Abstract
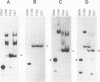
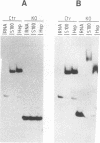
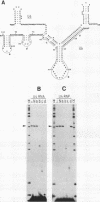
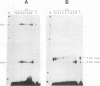
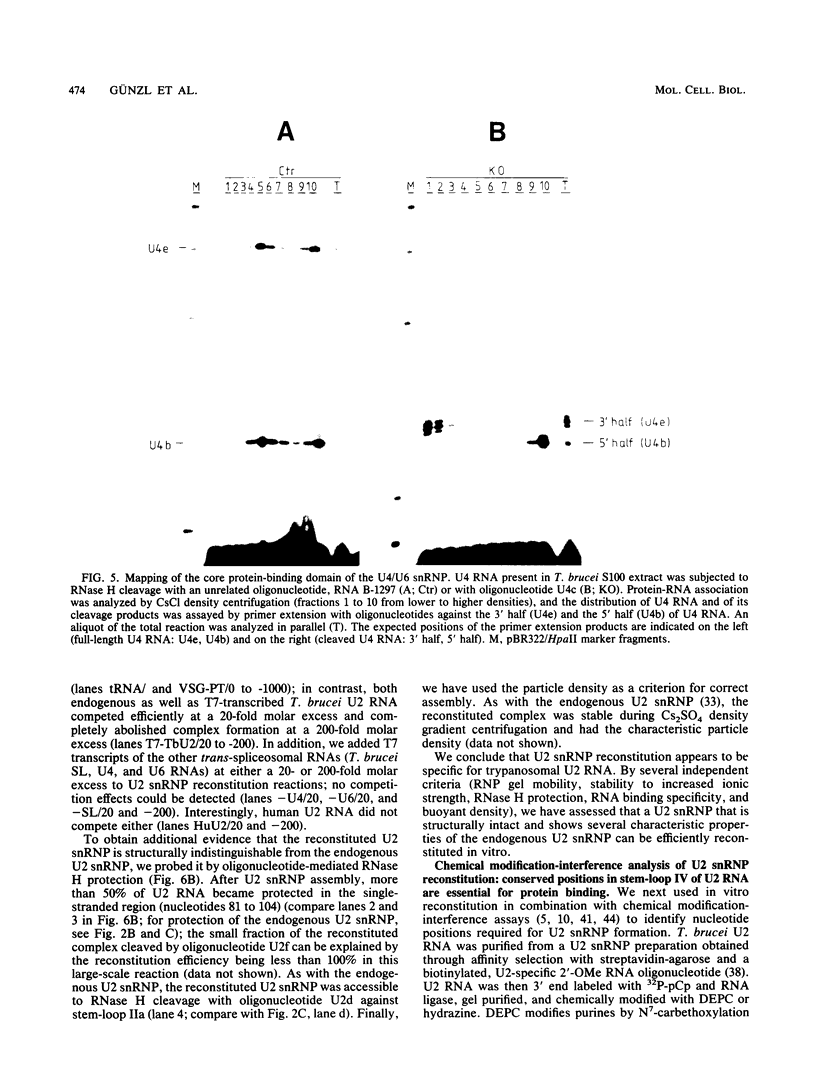
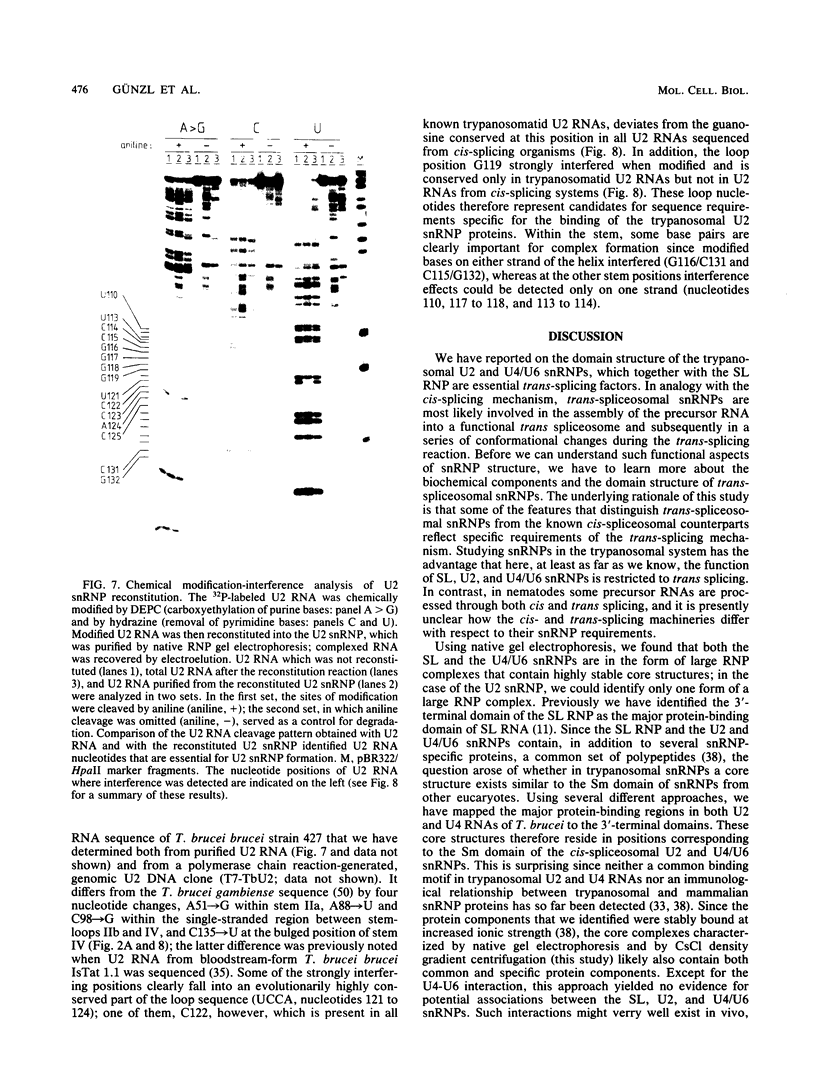
Maturation of mRNAs in trypanosomes involves trans splicing of the 5' end of the spliced leader RNA and the exons of polycistronic pre-mRNAs, requiring small nuclear ribonucleoproteins (snRNPs) as cofactors. We have mapped protein-binding sites in the U2 and U4/U6 snRNPs by a combination of RNase H protection analysis, native gel electrophoresis, and CsCl density gradient centrifugation. In the U2 snRNP, protein binding occurs primarily in the 3'-terminal domain; through U2 snRNP reconstitution and chemical modification-interference assays, we have identified discrete positions within stem-loop IV of Trypanosoma brucei U2 RNA that are essential for protein binding; significantly, some of these positions differ from the consensus sequence derived from cis-spliceosomal U2 RNAs. In the U4/U6 snRNP, the major protein-binding region is contained within the 3'-terminal half of U4 RNA. In sum, while the overall domain structure of the U2 and U4/U6 snRNPs is conserved between cis- and trans-splicing systems, our data suggest that there are also trans-spliceosomal specific determinants of RNA-protein binding.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Igel A. H. Lethal and temperature-sensitive mutations and their suppressors identify an essential structural element in U2 small nuclear RNA. Genes Dev. 1990 Dec;4(12A):2132–2145. doi: 10.1101/gad.4.12a.2132. [DOI] [PubMed] [Google Scholar]
- Bach M., Winkelmann G., Lührmann R. 20S small nuclear ribonucleoprotein U5 shows a surprisingly complex protein composition. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6038–6042. doi: 10.1073/pnas.86.16.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley R. C., Keene J. D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A' protein. Mol Cell Biol. 1991 Apr;11(4):1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Wolff T., Green M. R. Discrete domains of human U6 snRNA required for the assembly of U4/U6 snRNP and splicing complexes. EMBO J. 1990 Jan;9(1):251–255. doi: 10.1002/j.1460-2075.1990.tb08102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Thomas J. Cis and trans mRNA splicing in C. elegans. Trends Genet. 1988 Nov;4(11):305–308. doi: 10.1016/0168-9525(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Boelens W., Scherly D., Beijer R. P., Jansen E. J., Dathan N. A., Mattaj I. W., van Venrooij W. J. A weak interaction between the U2A' protein and U2 snRNA helps to stabilize their complex with the U2B" protein. Nucleic Acids Res. 1991 Feb 11;19(3):455–460. doi: 10.1093/nar/19.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Splicing a spliceosomal RNA. Nature. 1989 Jan 5;337(6202):14–15. doi: 10.1038/337014a0. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Steitz J. A. Spliced leader RNA sequences can substitute for the essential 5' end of U1 RNA during splicing in a mammalian in vitro system. Cell. 1990 Sep 7;62(5):889–899. doi: 10.1016/0092-8674(90)90264-f. [DOI] [PubMed] [Google Scholar]
- Conway L., Wickens M. Analysis of mRNA 3' end formation by modification interference: the only modifications which prevent processing lie in AAUAAA and the poly(A) site. EMBO J. 1987 Dec 20;6(13):4177–4184. doi: 10.1002/j.1460-2075.1987.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Günzl A., Palfi Z., Bindereif A. Analysis of small nuclear ribonucleoproteins (RNPs) in Trypanosoma brucei: structural organization and protein components of the spliced leader RNP. Mol Cell Biol. 1991 Nov;11(11):5516–5526. doi: 10.1128/mcb.11.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta B., Weiner A. M. Genetic evidence for base pairing between U2 and U6 snRNA in mammalian mRNA splicing. Nature. 1991 Aug 29;352(6338):821–824. doi: 10.1038/352821a0. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Fabrizio P., McPheeters D. S., Abelson J. In vitro assembly of yeast U6 snRNP: a functional assay. Genes Dev. 1989 Dec;3(12B):2137–2150. doi: 10.1101/gad.3.12b.2137. [DOI] [PubMed] [Google Scholar]
- Fresco L. D., Harper D. S., Keene J. D. Leucine periodicity of U2 small nuclear ribonucleoprotein particle (snRNP) A' protein is implicated in snRNP assembly via protein-protein interactions. Mol Cell Biol. 1991 Mar;11(3):1578–1589. doi: 10.1128/mcb.11.3.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröning K., Palfi Z., Gupta S., Cross M., Wolff T., Bindereif A. A new U6 small nuclear ribonucleoprotein-specific protein conserved between cis- and trans-splicing systems. Mol Cell Biol. 1991 Apr;11(4):2026–2034. doi: 10.1128/mcb.11.4.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hamm J., Dathan N. A., Mattaj I. W. Functional analysis of mutant Xenopus U2 snRNAs. Cell. 1989 Oct 6;59(1):159–169. doi: 10.1016/0092-8674(89)90878-7. [DOI] [PubMed] [Google Scholar]
- Hartshorne T., Agabian N. A new U2 RNA secondary structure provided by phylogenetic analysis of trypanosomatid U2 RNAs. Genes Dev. 1990 Dec;4(12A):2121–2131. doi: 10.1101/gad.4.12a.2121. [DOI] [PubMed] [Google Scholar]
- Hausner T. P., Giglio L. M., Weiner A. M. Evidence for base-pairing between mammalian U2 and U6 small nuclear ribonucleoprotein particles. Genes Dev. 1990 Dec;4(12A):2146–2156. doi: 10.1101/gad.4.12a.2146. [DOI] [PubMed] [Google Scholar]
- Igel A. H., Ares M., Jr Internal sequences that distinguish yeast from metazoan U2 snRNA are unnecessary for pre-mRNA splicing. Nature. 1988 Aug 4;334(6181):450–453. doi: 10.1038/334450a0. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Guthrie C. Unexpected flexibility in an evolutionarily conserved protein-RNA interaction: genetic analysis of the Sm binding site. EMBO J. 1990 Aug;9(8):2555–2561. doi: 10.1002/j.1460-2075.1990.tb07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Laird P. W. Trans splicing in trypanosomes--archaism or adaptation? Trends Genet. 1989 Jul;5(7):204–208. doi: 10.1016/0168-9525(89)90082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B., Ryder U., Hamm J. Probing the structure and function of U2 snRNP with antisense oligonucleotides made of 2'-OMe RNA. Cell. 1989 Jul 28;58(2):383–390. doi: 10.1016/0092-8674(89)90852-0. [DOI] [PubMed] [Google Scholar]
- Lelay-Taha M. N., Reveillaud I., Sri-Widada J., Brunel C., Jeanteur P. RNA-protein organization of U1, U5 and U4-U6 small nuclear ribonucleoproteins in HeLa cells. J Mol Biol. 1986 Jun 5;189(3):519–532. doi: 10.1016/0022-2836(86)90321-9. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Lossky M., Anderson G. J., Jackson S. P., Beggs J. Identification of a yeast snRNP protein and detection of snRNP-snRNP interactions. Cell. 1987 Dec 24;51(6):1019–1026. doi: 10.1016/0092-8674(87)90588-5. [DOI] [PubMed] [Google Scholar]
- McPheeters D. S., Fabrizio P., Abelson J. In vitro reconstitution of functional yeast U2 snRNPs. Genes Dev. 1989 Dec;3(12B):2124–2136. doi: 10.1101/gad.3.12b.2124. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Roberts T. G., Watkins K. P., Agabian N. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J Biol Chem. 1990 Jun 25;265(18):10582–10588. [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Sather S., Selkirk M., Agabian N. Identification of a small RNA containing the trypanosome spliced leader: a donor of shared 5' sequences of trypanosomatid mRNAs? Cell. 1984 Oct;38(3):721–729. doi: 10.1016/0092-8674(84)90267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram J., Perry K. L., Lizardi P. M., Lührmann R., Agabian N., Nelson R. G. Isolation and sequence of four small nuclear U RNA genes of Trypanosoma brucei subsp. brucei: identification of the U2, U4, and U6 RNA analogs. Mol Cell Biol. 1989 Mar;9(3):1212–1223. doi: 10.1128/mcb.9.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. K., Green M. R. Splice site selection and ribonucleoprotein complex assembly during in vitro pre-mRNA splicing. Genes Dev. 1988 Mar;2(3):319–329. doi: 10.1101/gad.2.3.319. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing in nematodes. Exp Parasitol. 1989 Nov;69(4):413–416. doi: 10.1016/0014-4894(89)90191-4. [DOI] [PubMed] [Google Scholar]
- Palfi Z., Günzl A., Cross M., Bindereif A. Affinity purification of Trypanosoma brucei small nuclear ribonucleoproteins reveals common and specific protein components. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9097–9101. doi: 10.1073/pnas.88.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. Q., Prives C. U2 snRNA sequences that bind U2-specific proteins are dispensable for the function of U2 snRNP in splicing. Genes Dev. 1989 Dec;3(12A):1887–1898. doi: 10.1101/gad.3.12a.1887. [DOI] [PubMed] [Google Scholar]
- Patzelt E., Perry K. L., Agabian N. Mapping of branch sites in trans-spliced pre-mRNAs of Trypanosoma brucei. Mol Cell Biol. 1989 Oct;9(10):4291–4297. doi: 10.1128/mcb.9.10.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikielny C. W., Bindereif A., Green M. R. In vitro reconstitution of snRNPs: a reconstituted U4/U6 snRNP participates in splicing complex formation. Genes Dev. 1989 Apr;3(4):479–487. doi: 10.1101/gad.3.4.479. [DOI] [PubMed] [Google Scholar]
- Reveillaud I., Lelay-Taha M. N., Sri-Widada J., Brunel C., Jeanteur P. Mg2+ induces a sharp and reversible transition in U1 and U2 small nuclear ribonucleoprotein configurations. Mol Cell Biol. 1984 Sep;4(9):1890–1899. doi: 10.1128/mcb.4.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymond B. C., Rosbash M. A chemical modification/interference study of yeast pre-mRNA spliceosome assembly and splicing. Genes Dev. 1988 Apr;2(4):428–439. doi: 10.1101/gad.2.4.428. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Scherly D., Dathan N. A., Boelens W., van Venrooij W. J., Mattaj I. W. The U2B'' RNP motif as a site of protein-protein interaction. EMBO J. 1990 Nov;9(11):3675–3681. doi: 10.1002/j.1460-2075.1990.tb07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster E. O., Guthrie C. Two conserved domains of yeast U2 snRNA are separated by 945 nonessential nucleotides. Cell. 1988 Oct 7;55(1):41–48. doi: 10.1016/0092-8674(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Tschudi C., Krainer A. R., Ullu E. The U6 small nuclear RNA from Trypanosoma brucei. Nucleic Acids Res. 1988 Dec 9;16(23):11375–11375. doi: 10.1093/nar/16.23.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Richards F. F., Ullu E. The U2 RNA analogue of Trypanosoma brucei gambiense: implications for a splicing mechanism in trypanosomes. Nucleic Acids Res. 1986 Nov 25;14(22):8893–8903. doi: 10.1093/nar/14.22.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Destruction of U2, U4, or U6 small nuclear RNA blocks trans splicing in trypanosome cells. Cell. 1990 May 4;61(3):459–466. doi: 10.1016/0092-8674(90)90527-l. [DOI] [PubMed] [Google Scholar]
- Tschudi C., Williams S. P., Ullu E. Conserved sequences in the U2 snRNA-encoding genes of Kinetoplastida do not include the putative branchpoint recognition region. Gene. 1990 Jul 2;91(1):71–77. doi: 10.1016/0378-1119(90)90164-m. [DOI] [PubMed] [Google Scholar]
- Wu J. A., Manley J. L. Base pairing between U2 and U6 snRNAs is necessary for splicing of a mammalian pre-mRNA. Nature. 1991 Aug 29;352(6338):818–821. doi: 10.1038/352818a0. [DOI] [PubMed] [Google Scholar]