Abstract
The three major human apoE isoforms (apoE2, apoE3, and apoE4) are encoded by distinct alleles (ε2, ε3, and ε4). Compared to ε3, ε4 is associated with increased risk to develop Alzheimer’s disease (AD), cognitive impairments in Parkinson’s disease (PD), and other conditions. In contrast, a recent study indicated an increased susceptibility to the recurring and re-experiencing symptom cluster of Post Traumatic Stress Disorder (PTSD), as well as related memory impairments, in patients carrying at least one ε2 allele. Contextual fear conditioning and extinction are used in human and animal models to study this symptom cluster. In this study, acquisition (day 1, training), consolidation (day 2, first day of re- exposure) and extinction (days 2–5) of conditioned contextual fear in human apo 2, apo 3, and apo 4 targeted replacement (TR) and C57BL/6J wild-type (WT) mice was investigated. Male and female apo 2 mice showed acquisition and retrieval of conditioned fear, but failed to exhibit extinction. In contrast, WT, apoE3 and apoE4 mice showed extinction. While apoE2 mice exhibited lower freezing in response to the context on day 2 than apoE3 and apoE4 mice, this cannot explain their extinction deficit as WT mice exhibited similar freezing levels as apoE2 mice on day 2 but still exhibited extinction. Elevating freezing through extended training preserved extinction in controls, but failed to ameliorate extinction deficits in apoE2 animals. These data along with clinical data showing an association of apoE2 with susceptibility to specific symptom clusters in PTSD supports an important role for apoE isoform in the extinction of conditioned fear.
Keywords: apoE, PTSD, extinction, acquisition
Introduction
Post-traumatic stress disorder (PTSD) is one of the most common (Kessler et al., 2007) and debilitating anxiety disorders (Kessler, 2000). It is frequently comorbid with other mental disorders (Koenen et al., 2008) and has been identified as a major unique identifier for suicidality (Sareen et al., 2005; Cougle et al., 2009). The prevalence of PTSD suggests the involvement of environmental risk factors (Breslau et al., 1998) (Kessler et al., 1995) including socioeconomic status and education (Breslau, et al., 1995), and associations between intensity and number of traumatic events (Sledjeski et al., 2008). In addition, twin studies have provided support for heritable and genetic risk factors increasing PTSD susceptibility (Stein et al., 2002; Skre, et al., 1993; Chantarujikapong et al., 2001).
ApoE plays an important role in the metabolism and redistribution of lipoproteins and cholesterol. The three major human apoE isoforms are encoded by distinct alleles (ε2, ε3, and ε4). They differ in having cysteine (Cys) or arginine (Arg) at positions 112 and 158, and differ in metabolic properties (Mahley, 1988). In the brain, apoE has been implicated in development, regeneration, neurite outgrowth, and neuroprotection (Mahley, 1988). Compared with ε2 and ε3, ε4 increases the risk of developing Alzheimer’s disease (AD), cognitive impairments following neurotrauma (Nathoo, 2003) those occurring with normal aging (Howieson et al., 2003) or in the context of Parkinson Disease (PD) (Tang, 2002; Zareparsi, 2002). While ε2 is generally considered neuroprotective, compared to ε4, ε2 may increase the risk of developing sporadic PD (Huang et al., 2004). Apo 2 might also be a critical determinant of susceptibility to specific symptom clusters within the PTSD diagnosis. In a study of veterans with combat-related PTSD, compared to apoE3 and apoE4, apo 2 was associated with worse re-experiencing symptoms and memory impairments, but was not associated with a risk to develop PTSD itself (Freeman et al., 2005).
The phenomenon of recurring and persistent recall of traumatic memories in PTSD may be related to a failure of extinction learning (Charney et al., 1993) or a failure to modify or acquire new associations to contextual stimuli (Corcoran et al., 2005). Therefore, contextual fear conditioning has been widely used to study aspects of PTSD in humans and animal models (Siegmund et al., 2006). In particular, fear conditioning is used to study the inhibition or extinction of learned fear, a process believed to underlie the recurring and re-experiencing symptoms of PTSD (Cannistraro and Rauch, 2003). The present study investigated the extinction of conditioned contextual fear in human apo 2, apo 3, and apo 4 targeted replacement (TR) and C57BL/6J wild-type (WT) mice. In a separate cohort of animals, open field and light-dark tests were used to assess anxiety-like behavior.
Material and Methods
Animals
Three to six-month-old TR apo 2 (Sullivan, et al., 1998), apo 3 (Sullivan et al., 1997), and apo 4 (Knouff et al., 1999) mice, expressing human apoE under control of the mouse apoE promoter on a C57BL/6J background, and C57BL/6J WT mice (n = 219 mice) were used in this study. The breeder mice to generate 4–6 month-old TR mice for this study were generously provided by Dr. Patrick Sullivan, who maintains his colony by heterozygous and homozygous matings. The human TR mice we received from Dr Sullivan were backcrossed to C57BL/6J mice at least eight times and therefore are at least 99.6% C57BL/6J. Breeder mice to generate the WT mice were obtained from Jackson Laboratories, Bar Harbor, ME. Homozygous breeding of the mice was used to generate the experimental mice for this study. Throughout testing, all the mice were singly housed. This study was performed over a 2-year period and involved 19 apoE2, 16 apoE3, 18 apoE4, and 15 WT litters. Animals were maintained on a 12 h light/dark schedule (lights on at 06:00). Laboratory chow (PicoLab Rodent diet 20, # 5,053; PMI Nutrition International, St. Louis MO, USA) and water were provided ad libitum. Behavioral testing took place during the light cycle. All procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with IACUC approval at Oregon Health & Sciences University. Experimenters were blinded to the genotype and sex of the animals.
Fear conditioning
Freezing in mice, defined as a lack of movement except for respiration, is commonly used to assess conditioned fear (Maren, 2001). The fear conditioning apparatus consisted of a Plexiglas chamber (21 cm W × 21 cm D × 24 cm H) containing a shock grid (21cm × 21cm, 30 bars 0.7cm apart) inside a sound-attenuating chamber (55.9 cm W × 35.6 cm D × 38.1 cm H, MedAssociates ENV-022V Standard Expanded PVC). Cameras and Noldus Ethovision XT 7.0 video tracking software (Noldus Information Technology, Leesburg, VA) were used to automate behavioral scoring. This software has been used to measure freezing (Bast et al., 2009) and selected tracking parameters optimally correlate with traditional hand-scoring (Pham et al., 2009). Fidelity to hand-scoring is preserved under these experimental conditions (as described in Villasana et al., 2010). Lights (9.33 ± 0.88 lux) and administration of shock (scrambled) were under control of a Hamilton Kinder programmable shocker using Kinder Scientific MotorMonitor software (Kinder Scientific, Poway, CA). External noise cues were further attenuated by a white noise generator running at 50 dB.
This study consisted of two contextual fear conditioning and extinction paradigms containing one day of training and four subsequent days of extinction, assessed by re-exposure to the context. In experiment 1, the mice (WT: n = 29 males and n = 14 females; apo 2: n = 11 males and n = 19 females; apo 3: n = 10 males and n = 9 females; and apo 4: n = 14 males and n = 13 females) were placed into the dark fear conditioning enclosure on the training day (Day 1). Lights came on at 0:00 and went off at 5:00. 148 seconds of exposure to the context was immediately followed by a 2-sec 0.5 mA shock. After another 148 sec, a second 2-sec 0.5 mA shock was delivered. Animals received 5-min extinction trials on Days 2–5. Enclosures, chambers and shock grids were cleaned with 5% glacial acetic acid between mice.
Because of relatively lower freezing levels in apo 2 and WT mice, in Experiment 2, 4–7 month-old male mice (WT: n = 12; apo 2: n = 6; apo 3: n = 6; and apo 4: n = 16) received four shocks (0.5 mA, 2-second shocks at 2:28, 4:58, 7:28, and 9:58) during a 10-min trial on the training day and 10-min extinction trials on Days 2–5. Female mice were not included as there were no sex differences observed during the extinction phase of experiment 1.
Baseline freezing, prior to the first shock in the training trial on Day 1 was analyzed and compared with post-training freezing (acquisition of fear response) and freezing 24 hour after training (consolidation and recall). Extinction of conditioned fear was measured as a decrease in freezing over time compared to the first contextual re-exposure 24 hour following training.
Baseline motion and Shock sensitivity
To assess differences in shock sensitivity, motion during shock, recorded using a Med Associates (Med Associates, St. Albans, Vermont) fear conditioning system (see (Anagnostaras, et al. 2010) for details) was analyzed in 60 3–4 month old male mice (WT = 20, apoE2 = 14, apoE3 = 19, apoE4 = 21) over ten 0.35 mA shocks, separated by 60-seconds. A lower amperage was chosen in case threshold differences would not be apparent at 0.5 mA. Med Associates reports motion using a proprietary index based on pixel change. Baseline motion before shock administration was also assessed.
Anxiety
To assess potential contributions of anxiety-like behavior, six-month old male apoE2 (N = 10), apoE3 (N = 8) and apoE4 (N = 9) mice were tested in both open field and light-dark mazes, as described in Villasana et al., 2006.
Statistical analyses
Data are reported as averages ± the standard error of the mean and were analyzed using SPSS 16.0 software (IBM, Armonk, NY). Two-way ANOVAs were conducted using sex and genotype as between-subject variables. Extinction curves were analyzed using repeated-measures ANOVAs with testing day as a within subjects variable and genotype and sex as a between-subjects variables. Non-normal data were log-transformed if possible; else they were analyzed using non-parametric methods. If Mauchly’s test of sphericity (for repeated measures) was not satisfied, multivariate statistics using Wilk’s lambda (λ) were reported. Bonferroni adjustments were used for post-hoc multiple comparisons. Results were considered significant at an α level of 0.05. Video failure resulted in the loss of Day 1 data for four apo 4 mice (2 male and 2 female mice). These data were removed from the baseline and acquisition freezing level analysis. A software failure on day three resulted in the loss of data of 13 WT mice (5 female and 8 male mice) from this data point. The freezing levels of these mice on day 2 were carried over into day 3.
Results
Experiment 1
There was an effect of genotype on baseline freezing levels (F(3,99) = 3.492, p = 0.018)(Fig. 1a). Apo 3 mice showed higher freezing levels (9.52 ± 0.86) than WT (6.54 ± 0.70) (p = 0.037) or apo 2 (6.43 ± 0.60) (p = 0.038) mice. The freezing levels of apo 4 mice (7.88 ± 0.88) did not differ from those in any other group.
Fig 1.
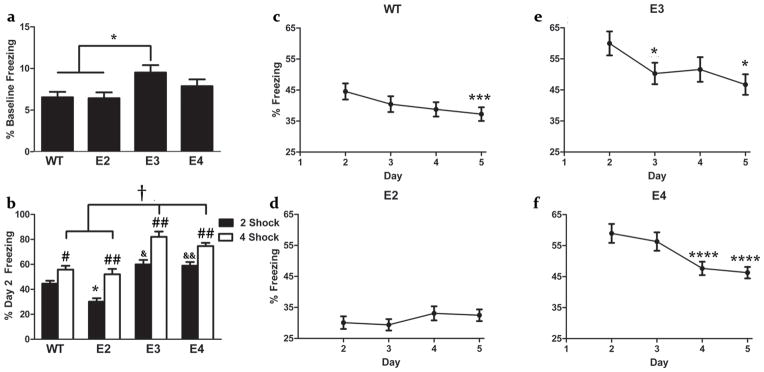
Baseline Freezing (a), contextual fear conditioning 24 hr after training (b) and extinction (c–f) of WT, apoE2, apoE3, and apoE4 mice. (a) WT and apoE2 mice show less freezing prior to the shock than apoE3 mice (*p < 0.05). (b) ApoE2 mice freeze less than WT, apoE3, and apoE4 mice (*p < 0.0001), and WT mice freeze less than apoE3 (&p < 0.05) and apoE4 (&&p < 0.01) 24 hours after training with two shocks. WT and apoE2 mice exhibit lower freezing than apoE3 and apoE4 mice 24 hours after training with four shocks (†p < 0.0001). All genotypes exhibit greater freezing in experiment 2 than experiment 1 (#p < 0.05, ##p < 0.01). (c) WT mice show extinction (p < 0.005), with lower freezing on Day 5 (***p < 0.005) than Day 2. (d) ApoE2 mice exhibit no extinction. (e) ApoE3 mice show extinction (p < 0.05), with lower freezing on Day 3 and 5 (*p < 0.05) than Day 2. (f) ApoE4 mice show extinction (p < 0.0001), with lower freezing on Day 4 and Day 5 (****p < 0.005) than Day 2). Example extinction curves shown in panels c-f were taken from Experiment 1.
All groups acquire contextual fear
Acquisition of contextual conditioned fear was analyzed comparing the percentage of time spent freezing during baseline (0:00 – 2:28) and the time between after the first shock (2:30–4:58). There was a main effect of training (F(1,94) = 141.37, p < 0.0001), and a significant interaction between training and genotype (F(3,94) = 3.900, p = 0.011), though all groups exhibited increased freezing (table 1a.), (WT: F(1,29) = 33.288, p < 0.0001; apo 2: F(1,29) = 41.674, p < 0.0001; apo 3: F(1,18) = 14.064, p = 0.001; and apo 4: F(1,22) = 127.493, p < 0.0001), suggesting intact acquisition of conditioned fear.
Table 1.
Acquisition and extinction of conditioned fear in Experiment 1.
| Acquisitiona | Extinction (% Freezing)b | ||||
|---|---|---|---|---|---|
|
| |||||
| Genotype | (% Increase in Freezing) | Day 2 | Day 3 | Day 4 | Day 5 |
| apoE2 | 249.45 ± 28.23 | 30.09 ± 2.03 | 29.38 ± 1.84 | 33.07 ± 2.26 | 32.47 ± 1.89 |
| apoE3 | 277.86 ± 49.00 | 60.00 ± 3.87 | 50.30 ± 3.44* | 54.59 ± 3.96 | 46.73 ± 3.30* |
|
apoE4
|
395.10 ± 67.14 | 58.94 ± 3.06 | 56.32 ± 2.97† | 47.64 ± 2.16**** | 46.27 ± 1.86**** |
| WT | 344.08 ± 59.91 | 44.54 ± 2.6 | 40.47 ± 2.54 | 38.77 ± 2.32 | 37.26 ± 2.19*** |
All genotypes exhibited acquisition of fear, as demonstrated by a significant increase in freezing following the first training shock
ApoE3, ApoE4 & WT mice exhibit extinction (significant decrease in freezing relative to day 2).
p < 0.05,
p < 0.005,
p < 0.0001,
p = 0.059. ApoE2 mice do not exhibit extinction.
Compared to apoE3 and apoE4, apoE2 and WT mice exhibit reduced contextual fear 24 hours after training
Consolidation and recall of conditioned contextual fear was measured as the percent of time freezing during the 300 second trial on Day 2 (first day of re-exposure) (Fig. 1b). There was an effect of genotype (F(3,111) = 23.171, p < 0.0001), as well as a significant interaction of sex and genotype (F(3,111) = 3.985, p = 0.010). Therefore, the sexes were then analyzed separately. There was an effect of genotype in males (F(3,60) = 13.008, p < 0.0001), with apo 2 mice freezing less than WT, apo 3, and apo 4 mice (p < 0.0001). There was also an effect of genotype in females (F(3,51) = 11.158, p < 0.0001), with female apo 4 and apo 3 mice exhibiting greater freezing than WT (WT vs apo 3: p = 0.027; WT vs apo 4: p < 0.001) and apo 2 mice (apo 2 vs apo 3: p < 0.003; apo 2 vs apo 4: p = 0.0001). Overall (fig 1. B), apoE2 mice froze less than WT, apoE3, & apoE4 mice (p < 0.0001), and WT mice froze less than apoE3 (p = 0.011) & apoE4 mice (p = 0.006).
ApoE2 mice exhibit impaired extinction compared to controls
Freezing during extinction trials (fig 1. c–f, table 1b.) showed an effect of day (λ = 0.751, F(3,109) = 12.034, p < 0.0001), and a significant interaction of day and genotype (λ = 0.749, F(9,333) = 3.581, p < 0.0001). There was no significant interaction with sex and day or main effect of sex in extinction. Next, each genotype was analyzed separately. There was an effect of day in WT (λ = 0.689, F(3,39) = 5.879, p = 0.002; with lower freezing on day 5 compared to day 2 (p = 0.004)), apo 3 (λ = 0.541, F(3,15) = 4.240, p = 0.023; with lower freezing on day 5 and 3 compared to day 2 (p = 0.015 and p = 0.022, respectively), and apo 4 mice (λ = 0.454, F(3,23) = 9.217, p < 0.0001; with lower freezing on day 5 vs Day 2 (p < 0.0001) and Day 4 vs Day 2 (p < 0.0001), with a trend towards a significant decrease on Day 3 compared to Day 2 (p = 0.059)). There was no effect of day, and thus no decrease in freezing, within apo 2 mice.
Experiment 2
Increasing the number of shocks during training elevates the fear response to the context 24 hours after training
Doubling the length of the trial and the number of shocks during training on Day 1 elevated freezing levels on Day 2 above those seen in the first experiment in all genotypes (Mann-Whitney U test, WT: Md1 = 25.72, Md2 = 36.17, U = 160, z = −1.997, p = 0.046; apo 2: Md1 = 16.13, Md2 = 30.33, U = 19, z = −3.014, p = 0.003; apo 3: Md1 = 10.58, Md2 = 20.67, U = 11, z = −2.927, p = 0.003; and apo 4; Md1 = 17.07, Md2 = 30.31, U = 160, z = −3.342, p = 0.001) (Fig. 1b). There was an effect of genotype on freezing levels on Day 2 (F(3,36) = 15.221, p < 0.0001), similar to that seen in Experiment 1; apo 3 and apo 4 mice exhibited greater freezing than WT or apo 2 mice (p < 0.0001).
Elevating freezing does not ameliorate extinction deficits in apoE2 mice
Freezing during extinction trials exhibited an effect of day (F(3,108) = 25.225, p < 0.0001) and an interaction between day and genotype (F(9,108) = 2.589, p = 0.010)(Table 2). When each genotype was analyzed separately, there was an effect of day in WT (F(3,33) = 7.511, p = 0.001; Day 5 vs Day 2, (p = 0.040); Day 4 vs Day 3 (p = 0.046)), apo 3 (F(3,15) = 12.992, p < 0.0001; Day 5 vs Day 2, p = 0.038; Day 5 vs Day 3: p = 0.005), and apo 4 mice (F(3,45) = 26.503, p < 0.0001; Days 4 (p < 0.000) and 5 (p = 0.003) vs Day 2; and a trend toward a difference between Days 3 and Day 2 (p = 0.059)). Changes in freezing in apoE2 mice did not reach significance.
Table 2.
Extinction of Conditioned fear in Experiment 2.
| Extinction (% Freezing)a | ||||
|---|---|---|---|---|
|
| ||||
| Genotype | Day 2 | Day 3 | Day 4 | Day 5 |
| apoE2 | 52.06 ± 5.01 | 53.61 ± 438 | 48.71 ± 5.58 | 45.39 ± 3.25 |
| apoE3 | 82.00 ± 3.62 | 72.68 ± 6.14 | 63.75 ± 7.61 | 60.17 ± 6.96* |
| apoE4 | 74.59 ± 2.01 | 66.35 ± 2.41 | 60.53 ± 1.66** | 63.69 ± 1.83** |
| WT | 55.82 ± 3.80 | 56.61 ± 3.32 | 50.04 ± 4.18 | 45.83 ± 4.43* |
ApoE3, ApoE4 & WT mice exhibit extinction (significant decrease in freezing relative to day 2).
p < 0.05,
p < 0.01. ApoE2 mice do not exhibit extinction.
Freezing differences are not due to shock sensitivity
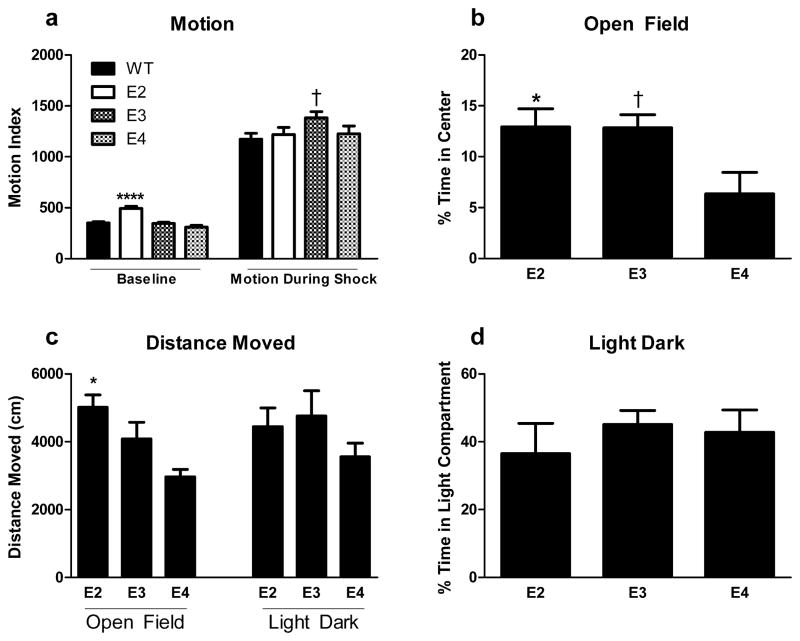
ApoE2 mice demonstrate greater baseline motion (492.59 ± 21.85) than all other groups (effect of genotype: F(3,70) = 20.726, p < 0.0001); WT: 350.89 ± 12.00, apoE3: 346.17 ± 12.50, apoE4: 309.27 ± 18.72; p < 0.0001 for apoE2 versus any other genotype) (Fig. 2a). With regards to shock sensitivity, there was only a trend of an effect on average motion during shock (F(3,70) = 2.381, p = 0.077). This was driven by a trend for WT mice (1172.70 ± 58.02) to display less motion (p = 0.082) than E3 (1383.037 ± 59.52) mice. No differences were found between apoE4 (1225.66 ± 76.42) and E2 (1219.98 ± 69.34) mice and any other groups (Fig 2a).
Fig 2.
(a) Baseline motion and motion during shock of WT, apoE2, apoE3, and apoE4 mice. (b) Measures of anxiety of apoE2, apoE3, and apoE4 mice in the open-field test. (c) Distance moved in the open field and light-dark tests. (d) Measures of anxiety of apoE3, apoE3, and apoE4 mice in the light-dark test. ***p < 0.0001 versus any other genotype; *p < 0.05 versus apoE4; † P = 0.061.
ApoE2 mice do not exhibit elevated anxiety-like behavior compared to apoE3 or apoE4 mice
There was an effect of genotype on time spent in the open (anxiety-inducing) portion of the open-field arena (F(2,24) = 4.440, p = 0.023). ApoE4 mice spent less time in the center of the arena compared to apoE2 counterparts (p = 0.041), with a trend towards greater anxiety-like behavior relative to apoE3 mice (p = 0.061)(Fig. 2b). An effect of genotype on distance moved was also noted (F(2,24) = 8.266, p = 0.002, with apoE2 mice exhibiting higher activity than apoE4 mice (p = 0.001)(Fig. 2c). There were no effects of genotype on time spent in the light (anxiety-inducing) portion of the light-dark maze (Fig. 2d), nor on distance moved (Fig. 2c).
Discussion
In the present study, male and female ApoE2 mice showed acquisition of conditioned fear but failed to exhibit extinction. ApoE2 mice also exhibited lower freezing in response to re-exposure to the context on Day 2 than apoE3 or apoE4 mice. This cannot explain their extinction deficit, as WT mice exhibited similar freezing levels as apoE2 mice on Day 2 but still exhibited extinction. Increased anxiety has been associated with greater activation of the amygdala during extinction, and a deficit thereof. As apoE2 mice did not show higher anxiety levels than apoE3 or apoE4 mice in the open field or light-dark tests, enhanced anxiety levels cannot explain the extinction deficit in apoE2 mice. In contrast to apoE2 mice, WT, apoE3 and apoE4 mice, showed extinction. This deficit seems specific to fear conditioning and extinction learning, as apoE2 mice show comparable spatial learning and memory in the water maze as compared to apoE3 mice (Villasana et al., 2006; Siegel et al., 2012). These data, along with clinical data showing an association of apoE2 with susceptibility to specific symptom clusters in PTSD (Freeman et al., 2005), support an important role of ApoE isoform in the extinction of conditioned fear.
ApoE2 mice demonstrate greater baseline motion than all other groups. It is unlikely that greater activity would significantly affect freezing levels if memory and fear response were intact, as freezing is a specific behavior rather than simply reduced activity levels. This is also indicated by the distinct pattern of genotype differences in baseline motion as compared to that of baseline freezing. Nevertheless, elevating freezing levels on day 2 failed to produce extinction in the same time-frame as was observed in the other genotypes. Increased activity would not affect a decrease in freezing over time, and one might even expect to see accelerated reduction in freezing over time if greater activity was present. Furthermore, reduced fear on day 1 cannot explain the deficit in extinction, as elevating freezing levels in the second paradigm did not ameliorate this apparent deficit.
ApoE4 mice showed higher levels of contextual freezing than other genotypes. Consistent with this result, apoE4 mice require fewer trials to learn the passive avoidance task compared to apoE2 and E3 mice (Villasana et al, 2006; Villasana et al, 2008). ApoE2 mice did not show elevated anxiety levels as compared to apoE3 or apoE4 mice in the open field or light-dark tests. Given the lack of differences in fear conditioning between apoE4 mice and other genotypes (E2 notwithstanding), differences in emotional learning and memory and anxiety may be unrelated to the extinction deficits of apoE2 mice in this study.
Contrary to our results, Kornecook et al. (2010) found reduced freezing levels in apoE4 compared to apoE3 mice in animals also over-expressing human APP. Aβ accumulation in these mice is apoE isoform-dependent (apoE4>apoE3) (Holtzman et al, 2000) and differential interactions between hAPP and apoE, rather than apoE isoform might affect contextual fear conditioning in this model.
In the apoE TR model in which apoE is expressed under control of the mouse apoE promoter, apoE4 mice do not show reduced cognitive performance under baseline conditions but enhanced susceptibility to cognitive impairments following environmental challenges such as cranial irradiation (Villasana et al., 2006). In contrast, in mice expressing apoE in either neurons or astrocytes, apoE4 mice do show cognitive impairments compared to apoE3 mice (Raber et al., 1998; Hartman et al., 2001; van Meer et al., 2007). Part of these convergent data might be due to the fact that apoE4 TR mice outperform GFAP-apoE4 mice in spatial learning and memory in the water maze (Siegel et al., 2012).
Contextual fear conditioning is frequently used to assess hippocampus-dependent memory (Anagnostaras et al., 2001), particularly in the context of acquisition and consolidation. Extinction processes similar to the initial learning have been proposed, involving acquisition, retrieval and consolidation, to mediate the formation of new, or modulation of original, fear memories (Ji et al., 2007). Hippocampal long-term-potentiation (LTP) has been implicated in the acquisition of conditioned fear (Maren et al., 1994). Attenuation of LTP in the Prefrontal Cortex-Hippocampus pathway has been shown to impair extinction in conditioned fear (Farinelli et al., 2006; Herry et al., 2002). Hippocampal LTP is reduced in apoE2 mice compared to ApoE4 mice (Trommer et al., 2004), and this might contribute to the extinction deficit of E2 mice. In addition to the hippocampus (Corocan et al., 2005), lesion studies have implicated the amygdala in the formation of new associations between stimuli (Knight et al., 2004) as well as in the expression of the fear response (Cheng et al., 2006) and a role of the prefrontal cortex in retrieval of the extinction memory (Quirk et al., 2008), and there is support for interdependence of these three brain regions in fear conditioning and extinction as well (Ji et al., 2007).
Conclusions
Consistent with clinical data showing an association of apoE2 with susceptibility to specific symptom clusters in PTSD, these data support an important role for apoE isoform in the extinction of conditioned fear. Deficits in extinction in apoE2 mice provide an example of aberrant processing of fear memory that may represent extant differences prior to developing the condition. Future studies are warranted to determine the brain region(s) involved in the isoform-dependent effects of apoE on contextual fear conditioning and extinction.
Acknowledgments
This work was supported by NNJ05HE63G (NASA) and the OHSU Development Account of Dr. Jacob Raber. The assistance of research intern Karen Li is appreciated.
Footnotes
There are no conflicts of interest to declare.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P. Risk factors for PTSD-related traumatic events: a prospective analysis. American Journal of Psychiatry. 1995;152:529–535. doi: 10.1176/ajp.152.4.529. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatr. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- Chantarujikapong SI, Scherrer JF, Xian H, Eisen S, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatr Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatr. 1993;50:294–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav Neurosci. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Keough ME, Riccardi CJ, Sachs-Ericsson N. Anxiety disorders and suicidality in the National Comorbidity Survey-Replication. J Psychiatr Res. 2009;43:825–829. doi: 10.1016/j.jpsychires.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learning Memory. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WST. Neuropsychiatric Associations of Apolipoprotein E Alleles in Subjects With Combat- Related Posttraumatic Stress Disorder. J Neuropsychiatr Clin Neurosci. 2005;17:541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and –apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan A, Parsadanian M, Sartorius LJ, Mackey B, Olney JW, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform dependent amyloid deposition and neuritic dgenratin in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson DB, Camicoli R, Quinn J, Silbert LC, Care B, Moore MM, Dame A, Sexton G, Kaye JA. Natural history of cognitive decline in the old old. Neurology. 2003;60:1489–2494. doi: 10.1212/01.wnl.0000063317.44167.5c. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen PC, Poole C. APOE-ε2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62:2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;758:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatr. 2000;61:4–14. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatr. 2007;6:168–176. [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatr. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognit Affect Behav Neurosci. 2004;4:317–235. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. ApoE structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1186. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffit TE, Caspi A, Gregory A, Harrington H, Pouton R. The developmental mental-disorder histories of adults with posttraumatic stress disorder: A prospective longitudinal birth cohort study. J Abnormal Psychol. 2008;117:460–466. doi: 10.1037/0021-843X.117.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornecook TJ, McKinney AP, Ferguson MT, Dodart JC. Isoform-specific effects of apolipoprotein E on cognitive performance in targeted-replacement mice overexpressing human APP. Genes Brain Behav. 2010;9:182–192. doi: 10.1111/j.1601-183X.2009.00545.x. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Ann Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology. 2001;56:1094–1098. doi: 10.1212/wnl.56.8.1094. [DOI] [PubMed] [Google Scholar]
- Pham J, Cabrera S, Sanchis-Segura C, Wood MA. Automated scoring of fear-related behavior using EthoVision software. J Neurosci. 2009;178:323–326. doi: 10.1016/j.jneumeth.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: Increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J, Houlahan T, Cox BJ, Asmundson GJG. Anxiety Disorders Associated With Suicidal Ideation and Suicide Attempts in the National Comorbidity Survey. J Nervous Mental Dis. 2005;193:450–454. doi: 10.1097/01.nmd.0000168263.89652.6b. [DOI] [PubMed] [Google Scholar]
- Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2010;33:345–58. doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Annals of the NY Acad Sci. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Lygren S, Kringlen E. A twin study of DSM-III-R anxiety disorders. Acta Psychiatr Scandinav. 1993;88:85–92. doi: 10.1111/j.1600-0447.1993.tb03419.x. [DOI] [PubMed] [Google Scholar]
- Sledjeski EM, Speisman B, Dierker LC. Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R) J Behav Med. 2008;31:341–349. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesly WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatr. 2002;159:1675–1181. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Investig. 1998;102(1):130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surís A, North C, Powell C, Greene R. Effects of exogenous glucocorticoids on combat-related PTSD symptoms. Ann Clin Psychiatr. 2010;22:274–279. [PMC free article] [PubMed] [Google Scholar]
- Tang G, Xie H, Xu L, Hao Y, Lin D, Ren D. Genetic study of Apolipoprotein E gene, alpha-1 antichymotrypsin gene in sporadic Parkinson disease. Am J Med Gen. 2002;114:446–449. doi: 10.1002/ajmg.10249. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM, Pasternak JF, LaDu MJ. ApoE isoform affects LTP in human targeted replacement mice. NeuroReport. 2004;15:2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- van Meer P, Acevedo S, Raber J. Impairments in spatial memory retention of GFAP-apoE4 female mice. Behav Brain Res. 2007;176:372–375. doi: 10.1016/j.bbr.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Rosenberg J, Raber J. Sex-dependent effects of 56Fe irradiation on contextual fear conditioning in C57BL/6J mice. Hippocampus. 2010;20:19–23. doi: 10.1002/hipo.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- Villasana L, Poage C, van Meer P, Raber J. Passive avoidance learning and memory of 56Fe sham-irradiated and irradiated human apoE transgenic mice. Radiat Biol Radioecol. 2008;48:191–194. [PubMed] [Google Scholar]
- Zareparsi S, Camicioli R, Sexton G, Bird T, Swanson P, Kaye J, Nutt J, Payami H. Age at onset of Parkinson disease and Apolipoprotein E genotypes. Am J Med Gen. 2002;107:156–161. doi: 10.1002/ajmg.10111. [DOI] [PubMed] [Google Scholar]