were also imputed for cigarettes in which topography data were not captured. Approximately 6% of the total cannabis cigarettes consumed (927 cigarettes) over the course of 4 days for 20 participants had values that were either out of range or missing and had to be imputed. Fourteen out of 20 participants had at least one cigarette that required value imputations, and most cigarette imputations were evenly distributed across participants and days.
A repeated-measures regression was conducted to determine whether cannabis smoking topography measures differed in the two residential admission periods (first versus second inpatient stay) and across days (day 1 versus day 2) of cannabis access within each inpatient admission. Repeated-measures regression was also conducted to examine differences in puff characteristics within and between the first, second, and last cigarettes smoked each day. Correlations were conducted to assess the association between smoking topography characteristics and other study outcome measures. Specifically, correlations between puff volume and the MWC, MCQ, and objective and subjective sleep measures assessed during the abstinence period in which participants received placebo medication were conducted to assess dose effects of cannabis on withdrawal, craving, and sleep. Correlations were also conducted between smoking topography and cognitive performance assessed on ad libitum cannabis self-administration days to determine whether smoking characteristics predicted cognitive performance when not acutely intoxicated. Correlations were assessed using Pearson’s correlation test, and the alpha level was set to p<0.05.
Results
Cannabis smoking topography daily measures
Daily cannabis use during the study ranged from 1 to 25 cigarettes per day. On average (SD), participants smoked 12 (5) cigarettes during each 9-h cannabis access period and took an average of 13 (4) puffs from each cigarette (range, 5 to 34 puffs in one cigarette). No significant differences were found in smoking topography measures grouped by inpatient admission period (admission 1 vs. admission 2), but differences by study day were found for a number of topography characteristics (see Table 1). A main effect of day was found for total cigarettes smoked (F=5.9, p<0.05), volume of smoke inhaled over the entire ad libitum period (F=9.3, p<0.01), volume of smoke inhaled per cigarette (F=6.0, p<0.05), volume of smoke inhaled per puff (F= 6.4, p<0.05), and puff velocity (F=12.8, p<0.01). Values from ad libitum smoking on day 2 were consistently higher for the aforementioned measures compared to day 1, indicating that participants were smoking more cigarettes, inhaling more smoke, and with greater velocity on the days immediately preceding cannabis abstinence phases.
Table 1.
Cannabis smoking topography measures
| Admission 1
|
Admission 2
|
|||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | |
| Total cigarettes* | 11 (1.3) | 13 (1.3) | 11 (1.2) | 12 (1.0) |
| Puffs per cigarette | 13 (1.0) | 13 (1.0) | 13 (0.9) | 14 (1.0) |
| Total volume (ml)* | 7,136 (1,326) | 9,048 (1,372) | 6,390 (907) | 8,789 (994) |
| Total volume per cigarette (ml)* | 648 (59) | 719 (58) | 628 (70) | 814 (100) |
| Average volume per puff (ml)* | 51 (4.3) | 58 (4.9) | 52 (6.2) | 61 (7.3) |
| Average puff duration (s) | 1.3 (0.1) | 1.3 (0.1) | 1.3 (0.1) | 1.3 (0.1) |
| Maximum puff duration (s) | 3.4 (0.3) | 3.2 (0.3) | 3.0 (0.3) | 3.5 (0.4) |
| Puff velocity (ml/s)* | 41 (2.8) | 46 (2.9) | 40 (2.5) | 47 (3.6) |
| Time to peak velocity (s) | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
p<0.05, main effect of day
Topography within-cigarette
Topography characteristics (puff volume, puff duration, velocity, and time to peak velocity) for the first four puffs and last four puffs of the first, second, and last cigarettes of the day were averaged across all 4 days of ad libitum administration. All participants puffed at least 8 times per cigarette for each of these 3 cigarettes, with the average (SD) across participants being 13 (4) puffs per cigarette. Puff volume and puff duration are shown in Fig. 1 for all 20 participants for the first and second cigarettes of the day. The second cigarette of the day was typically smoked 5 to 10 min following completion of the first. One participant only smoked two cigarettes per day and was excluded from last cigarette data also shown in Fig. 1.
Fig. 1.
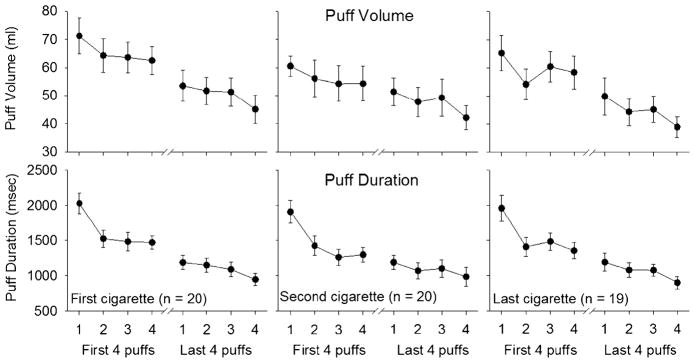
Mean volume of smoke inhaled and puff duration during the first, second, and the last cannabis cigarettes smoked each day. Error bars represent standard error of the mean. Data are averaged over 4 days. Only data for the first four puffs and last four puffs are shown due to variability in the number of puffs taken across cigarettes
Puff volume and puff duration steadily declined throughout the course of each cigarette. Puff velocity measures, however, increased over subsequent puffs. Time to peak velocity decreased sharply from the first to second puffs and then continued a gradual decrease throughout the remainder of the cigarette. Significant main effects for puff volume (F=39.2, p<0.0001), puff duration (F=75.9, p<0.0001), puff velocity (F=18.0, p< 0.0001), and time to peak velocity (F=32.0, p<0.0001) were observed when comparing the first four versus the last four puffs of all cigarettes. Significant main effects were also observed for all puff characteristics when puffs were aggregated across the first four and last four puffs of the cigarette (puff volume: F=13.8, p<0.0001; puff duration: F=38.8, p<0.0001; puff velocity: F=17.4, p< 0.0001; time to peak velocity: F=30.8, p<0.0001). A significant interaction was found between the first four and last four puffs of all cigarettes and puff count for puff duration (F=9.4, p<0.0001), puff velocity (F=5.4, p< 0.01), and time to peak velocity (F=10.5, p<0.0001), indicating a steep increase or decrease initially in the cigarette for these puffing characteristics.
Planned comparisons were also conducted between the first four and last four puffs within a cigarette. The volume inhaled in the first four puffs of the first and last cigarettes was higher compared with the last four puffs of those cigarettes (cigarette 1: t=3.4, p<0.01; last cigarette: t=3.5, p<0.01). Puff duration was longer for the first four puffs of the cigarette compared to the last four puffs for cigarette 1 (t=5.0, p<0.0001), cigarette 2 (t=3.6, p<0.001), and the last cigarette of the day (t=4.5, p<0.0001). Puff velocity was higher in the last four puffs for cigarette 1 (t=−2.2, p< 0.05) and cigarette 2 (t=−2.5, p<0.05). Time to peak velocity was longer in the first four puffs for cigarette 1 (t= 3.5, p<0.01), cigarette 2 (t=2.5, p<0.05), and the last cigarette of the day (t=2.8, p<0.01).
Since the first measured puff on the cigarette also served to facilitate lighting, the topography within-cigarette analyses were replicated excluding the first puff (compared puffs 2, 3, and 4 to the last three puffs on the cigarette). With the first puff excluded, significant main effects remained for puff volume (F=43.9, p<0.0001), puff duration (F=64.3, p<0.0001), puff velocity (F=9.7, p< 0.01), and time to peak velocity (F=19.8, p<0.001). Main effects of aggregated puffs also remained significant for puff volume (F=5.4, p<0.01), puff duration (F=6.8, p< 0.01), and time to peak velocity (F=9.2, p<0.01). However, the interactions observed in the initial analyses were not significant. Thus, the initial puff contributed to the steep increase or decrease in puffing characteristics from the first to second puff, but significant differences in puffing behavior were still observed, indicating a decrease in smoking intensity with progressive puffs.
Smoking topography and cannabis use history
Significant correlations observed between smoking topography measures and cannabis use history are shown in Table 2. The age of first cannabis use was positively correlated with time to peak velocity, but no other topography measures. Positive correlations were found between years of frequent cannabis use and puff volume, puff duration, and puff velocity. The self-reported number of times per day that cannabis was used in the 30 days prior to study admission was also positively correlated with puff volume and puff duration. The positive correlation between years of regular cannabis use and the volume inhaled per puff represents one of the largest correlation coefficients found in these analyses and the relationship is shown in Fig. 2a.
Table 2.
Correlations between smoking topography and cannabis-related outcome measures
| Total cigarettes | Total volume (ml) | Total volume per cigarette (ml) | Average volume per puff (ml) | Average puff duration (s) | Maximum puff duration (s) | Puff velocity (ml/s) | Time to peak velocity (s) | |
|---|---|---|---|---|---|---|---|---|
| Cannabis use history | ||||||||
| Age of first cannabis use | – | – | – | – | – | – | – | 0.55* |
| Years of frequent cannabis use | – | – | 0.58** | 0.61** | 0.49* | – | 0.46* | – |
| Times per day cannabis used | – | 0.43* | – | – | – | 0.47* | – | – |
| Craving during abstinence | ||||||||
| MCQ—compulsivity | 0.57** | 0.64** | – | – | – | – | – | – |
| MCQ—purposefulness | – | 0.49* | – | – | – | – | – | – |
| Withdrawal during abstinence | ||||||||
| Withdrawal (WDS) | 0.44* | – | – | – | – | – | – | – |
| Peak withdrawal (WDS) | 0.50* | – | – | – | – | – | – | – |
| Sleep during abstinence | ||||||||
| Sleep efficiency (PSG) | – | – | −0.58** | −0.49* | – | – | – | – |
| Sleep latency (PSG) | – | 0.53* | – | – | – | – | – | – |
| Wake after sleep onset (PSQI) | – | – | 0.58** | – | – | 0.50* | – | – |
| Sleep latency (PSQI) | – | – | – | – | 0.48* | – | – | – |
| Sleep quality (PSQI) | – | – | – | – | – | −0.59** | – | – |
| Morning mood (PSQI) | – | – | – | – | – | −0.50* | – | – |
| Cognitive during ad libitum | ||||||||
| DSST | ||||||||
| Trials attempted | – | – | −0.46* | −0.50* | – | – | – | – |
| % correct | 0.46* | – | – | −0.57** | – | – | – | 0.54* |
| TOL | ||||||||
| Reaction time | – | – | – | 0.44* | – | – | – | – |
p<0.05;
p<0.01
Fig. 2.
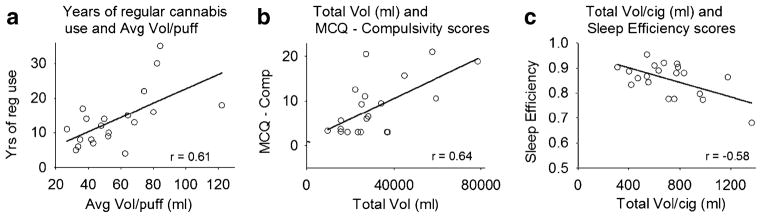
Correlations between a years of regular cannabis use and average volume inhaled per puff, b total puff volume inhaled (in milliliters) and scores on the compulsivity subscale of the MCQ measured during 3 days of cannabis abstinence, and c total puff volume inhaled per cigarette (in milliliters) and sleep efficiency scores measured via PSG during 3 days of cannabis abstinence
Smoking topography and craving during abstinence
Relationships between smoking topography measures and ratings of craving during supervised abstinence periods are summarized in Table 2. The number of cigarettes smoked and volume of smoke inhaled during cannabis self-administration were positively correlated with scores on the compulsivity and purposefulness scales of the MCQ. The correlation between total volume inhaled and compulsivity ratings via the MCQ are illustrated in Fig. 2b.
Smoking topography and cannabis withdrawal
Correlations between measures of cannabis withdrawal, including objective sleep assessments, obtained during the brief period of cannabis abstinence are also summarized in Table 2. The total number of cigarettes smoked was positively correlated with peak ratings of total withdrawal (WDS) during cannabis abstinence. Puff volume was negatively correlated with sleep efficiency (time asleep/time in bed) (see Fig. 2c) and positively correlated with sleep latency (time to fall asleep), assessed via PSG measures. Puff volume and puff duration were positively correlated with total time awake after sleep onset. Puff duration was positively correlated with subjective reports of sleep latency and negatively correlated with sleep quality and mood on awakening in the morning (measured by the PSQI).
Smoking topography and cognitive performance
Few significant correlations were observed between smoking topography characteristics and cognitive performance (bottom of Table 2). Puff volume was negatively correlated with performance on the DSST and positively correlated with reaction time on the TOL. Total cigarettes smoked and time of peak velocity were positively correlated with DSST performance.
Controlling for self-reported cannabis use
Since significant correlations were observed between self-reported cannabis use and smoking topography, additional analyses were conducted to assess whether topography and self-reported cannabis use measures independently predicted withdrawal, craving, and cognitive performance. Partial correlations controlling for self-reported cannabis use (times used per day and years of frequent cannabis use) yielded outcomes confirming that cannabis smoking topography measures significantly predicted severity of withdrawal and craving during abstinence, but generally were not associated with cognitive performance as was observed in the primary analyses. In contrast, none of the self-reported cannabis use measures (times per day, age of first cannabis use, or years of frequent cannabis use) were significantly correlated with craving during abstinence. Self-reported number of times cannabis was used each day was associated with increased total withdrawal (r= 0.50, p<0.05), and years of frequent cannabis use was associated with sleep disturbance (decreased PSG sleep efficiency: r=−0.78, p<0.01; increased self-reported sleep latency: r=0.65, p<0.01) during abstinence.
Discussion
The aim of the current study was to explore ways in which objective measures of cannabis smoking topography could be used to better understand the behavioral pharmacology of cannabis use and dependence. In prior research, smoking topography measures have been used to demonstrate that tobacco smokers adjust the manner in which they smoke as a means to titrate nicotine dose (Collins et al. 2010; Gust et al. 1983; Guyatt et al. 1989; Kolonen et al. 1992; Nemeth-Coslett and Griffiths 1985), and specific features of smoking behavior have been shown to predict increased dependence severity (Veilleux et al. 2011) and treatment outcomes (Strasser et al. 2004; Franken et al. 2006). Research examining the relationship of cannabis smoking characteristics and clinical outcomes is lacking. This study yielded a number of interesting and novel findings that extend this line of research.
Consistent with research in tobacco smokers, cannabis users in this study demonstrated a pattern of smoking in which inhalation volume and duration were high initially and progressively decreased with subsequent puffs on a given cannabis cigarette. This was also consistent with another study that showed decreases in puff volume during the second half of a cannabis cigarette compared to the first half of the cigarette (Wu et al. 1988b). Time to peak velocity also decreased throughout the course of the cigarette, while puff velocity increased throughout the cigarette.
The pharmacological mechanism for the topography patterns observed is unknown and was not directly tested in this study. One possibility is that cannabis users adjust their smoking to obtain an initial bolus of THC and then reduce puff volume as a means of titrating THC administration to achieve desired effects. An alternative explanation is that initial puffs may differ in potency from later puffs within a single cigarette. Two prior studies have suggested that THC concentration increases as more puffs are taken from a cigarette and that smaller puffs later in a cigarette actually deliver comparable THC doses as larger initial puffs (Davis et al. 1984; Wu et al. 1988b). Another possibility is that puff volume and duration decrease due to increased harshness of smoke with cumulative puffs from a cigarette (Tashkin et al. 1977, 1991b). Further work directly testing these hypotheses with cannabis is required.
The number of years a participant reported having been a frequent cannabis user was a strong predictor of objectively measured cannabis consumption and was associated with larger, longer, and more forceful puffs. This may indicate an adjustment in smoking behavior in order to compensate for increased drug tolerance that is likely to occur following prolonged frequent use of cannabis. Little association was found between smoking characteristics and the age of cannabis initiation or endorsement of cannabis abuse and dependence criteria in this study. However, there was very little variance on these measures across study participants. Evaluation of these relationships in a larger population representing a broader spectrum of cannabis use frequency is needed.
The positive association between self-reported cannabis use during the prior 30 days and the volume of cannabis consumed during ad libitum access periods in the present study supports the validity of the TLFB as a tool for quantifying cannabis use. As described previously, there are many features of cannabis use (varying potency, different methods of smoking, sharing cigarettes, blunts, or pipes with others) that make quantifying use difficult. This study suggests that, despite these obstacles, the TLFB appears to provide a good proxy for total cannabis consumption.
Another interesting finding was that smoking behavior was similar across study admission phases, but differed depending on whether the participant was able to administer cannabis the following day. The number of cannabis cigarettes consumed, volume of smoke inhaled, and velocity of inhalation was greater when participants were required to abstain from cannabis on the following day compared with when ad libitum cannabis access continued. This suggests that cannabis smokers respond to environmental restrictions to smoking by adjusting their drug intake. It is unknown whether tobacco smokers behave similarly.
Measures of smoking topography were also significantly correlated with increased craving, withdrawal severity, and worsened sleep during a brief period of abstinence. In prior studies, cannabis withdrawal, including sleep disturbances, and craving have been associated with relapse or increased use of other drugs among those trying to quit (Budney et al. 2008b; Copersino et al. 2006; Cornelius et al. 2008; Peters and Hughes 2010). Thus, these findings suggest the potential utility of cannabis smoking topography measures as predictors of clinical outcomes that could be used to inform treatment plans based on perceived relapse risk. Partial correlations controlling for self-reported cannabis use measures indicated that smoking topography measures were robust and independent predictors of craving and withdrawal. Prospective evaluation is needed to determine whether cannabis smoking topography characteristics (e.g., puff volume, duration, or velocity) could indeed predict abstinence and relapse rates among those entering treatment for cannabis use disorders and the comparative predictive ability with self-report assessments.
We hypothesized that increased amounts of cannabis smoked during ad libitum administration periods would be negatively correlated with cognitive performance. This was based on prior studies demonstrating that adults (Pope et al. 2001a, b) and adolescents (Shannon et al. 2010) with greater urinary cannabinoid concentrations performed more poorly on cognitive performance assessments. Few significant correlations were observed between cannabis smoking topography measures and cognitive performance in the present study. Greater puff volume was associated with worsened performance on the DSST and TOL tasks, but the total number of cannabis cigarettes smoked was also associated with improved performance on the DSST. Since only daily cannabis users were enrolled in the study, the ability to detect associations between cannabis use and cognitive performance may have been constrained.
Some limitations related to this study are important to discuss. First, as alluded to above, the study population consisted of a relatively small sample of heavy cannabis users that were homogeneous on many cannabis use measures. All participants used cannabis multiple times daily and most initiated cannabis use at a relatively young age. Thus, the generality of the study results are uncertain. Replication of these findings with a larger, more diverse population of cannabis users would be valuable. An additional limitation of this study is that cannabis consumption assessed by smoking topography was not validated against biological markers (e.g., plasma, urine) of cannabis exposure. While it is likely that the volume of cannabis smoke inhaled is a valid measure of delivered dose (Azorlosa et al. 1995), it cannot be assumed without confirmatory biological testing. It is possible that individual differences impact the bioavailability of smoked cannabis in a manner that could have affected the study results. Also, our smoking topography devices were not equipped to measure breath hold duration. Previous studies have shown that cannabis breath hold duration is associated with increased THC exposure, but no change in subjective effects (Zacny and Chait 1989, 1991; Azorlosa et al. 1995). It would be valuable to incorporate this measure in future studies relating topography with clinically relevant outcomes.
In summary, relatively little research has been conducted quantifying and characterizing the smoking topography of cannabis use. Initial studies of both cannabis and tobacco smoking topography suggest predictive relationships between topography measures, puff volume in particular, and clinically important outcomes such as dependence severity, craving, withdrawal, and relapse. Thus, the use of detailed smoking topography data within the context of treatment holds the possibility of incorporating topography assessments as a diagnostic tool to aid in personalizing treatment delivery options as a means to reduce the probability of relapse. Modification of smoking topography could also be explored as a method for improving clinical outcomes. Additional research in larger, more diverse populations of cannabis users in clinical settings is warranted.
Acknowledgments
The authors wish to thank Erin Curran, Jeannie Fry, and Elizabeth Girling for data collection and management, Linda Felch for statistical consulting services, and the recruiting, medical, and support staff of the Behavioral Pharmacology Research Unit at Johns Hopkins University.
Role of funding source This research was supported by grants R21-DA025794 and T32-DA07209 from the National Institute on Drug Abuse.
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
References
- Aung AT, Pickworth WB, Moolchan ET. History of marijuana use and tobacco smoking topography in tobacco-dependent adolescents. Addict Behav. 2004;29:699–706. doi: 10.1016/j.addbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther. 1995;272:560–569. [PubMed] [Google Scholar]
- Budney AJ, Novy P, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG. Health consequences of marijuana use. In: Brick J, editor. Medical consequences of drug abuse. 2. Haworth; New York: 2008a. pp. 251–301. [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abus Treat. 2008b;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Fearer SA. Cannabis. In: Ruiz P, Strain EC, editors. Lowinson & Ruiz’s substance abuse: a comprehensive textbook. 5. Lippincott Williams & Wilkins; Baltimore: 2011. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco-dependent adolescents. Nicotine and Tob Res. 2010;12:164–167. doi: 10.1093/ntr/ntp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KH, McDaniel IA, Cadwell LW, Moody PL. Some smoking characteristics of marijuana cigarettes. In: Agurell S, Dewey WL, Wallette RE, editors. The cannabinoids: chemical, pharmacologic, and therapeutic aspects. Academic; Orlando: 1984. pp. 97–109. [Google Scholar]
- Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers, and Prevention. 2006;15:154–157. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Christie DK. Challenges in quantifying marijuana use. Am J Addict. 2009;18(2):178–179. doi: 10.1080/10550490902772579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Gust SW, Pickens RW, Pechacek TF. Relation of puff volume to other topographical measures of smoking. Addict Behav. 1983;8:115–119. doi: 10.1016/0306-4603(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Guyatt AR, Kirkham AJT, Baldry AG, Dixon M, Cumming G. How does puffing behavior alter during the smoking of a single cigarette? Pharmacol Biochem Behav. 1989;33:189–195. doi: 10.1016/0091-3057(89)90449-8. [DOI] [PubMed] [Google Scholar]
- Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiology, Biomarkers, and Prevention. 2005;14:1370–1375. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior subjective reports and performance. Pharmacol Biochem Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak J, Helzer JE, Wetzel MW, Kessel KB, McBee B, Janca A, Przybeck P. The use of the DSM-III-R Checklist for initial diagnostic assessments. Comprehensive Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. Effects of smoked marijuana on heart rate, drug ratings, and task performance by humans. Behav Pharmacol. 1993;4:167–178. [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonen S, Tuomisto J, Puustinen P, Airaksinen MM. Puffing behavior during the smoking of a single cigarette in a naturalistic environment. Pharmacol Biochem Behav. 1992;41:701–706. doi: 10.1016/0091-3057(92)90215-2. [DOI] [PubMed] [Google Scholar]
- Mariani JL, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: blunts, joints, and pipes. Drug and Alcohol Dependence. 2011;113:249–251. doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Griffiths RR. Effects of cigarette rod length on puff volume and carbon monoxide delivery in cigarette smokers. Drug Alcohol Depend. 1985;15:1–13. doi: 10.1016/0376-8716(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of marijuana smoking on subjective ratings and tobacco smoking. Pharmacol Biochem Behav. 1986;25:659–665. doi: 10.1016/0091-3057(86)90156-5. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Owens S, Di Guiseppi S. The clinical pharmacology and dynamics of marihuana cigarette smoking. J Clin Pharmacol. 1981;21(8–9 suppl):201S–207S. doi: 10.1002/j.1552-4604.1981.tb02596.x. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Di Guiseppi S, Davis KH, Schindler VH, Cook CE. Comparison of effects of marihuana cigarettes to three different potencies. Clin Pharmacol Ther. 1982;31:617–624. doi: 10.1038/clpt.1982.86. [DOI] [PubMed] [Google Scholar]
- Peters EN, Hughes JR. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug and Alcohol Dependence. 2010;106:111–118. doi: 10.1016/j.drugalcdep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001a;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Current Psychiatry Reports. 2001b;3 (6):507–512. doi: 10.1007/s11920-001-0045-7. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Mathias CW, Dougherty DM, Liguori A. Cognitive impairments in adolescent cannabis users are related to THC levels. Addictive Disorders and Their Treatment. 2010;9 (4):158–163. [Google Scholar]
- Simmons MS, Tashkin DP. The relationship of tobacco and marijuana smoking characteristics. Life Science. 1995;56:2185–2191. doi: 10.1016/0024-3205(95)00206-l. [DOI] [PubMed] [Google Scholar]
- Simonson E, Brozek J. Flicker fusion frequency background and applications. Physiol Rev. 1952;32:349–378. doi: 10.1152/physrev.1952.32.3.349. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana; Totowa: 1992. pp. 41–72. [Google Scholar]
- Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiology, Biomarkers, and Prevention. 2004;13:1800–1804. [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug and Alcohol Dependence. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Reiss S, Shapiro BJ, Calvarese B, Olsen JL, Lodge JW. Bronchial effects of aerosolized Δ9-tetrahydrocannabinol in healthy and asthmatic subjects. American Review Of Respiratory Disease. 1977;115:57–65. doi: 10.1164/arrd.1977.115.1.57. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Gliederer F, Rose J, Chang P, Hui K, Yu JL, Wu TC. Effects of varying marijuana smoking profile on the deposition of tar and absorption of CO and delta-9 THC. Pharmacol Biochem Behav. 1991a;40:651–656. doi: 10.1016/0091-3057(91)90377-e. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Gliederer F, Rose J, Chang P, Hui K, Yu JL, Wu TC. Tar, CO and Δ9-THC delivery from the 1st and 2nd halves of a marijuana cigarette. Pharmacol Biochem Behav. 1991b;40 (3):657–661. doi: 10.1016/0091-3057(91)90378-f. [DOI] [PubMed] [Google Scholar]
- Timberlake DS. A comparison of drug use and dependence between blunt smokers and other cannabis users. Substance Use and Misuse. 2009;44:401–415. doi: 10.1080/10826080802347651. [DOI] [PubMed] [Google Scholar]
- UNODC. World drug report—2010. United Nations Office on Drugs and Crime; Vienna: 2010. [Google Scholar]
- Vandrey RG, Mintzer MZ. Performance and cognitive alterations. In: Cohen L, Collins FL, Young AM, McChargue DE, Leffingwell TR, editors. The pharmacology and treatment of substance abuse: an evidence-based approach. Lawrence Erlbaum Associates, Inc; Mahwah: 2009. pp. 41–62. [Google Scholar]
- Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug and Alcohol Dependence. 2011;117:38–44. doi: 10.1016/j.drugalcdep.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux JC, Kassel JD, Heinz AJ, Braun A, Wardle MC, Greenstein J, Evatt DP, Conrad M. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. J Adolesc Heal. 2011;48:176–181. doi: 10.1016/j.jadohealth.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M, Tunstall-Pedoe H. Self-titration of nicotine: evidence from the Scottish Heart Health Study. Addiction. 1993;88:821–830. doi: 10.1111/j.1360-0443.1993.tb02096.x. [DOI] [PubMed] [Google Scholar]
- Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoked marijuana as compared with tobacco. New England Journal of Medicine. 1988a;318:347–351. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]
- Wu TC, Tashkin DP, Rose JE, Djahed B. Influence of marijuana potency and amount of cigarette consumed on marijuana smoking pattern. Journal of Psychoactive Drugs. 1988b;20 (1):43–46. doi: 10.1080/02791072.1988.10524370. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Chait LD. Breathhold duration and response to marijuana smoke. Pharmacol Biochem Behav. 1989;33(2):481–484. doi: 10.1016/0091-3057(89)90534-0. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Chait LD. Response to marijuana as a function of potency and breathhold duration. Psychopharmacology. 1991;103 (2):223–226. doi: 10.1007/BF02244207. [DOI] [PubMed] [Google Scholar]


