Abstract
BACKGROUND
We previously reported that prostatic stem/progenitor cells are concentrated in the proximal region of prostatic ducts and express stem cell antigen 1 (Sca-1). As Wnt signaling is important for the maintenance of stem cells, we determined whether Sca-1 expressing cells also express Axin2, as Axin2 expression is highly suggestive of active Wnt signaling.
METHODS
Axin2 promoter reporter mice were used for whole mount and fluorescence activated cell sorting (FACS) analysis to determine its expression in the prostate. Axin2 expressing cells were also examined for the co-expression of Sca-1. We also used a chemical activator of Wnt signaling, BIO, to determine the effects of Wnt signaling on the growth of primary prostate cells in vitro.
RESULTS
We show that Axin2 expression is present in all lobes and is regulated by androgens with the highest Axin2 expression in the lateral and dorsal prostate. Furthermore, a fraction of Axin2 expressing cells co-express Sca-1, suggesting that some progenitor cells have active Wnt signaling. Lastly, we demonstrate that activation of the Wnt pathway may result in increased growth, consistent with a role for Wnt signaling in maintenance and/or expansion of the progenitor cell population.
CONCLUSION
Axin2 expressing cells that co-express Sca-1 are present in all prostate lobes suggesting that progenitor cells reside within the Wnt active population. An understanding of the basic biology of signaling pathways mediating growth in the prostate may lead to rational therapies to treat benign prostatic hyperplasia and prostate cancer.
Keywords: stem cell, Sca-1, androgen, castration, regeneration, Wnt, Axin2
BACKGROUND
Several intracellular-signaling pathways have been shown to be involved in many functions of stem cells including self-renewal [1–3], maintenance in their niche [4–7], and their transition to a transformed phenotype [8–10]. Activation of the Wnt pathway in adult hematopoietic stem/progenitor cells (HSC) results in an increase in HSC number [11–13], while inhibitors of the Wnt-signaling pathway inhibit HSC growth [14]. Similarly, stabilization of the Wnt activated β-catenin protein results in greater numbers of mammary stem cells in vivo and in vitro [15] eventually resulting in hyperplasia, implicating Wnts in the etiology of transformation. Likewise, Pinto and Clevers [16] demonstrate that Wnt-signaling results in renewal of intestinal crypt cells that replenish the intestinal epithelium, further demonstrating a role for Wnt signaling in the maintenance and/or differentiation of progenitor/stem cells.
In an unstimulated cell, the transcriptional coactivator, β-catenin, is phosphorylated and rapidly turned over via proteosome degradation [17,18]. Upon binding of Wnt ligands to their Frizzled receptor, β-catenin is not phosphorylated and is stabilized in the cytoplasm [19–21]. β-Catenin protein levels accumulate and translocate to the nucleus where they act as transcriptional coactivators for Wnt-signaling regulated genes [22–24]. Recent data strongly suggest that Axin2 is a direct transcriptional target upregulated after activation of Wnt signaling [25–27]. Therefore, analysis of Axin2 expression is likely to be a direct measure of active Wnt signaling.
Unlike the human prostate, the mouse prostate has distinct and separate lobes—ventral prostate (VP), lateral prostate (LP), and dorsal prostate (DP). The ductal architecture of each gland is divided into proximal, intermediate, and distal regions with histologically distinct features suggesting different biological functions in specific regions [28,29]. The proximal region of ducts contains a slow cycling population of stem cells that express high levels of Sca-1 [30–33]. As Wnts have roles in progenitor cell function in a number of tissues, they may also be important in regulating prostate stem and/or progenitor cell homeostasis.
To investigate a possible role for Wnt signaling in mouse prostate progenitor cells, we used two mouse models where a reporter molecule (green fluorescent protein (GFP) or lacZ) was inserted downstream of the Axin2 promoter. We demonstrate that Axin2 expression occurs in all lobes of the prostate in an androgen-dependent manner. We also show that Axin2 expressing cells co-express Sca-1, suggesting that Wnt-signaling occurs in progenitor cells of the prostate. Lastly, using a Wnt-signaling agonist we find that active Wnt signaling may result in increased growth of primary prostate cells in vitro. Together this data suggests that Wnt signaling may be involved in the maintenance/growth of progenitor cells in the prostate.
METHODS
Animals
C57BL/6 mice were obtained from Charles River (Wilmingon, MA). Axin2-GFP and Axin2+/lacZ mice were generated as previously described [26,27] and kindly provided by Dr Frank Costantini at Columbia University, New York. All mice were housed in climate controlled animal research facilities at New York University, and all experiments were performed in compliance with institutional review board requirements. In some instances, castrated animals were replenished with androgen by intraperitoneal injection of testosterone propionate (4 μg/g body weight/day).
Tissue Preparation and Analysis
Animals were sacrificed and the urogenital tract was removed en bloc and transferred to Hank’s balanced salt solution (HBSS) (Cellgro). The VP, LP, and DP were dissected in HBSS under a Leica S6D dissecting microscope using 25-gauge needles [29]. Images were acquired using an Optronics digital camera (Model 60800) and MagnaFire software (Optronics) attached to a Leica MZFLIII dissecting microscope.
To prepare single cell suspensions, prostates were removed from C57BL/6 and Axin2-GFP mice and individual lobes were separated into two regions: (i) the proximal region, which comprises those ducts nearest the urethra, and (ii) the remaining regions, which include the intermediate and distal regions [30]. Each sample was incubated in 0.5% collagenase (type II clostridiopeptidase A; Sigma–Aldrich) for 1 hr with periodic agitation followed by digestion in 0.25% trypsin (BD Biosciences) for 7 min at 37°C. Cells were suspended in growth medium containing conditioned medium from a prostatic smooth muscle cell line, PSMC1 [34,35], and used for further analysis.
LacZ Staining
Prostates were fixed in glutaraldehyde fixative (5 mM EGTA (Sigma), 2 mM MgCl2 (Sigma), 0.1 M NaPi pH 7.3 (Sigma), 0.1% glutaraldehyde (Fisher), 1.5% formaldehyde (Fisher)) for 30 min at room temperature. Prostates were removed from the fixative and washed three times for 30 min/wash in 0.02% NP-40 (Sigma) in 1 × PBS at room temperature. Prostates were then transferred to the staining solution (5 mM K3FE(CN)6 (Sigma), 5 mM K4Fe(CN)6 (Sigma), 2 mM MgCl2 (Sigma), 0.01% deoxycholate (Sigma), 0.02% NP-40 (Sigma), 1 mg/ml X-gal (Roche) in 1 × PBS) and monitored until adequate staining had developed (approximately 1 hr). Once staining was achieved, prostates were washed three times with 1 × PBS, post-fixed overnight in 4% paraformaldehyde (Electron Microscopy Sciences) in 1 × PBS, and photographed as previously described.
Cell Preparation and FACS Analysis
Single cell digests were resuspended in FACS buffer (HBSS containing 0.5% BSA (Sigma–Aldrich) and 2 mM EDTA (Sigma–Aldrich)). Fc receptors were blocked with mouse anti-mouse CD16/32 antibodies (Caltag) and rat IgG (Caltag) for 10 min on ice. The cells were then incubated with phycoerythrin (PE)-conjugated rat anti-mouse Sca-1 (Caltag) antibody (100 ng/ml) or control rat IgG2a-PE (100 ng/ml) (Caltag) for 30 min on ice then washed with FACS buffer. In all analyses, the dye 7-aminoactinomycin D (1 μg/ml) was added 5 min before analysis, to exclude dead cells [31]. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson), using CELLQUEST software (Becton Dickinson).
Cell Growth Assays
Each population of cells was seeded at 104 cells/well on collagen-coated 96-well plates (Falcon) [36]. Cells were cultured for the indicated number of days in medium containing 1 μM methylBIO or 1 μM BIO [37] compound kindly provided by Dr Ali Brivanlou (Rockefeller University, New York). Following culture, cells were removed from collagen-coated plates by a 20 min incubation with 0.5% collagenase (type II clostridiopeptidase A; Sigma–Aldrich) and disaggregated into a single cell suspension by a 7 min incubation in 0.25% trypsin (BD Biosciences). Trypan blue (Sigma–Aldrich) exclusion was used to determine cell viability.
RESULTS
Axin2 Expression Is Highest in the Lateral and Dorsal Lobes of the Mouse Prostate and Is Androgen Dependent
We have shown that prostatic stem cells reside in the proximal region of ducts and that progenitor cells express Sca-1 [30–32]. As Wnt signaling has a role in several aspects of stem cell and tumor biology [38,39], we determined whether evidence of Wnt signaling was present in prostate progenitor cells. This was accomplished using Axin2 expression in the prostate as an indication of Wnt-signaling using two Axin2 reporter mouse models. In these models, either a destabilized form of GFP (Axin2-GFP) or lacZ (Axin2+/lacZ) is expressed under control of the Axin2 promoter [26,27]. Whole mount analysis of the mouse prostate showed that the LP expressed the most Axin2 with less expression evident in the VP or DP (Figs. 1 and 2). This indicates that Axin2 (Wnt activity) is expressed in all lobes of the mouse prostate with the highest levels occurring in the LP.
Fig. 1.
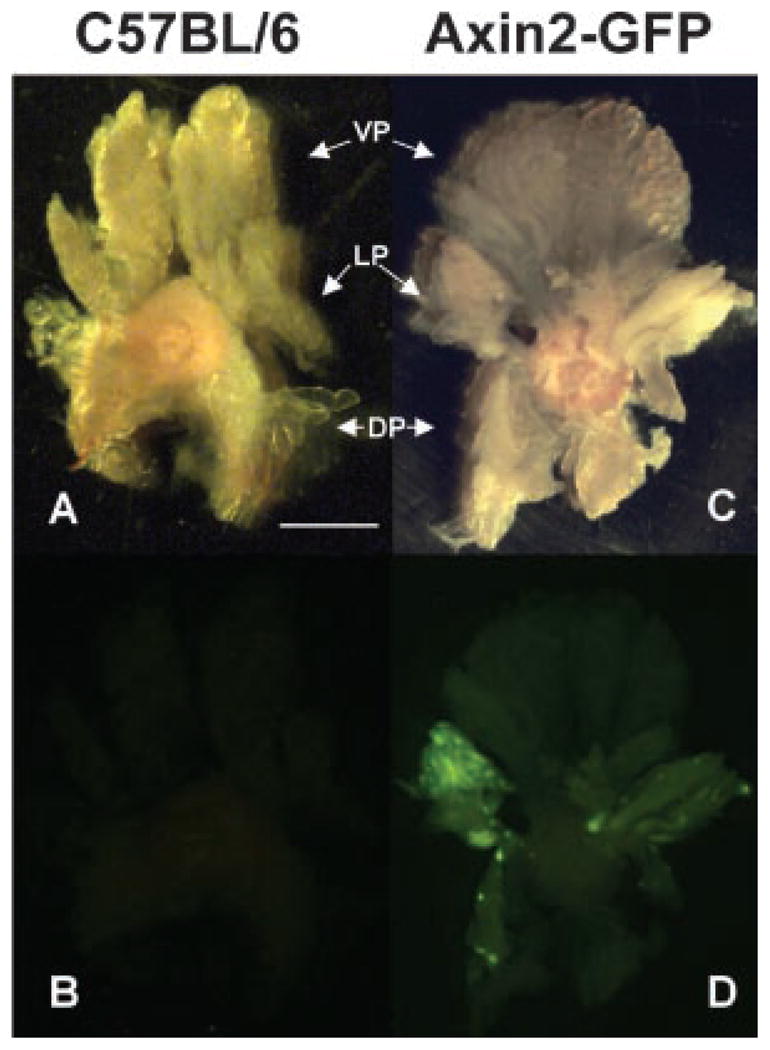
Axin2 is differentially expressed in lobes of the prostate from Axin2-GFP mice. Prostate glands from six Axin2-GFP mice were analyzed for GFP indicative of Axin2 expression. A and B represent brightfield and fluorescence images respectively of a control C57BL/6 prostate. C and D represent brightfield and fluorescence images, respectively, of an Axin2-GFP prostate. The LP has higher levels of Axin2 than the VP and DP (D). Scale bar: 2mm.
Fig. 2.
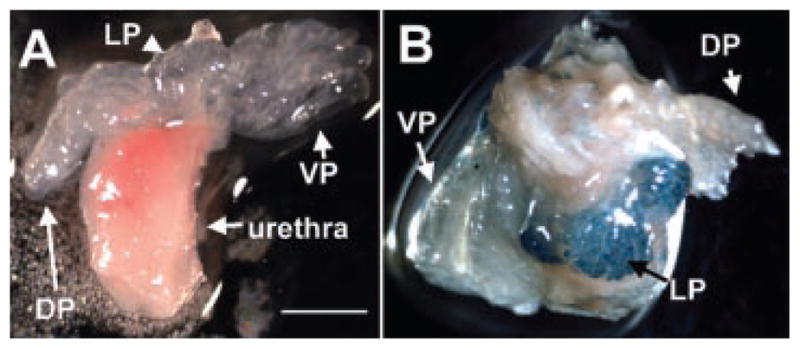
Axin2 is differentially expressed in lobes of the prostate from Axin+/lacZ mice. Prostate glands from three Axin2+/+ and Axin2+/lacZ mice were analyzed for β-galactosidase activity indicative of Axin2 expression. A and B represent prostate lobes (VP, LP, and DP) from Axin2+/+ and Axin2+/lacZ mice, respectively. The LP has higher levels of Axin2 than the VP and DP (B). Scale bar: 2mm.
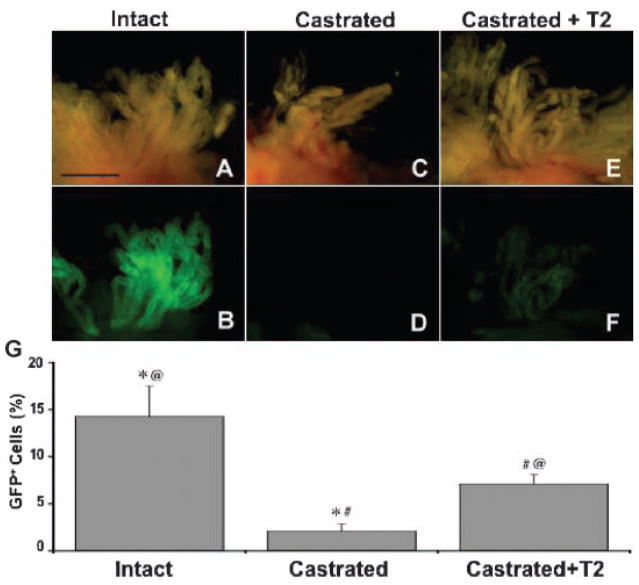
The prostate is an androgen-dependent gland able to undergo over 30 cycles of involution followed by regeneration [40] indicating the presence of a population of stem cells able to survive involution and promote regeneration upon androgen replenishment. Axin2-GFP mice were therefore castrated (androgen deficient) and androgens replenished in an alternate group of mice in order to determine the effect of androgens on Axin2 expression. In whole mounts of prostates of mice that had been castrated for 7 days, GFP is almost undetectable in all lobes of the prostate (see LP in Fig. 3D) demonstrating a dependence on androgens for Axin2 expression. Conversely, in mice that had been castrated for 7 days followed by 7 days of androgen replenishment, GFP expression was partially restored (Fig. 3F) 1 week after androgen supplementation, albeit still below levels seen in intact Axin2-GFP mice (Fig. 3B). The effects of androgen on Axin2 expression were also measured by determining the fraction of cells expressing GFP using the FACS (Fig. 3G). The incidence of Axin2-GFP positive cells was measured in the LP from intact Axin2-GFP mice, castrated Axin2-GFP mice, and Axin2-GFP mice castrated for 7 days followed by daily testosterone injections for 7 days. The LP of castrated mice contained 2.1 ± 0.7% Axin2-GFP expressing cells versus 7.1 ± 1.0% present in animals supplemented with androgen for 7 days (P <0.03) (Fig. 3G). It is possible that levels would have risen further had androgen supplementation been continued for a longer period of time and that they may have approached levels noted in intact animals. However, the levels present after 7 days of androgen supplementation (7.1 ± 1.0%) are not significantly different from those of intact mice (14.2 ± 3.3, P =0.22). This indicates that Axin2 expression is an androgen-dependent event.
Fig. 3.
Axin2 expression is positively regulated by androgens. Five Axin2-GFP mice were castrated (C and D) or castrated then given daily testosterone injections for 7 days (six mice) (castrated +T2) (E and F) and GFP fluorescence compared to that noted in intact Axin2-GFP mice (A and B). A,C, and E represent brightfield images of the LP and B,D, and F represent the corresponding fluorescence images. Scale bar:1mm. (G) FACS was performed to determine the incidence of Axin2-GFP positive cells in the LP from four intact Axin2-GFP mice, three castrated Axin2-GFP mice, and two Axin2-GFP mice castrated then given daily testosterone injections for 7 days. *P <0.03, #P <0.03, @P =0.22.
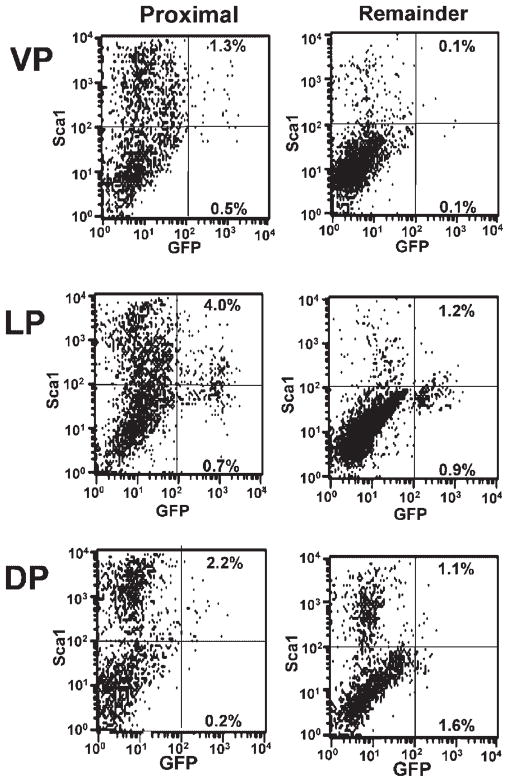
To quantitate the number of GFP+/Axin2 expressing cells in the prostate, proximal and remaining regions of individual lobes from the Axin2-GFP reporter mice were analyzed by FACS for GFP expression. As stem cells are concentrated in the proximal region of prostatic ducts, we examined the proximal and remaining regions of individual lobes to determine whether Axin2 expression was concentrated in cells of the proximal region. We found no significant difference in Axin2 expression along the proximal–distal ductal axis of the VP, LP, or DP (Table I), suggesting that Wnt signaling may not preferentially occur in the proximal stem cell enriched region. While no differences in Axin-2 expression among the remaining regions of ducts were noted, we found that the proximal region of the DP had more Axin2 expressing cells (6.8 ± 1.4%) than the proximal region of the VP (4.7 ± 1.0%) (P <0.01) (Table I). Furthermore, the proximal LP contained significantly more Axin2 expressing cells (12.3 ± 3.0%) than the proximal DP (6.8 ± 1.4%) (P <0.01) (Table I). This indicates that the proximal lobes may be phenotypically different from each other in terms of Wnt signaling which might imply different functions for Wnt in this region of the lobes.
TABLE I.
Axin2-GFP Expression by Cells in Different Regions of Prostate Lobes
| Region and lobe | a GFP+ cells (%) |
|---|---|
| pVP | 4.7 ± 1.0* |
| rVP | 1.9 ± 0.3 |
| pLP | 12.3 ± 3.0# |
| rLP | 9.2 ± 4.0 |
| pDP | 6.8 ± 1.4*,# |
| rDP | 6.8 ± 1.5 |
p, proximal; r, remainder.
Each data point represents the average expression of the indicated antigens from 12 individual mice.
P <0.01.
P <0.01.
Axin2 Expressing Cells Are Sca-1Positive
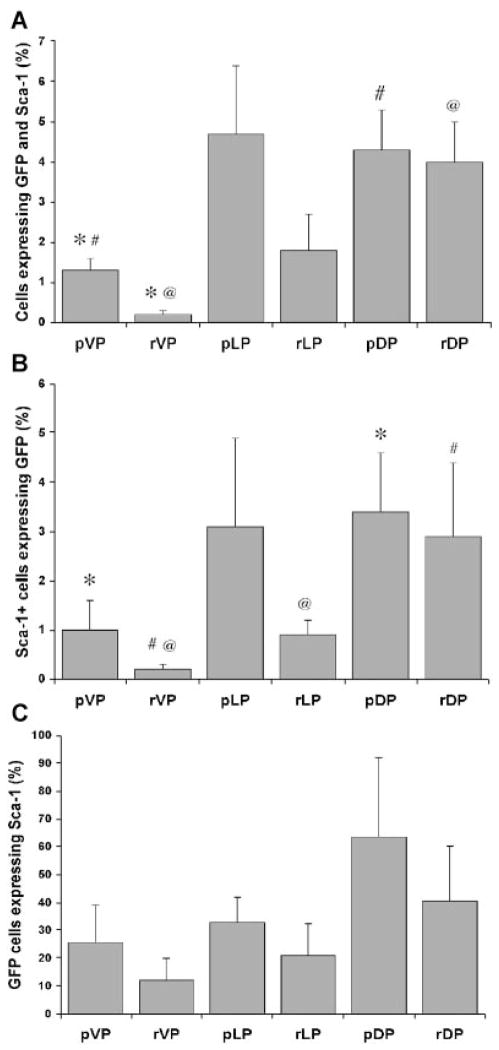
We examined Axin2 expressing cells for their co-expression of Sca-1 as progenitor cells of the prostate express this antigen [31,33]. When we compared the number of GFP+/Sca-1+ (double positive) expressing cells in the proximal region with remaining regions of ducts in individual lobes, we found that only the VP had significantly different numbers of GFP+/Sca-1+ cells in the pVP (1.3 ± 0.3%) compared to the rVP (0.2 ± 0.1%) (P <0.02) (Table IIa and Figs. 4 and 5A). This indicates that the proximal region of the VP contains more progenitor cells that may be undergoing Wnt signaling than the distal region. A comparison of either the proximal or remaining regions of the individual lobes with each other indicated that there were significantly more GFP+/Sca-1+ cells in the pDP (4.3 ± 1.0%) compared to the pVP (1.3 ± 0.3%) (P <0.03) and significantly more GFP+/Sca-1+ cells in the rDP (4.0 ± 1.0%) compared to the rVP (0.2 ± 0.1%) (P <0.02) (Table IIa and Figs. 4 and 5A). This indicates that the DP contains more progenitor (Sca-1+) cells that may have Wnt signaling than the VP.
TABLE II.
The Relationship Between Axin2-GFP and Sca-1 Expression in Different Regions of Prostate Lobes
| (a) Region and lobe | a Cells expressing GFP and Sca-1 (%) |
| pVP | 1.3 ± 0.3*,† |
| rVP | 0.2 ± 0.1*,‡ |
| pLP | 4.7 ± 1.7 |
| rLP | 1.8 ± 0.9 |
| pDP | 4.3 ± 1.0† |
| rDP | 4.0 ± 1.0‡ |
| (b) Region and lobe | a Sca-1+ cells expressing GFP (%) |
| pVP | 1.0 ± 0.6§ |
| rVP | 0.2 ± 0.1§ |
| pLP | 3.1 ± 1.8 |
| rLP | 0.9 ± 0.3§ |
| pDP | 3.4 ± 1.2§ |
| rDP | 2.9 ± 1.5§ |
| (c) Region and lobe | a GFP cells expressing Sca-1 (%) |
| pVP | 25.7 ± 13.4 |
| rVP | 12.2 ± 7.9 |
| pLP | 33.0 ± 9.0 |
| rLP | 21.1 ± 11.4 |
| pDP | 63.6 ± 28.5 |
| rDP | 40.7 ± 19.7 |
p, proximal; r, remainder.
Each data point represents the average expression of the indicated antigens from five individual mice.
P <0.02.
P <0.03.
P <0.02.
P <0.01.
Fig. 4.
Quantitation of Axin2-GFP and Sca-1 expressing cells. FACS analysis was performed to determine the incidence of Axin2-GFP and Sca-1 expressing cells in the proximal and remaining regions of the VP, LP, and DP in five mice. A representative dot plot indicating Wnt activity (GFP+) and Sca-1 expression in cells from different regions of each lobe is depicted.
Fig. 5.
The relationship between Axin2-GFP and Sca-1 expression in different regions of prostate lobes. FACS analysis was performed to determine the incidence of Axin2-GFP and Sca-1 expressing cells in the proximal and remaining regions of the VP, LP, and DP. Each bar represents the average expression of the indicated antigens from five mice. Each data point represents the average expression of the indicated antigens from five individual mice. (A) *P <0.02, #P <0.03, and @P <0.02; (B) *P <0.01, #P <0.01, and\@P <0.01.
As prostate stem and progenitor cells express Sca-1 [31,33], we determined the fraction of Sca-1 expressing cells that may contain an active Wnt pathway (GFP+) (Table IIb and Figs. 4 and 5B). A small fraction of Sca-1 expressing cells (Fig. 4, cells in upper right and upper left quadrants express Sca-1) displayed Axin2 expression suggesting that a subpopulation of progenitor cells in the prostate may utilize Wnt signaling. Comparison of the proximal with remaining regions indicated that the DP contained significantly more Sca-1 expressing cells with Axin2 expression (3.4 ± 1.2% in the pDP and 2.9 ± 1.5% in the rDP) compared to the VP (1.0 ± 0.6% in the pVP and 0.2 ± 0.1% in the rVP) (P <0.01 and 0.01, respectively). Furthermore, the rLP (0.9 ± 0.3%) contained significantly more Sca-1 expressing cells with Axin2 expression than the rVP (P <0.01). This indicates that there may be differences in the number of primitive cells utilizing Wnt signaling among the regions of the individual lobes.
Wnt signaling has a role in homeostasis of stem cells and also functions in more differentiated cells. As the regenerating ability of the prostate resides in the Sca-1 expressing population [31], we determined the fraction of cells that expressed Axin2 and also co-expressed Sca-1 and noted no significant differences in the regional expression of this population of cells (Table IIc and Figs. 4 and 5C). However, significant numbers of Axin2 expressing cells lacked Sca-1 expression, suggesting that not all Wnt active cells are progenitor cells.
Suppression of GSK3β Increases the Growth of Primary Prostate Epithelial Cells
Much work has demonstrated that activation of the Wnt-signaling pathway contributes to maintenance and/or self-renewal of stem cells in different tissues [2,7,14,39,41]. The binding of Wnt proteins to their frizzled receptors, results in altered gene expression and cellular functions with one of the major regulators of the Wnt pathway being GSK3β [42,43]. In an unstimulated cell, GSK3β phosphorylates β-catenin to promote its degradation. After stimulation GSK3β phosphorylation is inhibited, β-catenin is stabilized and available to activate transcription. In order to determine if GSK3β had an effect on cell proliferation, we mimicked the effects of an active Wnt signal in prostate cells by using a chemical inhibitor of GSK3β or its kinase inactive inhibitor analog, methylBIO [37]. The BIO compound and not methylBIO has been shown to decrease β-catenin phosphorylation and degradation [37], and is used to activate intracellular Wnt-signaling molecules by bypassing Wnt ligand binding [44–47]. Although consequences of the inhibition of GSKβ are not definitive proof of Wnt signaling as this protein also acts in other pathways, we determined the effect of BIO on the growth of primary prostate cells. Cells from the proximal and remaining regions of ducts from individual lobes were therefore cultured in the presence of BIO (1 μM) or methylBIO (1 μM) and an approximate twofold (P <0.01) increase in number was noted in BIO treated cells (Table III). This indicates that stimulation of Wnt signaling may result in an increase in the number of prostatic epithelial cells and is consistent with in vivo models showing increased proliferation when Wnt signaling is activated [14,16,38].
TABLE III.
Suppression of GSK3β Increases the Number of Primary Prostate Epithelial Cellsa
| Region and lobe | MethylBIO (cells)* | BIO (cells)* |
|---|---|---|
| pVP | 4,723 ± 342 | 7,980 ± 626 |
| rVP | 6,516 ± 742 | 11,724 ± 1,315 |
| pLP | 9,019 ± 1,171 | 17,804 ± 1,844 |
| rLP | 2,651 ± 349 | 6,081 ± 830 |
| pDP | 2,958 ± 437 | 8,225 ± 1,013 |
| rDP | 3,568 ± 457 | 10,963 ± 1,021 |
p, proximal; r, remainder.
Each data point represents the average number of cells from 12 replicates.
P <0.01 for each region cultured.
CONCLUSIONS
We demonstrate here that Axin2 expression, strongly suggestive of Wnt signaling, is present in all lobes of the mouse prostate and that this expression/Wnt activity is androgen dependent. These data support the idea of interplay between Wnt and androgen signaling. On the one hand, activation of the Wnt-signaling pathway has been shown to enhance or activate different steps in the androgen receptor-signaling pathway. Stimulation of LNCaP cells with Wnt3a can induce ligand independent nuclear accumulation of androgen receptor and androgen receptor-mediated transcription [48,49]. Buttyan and co-workers [50] demonstrated that three LEF1/TCF binding elements lying upstream of the human androgen receptor promoter confer robust transcriptional activation to the promoter after activation of the Wnt-signaling pathway. On the other hand, androgen receptor signaling is also able to alter the Wnt-signaling pathway, albeit usually in an inhibitory manner. Studies demonstrate that androgen receptor activation downregulates transcription from β-catenin inducible promoters [51,52] by using β-catenin at androgen response elements rather than TCF response elements [53,54]. These studies demonstrate a complex balance between Wnt and androgen receptor signaling and imply that there is likely a complex interaction between Wnt and androgen signaling in the different lobes of the mouse prostate. In our system, androgen deprivation results in decreased Wnt activity. Indeed, androgens are known to stimulate Wnt signaling in prostate cells through phosphorylation of GSK3β [55] and direct interaction with β-catenin [56,57]. The resulting lack of Wnt signaling we see after castration could be explained by any of these scenarios where removal of androgens would result in decreased Wnt signaling.
We found no difference in Axin2 expression (Wnt activity) between the proximal and remaining regions in the individual lobes of the prostate. However, we noted that the pLP had significantly more Axin2 expressing cells than the pDP, and the pDP had significantly more Axin2 expressing cells than the pVP. No significant differences in the fraction of Axin2 expressing cells among remaining regions were noted. Our observation that the pLP and pDP may contain the highest fraction of Wnt active cells may be relevant in light of the fact that the lobes of the prostate are associated with different functions. For example, Berquin et al. [58] used DNA array analysis to show that the gene expression pattern in the mouse dorso-lateral lobe, and not the ventral lobe, was closest to that of the human prostate peripheral zone. This supports the hypothesis that these prostate compartments are more similar to each other than they are to the VP [59–61]. As Wnt-signaling pathways are active in carcinogenesis [17,62,63], our data suggesting more Wnt activity in the LP and DP may result in a greater susceptibility of these lobes to the development of prostate cancer [50,64,65]. This may indicate that the LP and DP may serve as better models than the VP for studying prostate carcinogenesis as these lobes may be more closely related to the peripheral zone in which human prostate cancer primarily develops.
The cells in the murine prostate with prostate regenerating potential reside within the Sca-1 expressing population [31,33]. We show that a significant fraction of Axin2 expressing cells co-express Sca-1 and are therefore progenitor cells. These findings are similar to those of the hair follicle in which the strongest Wnt activity occurs in progenitor cells as they leave the cell cycle and begin terminal differentiation [66,67]. In addition, Wnt signaling in the brain is present in proliferating neural progenitors rather than in the most primitive neural stem cells [41]. Thus our data suggest that Wnt signaling in the prostate may be present in progenitor cells where it is likely to promote both proliferation and differentiation to a mature prostate epithelial cell. Indeed we show that the activation of Wnt signaling may result in increased cell numbers consistent with a role for Wnt in proliferation in the prostate.
While Wnt signaling has a role in regulating transcription, it is also associated with changes in cell adhesion [68,69] by promoting association of cytoplasmic β-catenin with cadherins to stabilize extracellular protein interactions between cells [20,68,70]. Therefore signals leading to Axin2 expression could also be related to changes in cell adhesion in addition to or independent of a role in transcriptional activation. It is therefore possible that the higher Axin2 expression observed in the LP could in part be involved in mediating different cell–cell contacts not observed in the VP and DP. Because progenitor cells are longer-lived than more differentiated cells, it is possible that primitive cells remain in their niche by establishing contacts with neighboring cells and extracellular matrix to facilitate their maintenance in their niche. Indeed Wnt signaling has been shown to be important for maintenance of cells in their normal location and migration within many tissues. Active Wnt signaling in the SW480 human colon carcinoma cell line leads to increased E-cadherin expression [71] suggesting a role for Wnt signaling in cell adhesion. Additionally, overexpression of a stable form of β-catenin in intestinal epithelium results in augmentation of E-cadherin at adherens junctions which slows migration of primitive crypt cells up the villus during their progression to a more differentiated phenotype [72]. It is therefore possible that Wnt signaling in the prostate is important for cell adhesive properties of prostate progenitors and that this confers greater growth potential on the cells.
While there have been several reports of alterations in Wnt signaling being associated with prostate cancer [50,65,73], a role for Wnt signaling in prostate homeostasis is not documented. Here we show that all lobes of the prostate may contain cells with androgen-dependent Wnt signaling. Furthermore, these cells have the characteristics of prostate progenitor cells and may use the Wnt-signaling pathway for their expansion and differentiation. Knowledge of the role of Wnt signaling in the normal prostate may facilitate an understanding of the processes involved in carcinogenesis as Wnt signaling is involved in the development of cancer [17,62,63,65]. It furthermore contributes to an understanding of the signals involved in the biology of prostate progenitor cells.
Acknowledgments
This work was supported by a postdoctoral National Research Service Award (NRSA) from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 5F32DK071468, DK 52634 and the National Cancer Institute (NCI), CA132641. We would like to thank Dr Frank Costantini at Columbia University, New York for Axin2-GFP mice. We also thank Dr Ali Brivanlou at Rockefeller University, New York for the supply of methlyBIO and BIO compounds. We also acknowledge support from the Helen L and Martin S Kimmel Center for Biology at NYU School of Medicine.
References
- 1.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multi-potent hematopoietic progenitors in culture. J Immunol. 2006;177(4):2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 2.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345(2):789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7(3):86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Neaves WB. Normal stem cells and cancer stem cells: The niche matters. Cancer Res. 2006;66(9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 6.Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28(1–2):81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 7.Niemann C. Controlling the stem cell niche: Right time, right place, right strength. Bioessays. 2006;28(1):1–5. doi: 10.1002/bies.20352. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett. 2006;580(12):2869–2874. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Ishikawa TO, Oshima M, Taketo MM. The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Res. 2005;65(19):8622–8627. doi: 10.1158/0008-5472.CAN-05-2145. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, Moon RT, Bhatia M. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA. 2003;100(6):3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: A WNT–WNT situation. Nat Rev Immunol. 2005;5(1):21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 13.Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: Expansion of multi-lineage progenitor cells. Blood. 1997;89(10):3624–3635. [PubMed] [Google Scholar]
- 14.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 15.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA. 2004;101(12):4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306(2):357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Karim R, Tse G, Putti T, Scolyer R, Lee S. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36(2):120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]
- 18.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7(6):1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 19.Seto ES, Bellen HJ. The ins and outs of Wingless signaling. Trends Cell Biol. 2004;14(1):45–53. doi: 10.1016/j.tcb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Es JH, Barker N, Clevers H. You Wnt some, you lose some: Oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev. 2003;13(1):28–33. doi: 10.1016/s0959-437x(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 22.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6(7):626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 23.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 24.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science. 1997;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 25.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98(26):14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jesik CJ, Holland JM, Lee C. An anatomic and histologic study of the rat prostate. Prostate. 1982;3(1):81–97. doi: 10.1002/pros.2990030111. [DOI] [PubMed] [Google Scholar]
- 29.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34(5):961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 30.Goto K, Salm SN, Coetzee S, Xiong X, Burger PE, Shapiro E, Lepor H, Moscatelli D, Wilson EL. Proximal prostatic stem cells are programmed to regenerate a proximal–distal ductal axis. Stem Cells. 2006;24(8):1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 31.Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA. 2005;102(20):7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: A model of prostatic homeostasis. J Cell Biol. 2002;157(7):1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102(19):6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salm SN, Koikawa Y, Ogilvie V, Tsujimura A, Coetzee S, Moscatelli D, Moore E, Lepor H, Shapiro E, Sun TT, Wilson EL. Transforming growth factor-beta is an autocrine mitogen for a novel androgen-responsive murine prostatic smooth muscle cell line, PS MC1. J Cell Physiol. 2000;185(3):416–424. doi: 10.1002/1097-4652(200012)185:3<416::AID-JCP12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Salm SN, Koikawa Y, Ogilvie V, Tsujimura A, Coetzee S, Moscatelli D, Moore E, Lepor H, Shapiro E, Sun TT, Wilson EL. Generation of active TGF-beta by prostatic cell cocultures using novel basal and luminal prostatic epithelial cell lines. J Cell Physiol. 2000;184(1):70–79. doi: 10.1002/(SICI)1097-4652(200007)184:1<70::AID-JCP7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL. TGF-β maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170(1):81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10(12):1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, III, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 39.Pazianos G, Uqoezwa M, Reya T. The elements of stem cell self-renewal: A genetic perspective. Biotechniques. 2003;35(6):1240–1247. doi: 10.2144/03356ss03. [DOI] [PubMed] [Google Scholar]
- 40.Isaacs JT. Control of cell proliferation and cell death in the normal and neoplastic prostate: A stem cell model. In: Rodgers DSCCH, Cunha G, Grayshack JT, Henman R Jr, Horton R, editors. Benign prostatic hyperplasia. 87-2881. Washington, DC: Department of Health and Human Services, NIH; 1985. pp. 85–94. [Google Scholar]
- 41.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 42.Cohen P, Frame S. The renaissance of G SK3. Nat Rev Mol Cell Biol. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 43.Cadigan KM, Liu YI. Wnt signaling: Complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 44.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10(1):55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 45.Besser D. Expression of nodal, lefty-a, and lefty-B in undifferentiated human embryonic stem cells requires activation of Smad2/3. J Biol Chem. 2004;279(43):45076–45084. doi: 10.1074/jbc.M404979200. [DOI] [PubMed] [Google Scholar]
- 46.Sinha D, Wang Z, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, Schwartz JH, Borkan SC. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am J Physiol Renal Physiol. 2005;288(4):F703–F713. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]
- 47.Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47(4):935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 48.Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64(24):8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- 49.Cronauer MV, Schulz WA, Ackermann R, Burchardt M. Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. Int J Oncol. 2005;26(4):1033–1040. doi: 10.3892/ijo.26.4.1033. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, Kitajewski J, de la Taille A, Benson MC, Guo Y, Buttyan R. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006;25(24):3436–3444. doi: 10.1038/sj.onc.1209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulholland DJ, Read JT, Rennie PS, Cox ME, Nelson CC. Functional localization and competition between the androgen receptor and T-cell factor for nuclear beta-catenin: A means for inhibition of the Tcf signaling axis. Oncogene. 2003;22(36):5602–5613. doi: 10.1038/sj.onc.1206802. [DOI] [PubMed] [Google Scholar]
- 52.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278(48):48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277(13):11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 54.Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME. Liganded androgen receptor interaction with beta-catenin: Nuclear co-localization and modulation of transcriptional activity in neuronal cells. J Biol Chem. 2002;277(23):20702–20710. doi: 10.1074/jbc.M200545200. [DOI] [PubMed] [Google Scholar]
- 55.Liu XH, Kirschenbaum A, Yao S, Liu G, Aaronson SA, Levine AC. Androgen-induced Wnt signaling in preosteoblasts promotes the growth of MDA-PCa-2b human prostate cancer cells. Cancer Res. 2007;67(12):5747–5753. doi: 10.1158/0008-5472.CAN-07-0478. [DOI] [PubMed] [Google Scholar]
- 56.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237(1):22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, Kitajewski J, de la Taille A, Benson MC, Guo Y, Buttyan R. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006;25(24):3436–3444. doi: 10.1038/sj.onc.1209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berquin IM, Min Y, Wu R, Wu H, Chen YQ. Expression signature of the mouse prostate. J Biol Chem. 2005;280(43):36442–36451. doi: 10.1074/jbc.M504945200. [DOI] [PubMed] [Google Scholar]
- 59.Bosland MC, Prinsen MK. Induction of dorsolateral prostate adenocarcinomas and other accessory sex gland lesions in male Wistar rats by a single administration of N-methyl-N-nitro-sourea, 7,12-dimethylbenz(a)anthracene, and 3,2′-dimethyl-4-aminobiphenyl after sequential treatment with cyproterone acetate and testosterone propionate. Cancer Res. 1990;50(3):691–699. [PubMed] [Google Scholar]
- 60.Yaono M, Tamano S, Mori T, Kato K, Imaida K, Asamoto M, Shirai T. Lobe specific effects of testosterone and estrogen on 3,2′-dimethyl-4-aminobiphenyl-induced rat prostate carcinogenesis. Cancer Lett. 2000;150(1):33–40. doi: 10.1016/s0304-3835(99)00370-5. [DOI] [PubMed] [Google Scholar]
- 61.Wikstrom P, Lindahl C, Bergh A. Characterization of the autochthonous transgenic adenocarcinoma of the mouse prostate (TRAMP) as a model to study effects of castration therapy. Prostate. 2005;62(2):148–164. doi: 10.1002/pros.20123. [DOI] [PubMed] [Google Scholar]
- 62.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 63.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103(2):311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 64.Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21(17):2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 65.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(2):119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 66.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126(20):4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 67.Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19(13):1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bienz M. beta-Catenin: A pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15(2):R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 69.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17(5):459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin–cadherin interactions: The roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163(4):847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998;141(3):765–777. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2005;237(1):22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]