Abstract
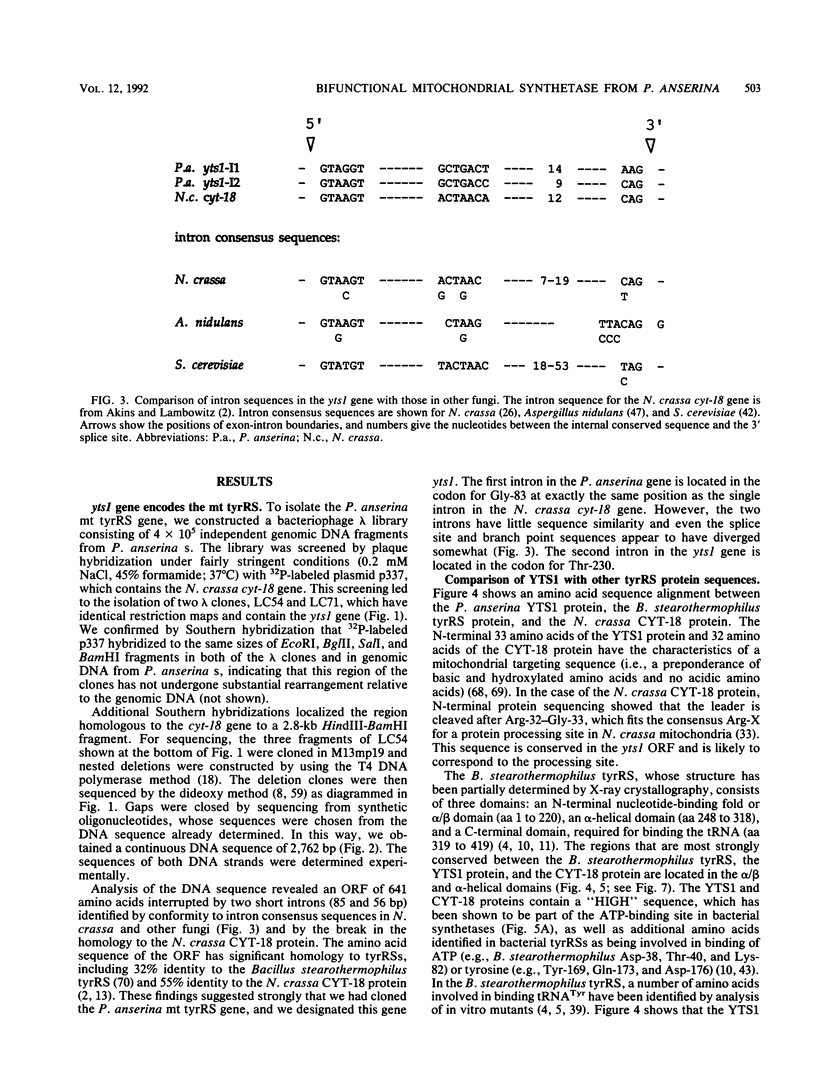
The Neurospora crassa mitochondrial tyrosyl-tRNA synthetase (mt tyrRS), which is encoded by the nuclear gene cyt-18, functions not only in aminoacylation but also in the splicing of group I introns. Here, we isolated the cognate Podospora anserina mt tyrRS gene, designated yts1, by using the N. crassa cyt-18 gene as a hybridization probe. DNA sequencing of the P. anserina gene revealed an open reading frame (ORF) of 641 amino acids which has significant similarity to other tyrRSs. The yts1 ORF is interrupted by two introns, one near its N terminus at the same position as the single intron in the cyt-18 gene and the other downstream in a region corresponding to the nucleotide-binding fold. The P. anserina yts1+ gene transformed the N. crassa cyt-18-2 mutant at a high frequency and rescued both the splicing and protein synthesis defects. Furthermore, the YTS1 protein synthesized in Escherichia coli was capable of splicing the N. crassa mt large rRNA intron in vitro. Together, these results indicate that YTS1 is a bifunctional protein active in both splicing and protein synthesis. The P. anserina YTS1 and N. crassa CYT-18 proteins share three blocks of amino acids that are not conserved in bacterial or yeast mt tyrRSs which do not function in splicing. One of these blocks corresponds to the idiosyncratic N-terminal domain shown previously to be required for splicing activity of the CYT-18 protein. The other two are located in the putative tRNA-binding domain toward the C terminus of the protein and also appear to be required for splicing. Since the E. coli and yeast mt tyrRSs do not function in splicing, the adaptation of the Neurospora and Podospora spp. mt tyrRSs to function in splicing most likely occurred after the divergence of their common ancestor from yeast.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Akins R. A., Lambowitz A. M. General method for cloning Neurospora crassa nuclear genes by complementation of mutants. Mol Cell Biol. 1985 Sep;5(9):2272–2278. doi: 10.1128/mcb.5.9.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Bruton C. J., Winter G. The tyrosyl-tRNA synthetase from Escherichia coli. Complete nucleotide sequence of the structural gene. FEBS Lett. 1982 Dec 27;150(2):419–423. doi: 10.1016/0014-5793(82)80781-3. [DOI] [PubMed] [Google Scholar]
- Bedouelle H. Recognition of tRNA(Tyr) by tyrosyl-tRNA synthetase. Biochimie. 1990 Aug;72(8):589–598. doi: 10.1016/0300-9084(90)90122-w. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Winter G. A model of synthetase/transfer RNA interaction as deduced by protein engineering. 1986 Mar 27-Apr 2Nature. 320(6060):371–373. doi: 10.1038/320371a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Bridge P., Collins R. A., Garriga G., Lambowitz A. M. RNA splicing in Neurospora mitochondria. Characterization of new nuclear mutants with defects in splicing the mitochondrial large rRNA. Cell. 1982 Jun;29(2):517–526. doi: 10.1016/0092-8674(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick P., Bhat T. N., Blow D. M. Structure of tyrosyl-tRNA synthetase refined at 2.3 A resolution. Interaction of the enzyme with the tyrosyl adenylate intermediate. J Mol Biol. 1989 Jul 5;208(1):83–98. doi: 10.1016/0022-2836(89)90090-9. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Starzyk R. M., Schimmel P. Understanding structural relationships in proteins of unsolved three-dimensional structure. Proteins. 1990;7(2):99–111. doi: 10.1002/prot.340070202. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Cherniack A. D., Garriga G., Kittle J. D., Jr, Akins R. A., Lambowitz A. M. Function of Neurospora mitochondrial tyrosyl-tRNA synthetase in RNA splicing requires an idiosyncratic domain not found in other synthetases. Cell. 1990 Aug 24;62(4):745–755. doi: 10.1016/0092-8674(90)90119-y. [DOI] [PubMed] [Google Scholar]
- Chow C. M., Metzenberg R. L., Rajbhandary U. L. Nuclear gene for mitochondrial leucyl-tRNA synthetase of Neurospora crassa: isolation, sequence, chromosomal mapping, and evidence that the leu-5 locus specifies structural information. Mol Cell Biol. 1989 Nov;9(11):4631–4644. doi: 10.1128/mcb.9.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. A., Lambowitz A. M. RNA splicing in Neurospora mitochondria. Defective splicing of mitochondrial mRNA precursors in the nuclear mutant cyt18-1. J Mol Biol. 1985 Aug 5;184(3):413–428. doi: 10.1016/0022-2836(85)90291-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., McNally K. L., Domenico J. M., Matsuura E. T. The complete DNA sequence of the mitochondrial genome of Podospora anserina. Curr Genet. 1990 May;17(5):375–402. doi: 10.1007/BF00334517. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fincham J. R. Transformation in fungi. Microbiol Rev. 1989 Mar;53(1):148–170. doi: 10.1128/mr.53.1.148-170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampel A., Nishikimi M., Tzagoloff A. CBP2 protein promotes in vitro excision of a yeast mitochondrial group I intron. Mol Cell Biol. 1989 Dec;9(12):5424–5433. doi: 10.1128/mcb.9.12.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga G., Bertrand H., Lambowitz A. M. RNA splicing in Neurospora mitochondria: nuclear mutants defective in both splicing and 3' end synthesis of the large rRNA. Cell. 1984 Mar;36(3):623–634. doi: 10.1016/0092-8674(84)90342-8. [DOI] [PubMed] [Google Scholar]
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Guo Q. B., Akins R. A., Garriga G., Lambowitz A. M. Structural analysis of the Neurospora mitochondrial large rRNA intron and construction of a mini-intron that shows protein-dependent splicing. J Biol Chem. 1991 Jan 25;266(3):1809–1819. [PubMed] [Google Scholar]
- Herbert C. J., Labouesse M., Dujardin G., Slonimski P. P. The NAM2 proteins from S. cerevisiae and S. douglasii are mitochondrial leucyl-tRNA synthetases, and are involved in mRNA splicing. EMBO J. 1988 Feb;7(2):473–483. doi: 10.1002/j.1460-2075.1988.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittle J. D., Jr, Mohr G., Gianelos J. A., Wang H., Lambowitz A. M. The Neurospora mitochondrial tyrosyl-tRNA synthetase is sufficient for group I intron splicing in vitro and uses the carboxy-terminal tRNA-binding domain along with other regions. Genes Dev. 1991 Jun;5(6):1009–1021. doi: 10.1101/gad.5.6.1009. [DOI] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Kubelik A. R., Turcq B., Lambowitz A. M. The Neurospora crassa cyt-20 gene encodes cytosolic and mitochondrial valyl-tRNA synthetases and may have a second function in addition to protein synthesis. Mol Cell Biol. 1991 Aug;11(8):4022–4035. doi: 10.1128/mcb.11.8.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kück U., Osiewacz H. D., Schmidt U., Kappelhoff B., Schulte E., Stahl U., Esser K. The onset of senescence is affected by DNA rearrangements of a discontinuous mitochondrial gene in Podospora anserina. Curr Genet. 1985;9(5):373–382. doi: 10.1007/BF00421608. [DOI] [PubMed] [Google Scholar]
- Labouze E., Bedouelle H. Structural and kinetic bases for the recognition of tRNATyr by tyrosyl-tRNA synthetase. J Mol Biol. 1989 Feb 20;205(4):729–735. doi: 10.1016/0022-2836(89)90317-3. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Perlman P. S. Involvement of aminoacyl-tRNA synthetases and other proteins in group I and group II intron splicing. Trends Biochem Sci. 1990 Nov;15(11):440–444. doi: 10.1016/0968-0004(90)90283-h. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M. Preparation and analysis of mitochondrial ribosomes. Methods Enzymol. 1979;59:421–433. doi: 10.1016/0076-6879(79)59103-4. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Fersht A. R., Winter G. Transition-state stabilization in the mechanism of tyrosyl-tRNA synthetase revealed by protein engineering. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7840–7844. doi: 10.1073/pnas.82.23.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Majumder A. L., Akins R. A., Wilkinson J. G., Kelley R. L., Snook A. J., Lambowitz A. M. Involvement of tyrosyl-tRNA synthetase in splicing of group I introns in Neurospora crassa mitochondria: biochemical and immunochemical analyses of splicing activity. Mol Cell Biol. 1989 May;9(5):2089–2104. doi: 10.1128/mcb.9.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Collins R. A., Green M. R., Lambowitz A. M. Defective splicing of mitochondrial rRNA in cytochrome-deficient nuclear mutants of Neurospora crassa. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2635–2639. doi: 10.1073/pnas.76.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G. S., Tsang M. L., Smith H., Fidel S., Morris N. R. Aspergillus nidulans beta-tubulin genes are unusually divergent. Gene. 1987;55(2-3):231–243. doi: 10.1016/0378-1119(87)90283-6. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Cummings D. J. Analysis of class I introns in a mitochondrial plasmid associated with senescence of Podospora anserina reveals extraordinary resemblance to the Tetrahymena ribosomal intron. Curr Genet. 1985;10(1):69–79. doi: 10.1007/BF00418495. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Miyata T., Miyazawa S., Yasunaga T. Two types of amino acid substitutions in protein evolution. J Mol Evol. 1979 Mar 15;12(3):219–236. doi: 10.1007/BF01732340. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Conrad B. Improved programs for DNA and protein sequence analysis on the IBM personal computer and other standard computer systems. Nucleic Acids Res. 1986 Jan 10;14(1):443–454. doi: 10.1093/nar/14.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Bertrand H., Werner S. A nuclear mutant of Neurospora crassa lacking subunit 1 of cytochrome c oxidase. J Biol Chem. 1978 Sep 25;253(18):6364–6369. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Riederer B., Mörl M., Schmelzer C., Stahl U. Self-splicing of the mobile group II intron of the filamentous fungus Podospora anserina (COI I1) in vitro. EMBO J. 1990 Jul;9(7):2289–2298. doi: 10.1002/j.1460-2075.1990.tb07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Klösgen R. B., Wienand U., Peterson P. A., Saedler H. The Spm (En) transposable element controls the excision of a 2-kb DNA insert at the wx allele of Zea mays. EMBO J. 1984 May;3(5):1021–1028. doi: 10.1002/j.1460-2075.1984.tb01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierny-Jamet C. Senescence in Podospora anserina: a possible role for nucleic acid interacting proteins suggested by the sequence analysis of a mitochondrial DNA region specifically amplified in senescent cultures. Gene. 1988 Dec 30;74(2):387–398. doi: 10.1016/0378-1119(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Winter G., Koch G. L., Hartley B. S., Barker D. G. The amino acid sequence of the tyrosyl-tRNA synthetase from Bacillus stearothermophilus. Eur J Biochem. 1983 May 2;132(2):383–387. doi: 10.1111/j.1432-1033.1983.tb07374.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van der Horst G., Tabak H. F. Self-splicing of yeast mitochondrial ribosomal and messenger RNA precursors. Cell. 1985 Apr;40(4):759–766. doi: 10.1016/0092-8674(85)90335-6. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]