Abstract
The insulin like growth factor receptor subtype 1(IGF-1R) plays an important role in cancers transformation and progression. The aim is to investigate the effects of sunitinib on IGF-1R cell signaling transduction, especially on receptor phosphorylation and ubiquitination. In HEK293 cells, IGF-1R signaling pathways are activated in response to IGF-1, which induces obvious phosphorylations of receptor tyrosine and Akt, ERK. However, the phosphorylations of receptor tyrosine, Akt and ERK were significant inhibited by sunitinib. We found that both IGF-1 and sunitinib obviously down regulated the IGF-1R expression. For analysis the ubiquitination, HEK293 cells were simulated with 100 ng/ml IGF-1 or 10 nM sunitinib for 10 min after serum starvation for 24 h. Both IGF-1 and sunitinib could obviously induce the IGF-1R ubiquitination at 10 min compared with control (only serum free, no stimulation), indicating IGF-1 and sunitinib down-regulate the IGF-1R by increasing the receptor degradation through ubiquitination dependent proteasome pathway. We also found that MDM2 combined to IGF-1R in response to sunitinib stimulation. To confirm it, HEK293 cells were transfected with human HA-MDM2 (+MDM2) or siRNA to MDM2 (−MDM2). Following 24 h serum starvation, cells were stimulated with 10 nM sunitinib for 10 min. In over-expressed MDM2 cells, IGF-1R was more ubiquitinated than that in mock-transfected cells (control), and no ubiquitination in −MDM2 cells. These results mean that sunitinib mediates ubiquitination of IGF-1R dependent on MDM2. In summary, sunitinib could block signaling transduction and mediate degradation of IGF-1R.
Keywords: Tyrosine kinase inhibitor, Insulin like growth factor, Phosphorylation and ubiquitination, MDM2
1. Introduction
The insulin like growth factor type 1receptor (IGF-1R) belongs to transmembrane, receptor tyrosine kinases families, which is known plays a crucial role in the development and progression of human cancers. Overexpression of IGF-1R is observed in many human malignancies [1], often involved in worse prognosis [2,3] The IGF-1R is a prominent target for anti-cancer therapy and the downregulation of its activity has been shown to inhibit the growth of many types of human tumor cells. Many researches on IGF-1R function inhibition have been investigated during the past years. The IGF-1R monoclonal antibodies, which mediate receptor downregulation, have been encouraging in cancer cell lines [4,5] and xenografts [6,7]. Another attempt to inhibit IGF-1R is the use of small molecules such as picropodophylin to inhibit kinase activity [8]. Ubiquitin was discovered in the 1970s to eliminating dysfunctional proteins, but it is known to be involved in numerous cellular processes like DNA repair, cell cycle, gene expression, regulation of signaling and protein internalization and trafficking recently. IGF-1R is also a substrate for ubiquitination [9–11], however, there is few research focusing on ubiquitination of the receptor.
Sunitinib is a small molecule and multi-target tyrosine kinase inhibitor, approved for treating the advanced and/or metastatic renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor (GIST). Sunitinib inhibits vascular endothelial growth factor receptors (VEGFR of −1, −2 and −3) [12] and the platelet-derived growth factor receptors (PDGFR in-α and-β) [12,13]. These receptors are implicated in angiogenesis and tumor progression [14–16]. In addition, sunitinib could inhibit colony-stimulating factor 1 receptor [17], stem-cell factor receptor [14], fms-like tyrosine kinase 3 [18], and glial cell line-derived neurotrophic factor [19], which play important roles in vascular endothelial cell growth and migration, vascular permeability, pericyte recruitment, lymphangiogenesis and tumor cells survival [20]. However, there is no any research focused on sunitinib wether inhibits the IGF-1R tyrosine kinase or not.
In this study, we aim to investigate the effects of sunitinib on IGF-1R cell signaling transduction. Especially we focus on whether sunitinib could inhibit the phosphorylation and induce the ubiquitination or not.
2. Materials and methods
2.1. Reagents
Anti-IGF-1R, anti-MDM2 and anti-ubiquitin antibodies were purchased from Santa Cruz Biotechnology Inc. The antibodies of phospho-IGF-1R tyrosine 1311, phosph-MAPK and phosph-Akt were from cell signaling Technology. Sunitinib (sutent11248) was from Pfizer Inc. All other reagents were from Sigma.
2.2. Cell cultures
HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium with sodium supplemented with 10% FBS.
2.3. Transient transfection
The HEK293 cells were put at 80–90% confluent density in 6-well plates, which was transiently transfected with 4 μg/ml DNA plasmids containing Mdm2 (HA-MDM2) using Lipofectamine 2000 (Invitrogen). After 24 h, the transfected cell were split into 24-well plates and cultured at serum free medium for another 24 h. Cells then were stimulated with 50 ng/ml IGF-1 and/or 2.5 nM sunitinib. Protein extracts were prepared for immunoprecipitation or Western blot.
2.4. Small Interfering RNA
Mdm2 siRNA targeting human MDM2 mRNA (5′-AAG CCA UUG CUU UUG AAG UUA-3′) supplied by Dharmacon. siRNA (300 pmol) was transfected into cells using RNAimax reagent (Invitrogen) according to the instructions of the manufacturer. A nonsilencing RNA duplex (5′-AAUUCUCCGAACGUGUCACGU-3′) (Dharmacon) was used as a control.
2.5. Immunoprecipitation
The isolated cells were lysed with IP lysis buffer supplied by Invitrogen, using 500 μl lysis buffer with 10 mM N-ethylmaleimide, 50 μM MG132, and protease inhibitor cocktail tablet (Roche) per well in the 6-well plates. One microgram antibody were added to each sample and incubated for 3 h at 4 °C on a rocker platform. Then fifteen microliters of Dynabeads Protein G were added. After overnight incubation at 4 °C on a rocker platform, the immunoprecipitated complexes were collected by magnet(Invitrogen). The pellet was washed thrice with IP lysis buffer and then dissolved in a sample buffer for SDS–PAGE whereupon the samples were heated for 10 min at 95 °C and further analyzed by western blot.
2.6. SDS–PAGE and western blotting
Protein samples were dissolved in a sample buffer containing 1 mM β-mercapethnol. Samples were analyzed by SDS–PAGE with a 12% separation gel. After SDS–PAGE, the proteins were transferred to nitrocellulose membranes (GE Healthcare) at 4 °C for 1 h and then blocked for 1 h at room temperature in a solution of 5% (w/v) skimmed milk powder and 0.02% (w/v) Tween 20 in PBS, pH 7.5. Incubation with appropriate primary antibodies overnight at 4 °C. This was followed by washes with PBST and incubation with a horseradish peroxidase-labeled secondary antibody (Pierce) for 1 h at room temperature. The detection was made with ECL (Pierce). The films were exposured; and then developed and fixed.
3. Results
3.1. IGF-1R signaling is activated in response to ligand stimulation
HEK293 cells were stimulated with 50 ng/ml IGF-1 for 2, 5, 10, 30 and 60 min after 24 h serum starvation. As expected, IGF-1R was phosphorylated at tyrosine residues Y1131 from 2 min to 60 min in response to IGF-1 stimulation (Fig. 1). Simultaneously, we used the same samples to detect the phosphorylation of the main signaling proteins, Akt and ERK. We found that ERK was phosphorylated from 5 min and max at 5 to 10 min and Akt was phosphorylated maximum at 30 min, as been shown in Fig. 1.
Fig. 1.

IGF-1 receptor is activated by IGF-1. HEK293 cells plated into 24-well plates were stimulated with 50 ng/ml IGF-1 for 0, 2, 5, 10, 30 and 60 min after serum starvation for 24 h. IGF-1R was phosphorylated at tyrosine residues Y1131 from 2 min to 60 min in response to IGF-1 stimulation. Simultaneously, the main signaling proteins, ERK was phosphorylated from 5 min and max at 5 to 10 min whereas Akt maximum at 30 min. The experiments were performed three times indepently.
3.2. IGF-1R tyrosine phosphorylation is inhibited by sunitinib
As shown in Fig. 2, following serum starvation for 24 h, cells were treated 50 ng/ml IGF-1 or first treated 2.5 nM sunitinib for 1 h and then simulated with 50 ng/ml IGF-1 for 2, 5, 10, 30 and 60 min. Compared with single IGF-1, sunitinib significantly decreased the IGF-1R tyrosine phosphorylation, even there was no phosphorylation after IGF-1 stimulation for 1 h.
Fig. 2.
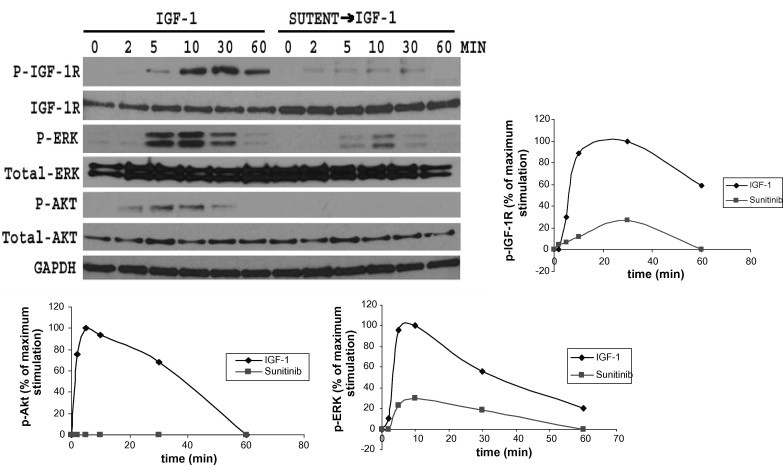
Sunitinib inhibits IGF-1R signaling transduction. Following serum starvation for 24 h, HEK293 cells were treated with 50 ng/ml IGF-1 or first treated with 2.5 nM sunitinib for 1 h and then simulated with 50 ng/ml IGF-1 for 0, 2, 5, 10, 30 and 60 min. Compared with single IGF-1, sunitinib significantly decreased the IGF-1R tyrosine phosphorylation. Both Akt and ERK phosphorylation was largely reduced. The phosphorylation signals were quantified by pIGF-1R, pAkt and pERK versus total IGF-R, Akt and ERK of western blotting. Data was shown as means and SEMs. The results were from 3 independent experiments.
3.3. Sunitinib inhibits IGF-1R signaling transduction
HEK293 cells were treated as above. To detect whether sunitinib affects the IGF-1R signaling, we used IGF-1 induced Akt and ERK phosphorylation as a measurement of PI3K and MAPK pathways activation, seperately. As can be seen in Fig. 2, Akt and ERK proteins were phosphorylated after IGF-1 stimulation without sunitinib. However, in cells treated with with 2.5 nM sunitinib for 1 h and then simulated with 50 ng/ml IGF-1, both Akt and ERK phosphorylation was largely reduced, indicating that sunitinib inhibits the IGF-1R signaling pathway.
3.4. IGF-1 down-regulates IGF-1R and induces IGF-1R ubiquitination
To determine IGF-1R degradation, HEK293 cells were treated 100 ng/ml IGF-1 in serum free medium for 1, 3, 6, 12 and 24 h. We found that IGF-1 obviously down regulated the IGF-1R expression (Fig. 3).
Fig. 3.
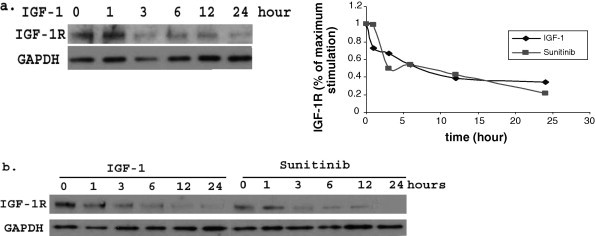
IGF-1R is down-regulated by IGF-1 and sunitinib. (a) IGF-1R is degradated after IGF-1 stimulation. HEK293 cells were treated with 100 ng/ml IGF-1 in serum free medium for 1, 3, 6, 12 and 24 h. IGF-1 obviously down regulated the IGF-1R expression according to stimulation time. (b) IGF-1R is down-regulated by sunitinib. HEK293 cells were treated with with 100 ng/ml IGF-1 or 5 nM sunitinib in serum free medium for 1, 3, 6, 12 and 24 h. Compared with IGF-1, Sunitinib also down regulated the IGF-1R expression, but its effects was postpone. The signals were quantified by densitometry and data was presented as means and SEMs, Which was from 3 different experiments.
For analysis the ubiquitination, HEK293 cells split in 6-well plates were simulated with 100 ng/ml IGF-1 for 10 min after 24 h serum starvation. Following IGF-1 stimulation, cells were harvested with IP buffer (see Section 2). The IGF-R antibody was used for immunoprecipitation, and immunoblotting with ubiquitin antibody. As shown in Fig. 4, IGF-1 could induce obvious IGF-1R ubiquitination at 10 min compared with control (only serum free, no stimulation), indicating IGF-1 down-regulates the IGF-1R by increasing the receptor degradation dependent on ubiquitination.
Fig. 4.
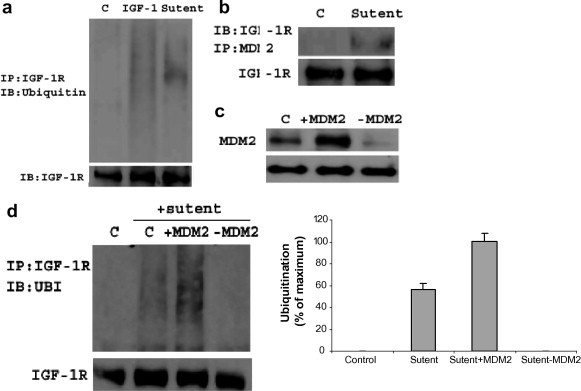
Sunitinib induces IGF-1R ubiquitination dependent on MDM2. (a) IGF-1 and sunitinib mediate IGF-1R ubiquitination. For analysis the ubiquitination, HEK293 cells split in 6-well plates were simulated with 100 ng/ml IGF-1 1 or 5 nM sunitinib for 10 min after serum starvation for 24 h. Following stimulation, cells were harvested with IP buffer. The IGF-R antibody was used for immunoprecipitation, and immunobloting with ubiquitin antibody. Both IGF-1 and sunitinib could induce obviously the IGF-1R ubiquitination at 10 min compared with C (control, only serum free, no stimulation); however, ubiquitination induced by sunitinib was weaker than that induced by IGF-1. (b) HEK293 cells were immunoprecipitated with IGF-1R antibody and detected with MDM2 antibody when stimulation with 5 nM sunitinib for 10 min after serum starvation for 24 h. There is MDM2 signal compared with C (control, only serum free, no stimulation). (c) The efficiency of transient transfection. C is the non-transfected cells. (d) Sunitinib mediate IGF-1R ubiquitination dependent on MDM2. HEK293 cells were transiently transfected with human HA-MDM2 (+MDM2) or siRNA to MDM2 (−MDM2). Following 24 h serum starvation, cells were stimulated with 10 nM sunitinib for 10 min. IGF-1R was immunoprecipitated and analyzed for ubiquitination. In over-expressed MDM2 cells IGF-1R was ubiquitinated more than that in mock-transfected cells (control), in response to 10 nM sunitinib. Further more, there was nearly no ubiquination in −MDM2 cells. These results mean that sunitinib mediates IGF-1R dependent on MDM2. The ubiquitins signals were quantified by densitometry and data was expressed as means and SEMs. These experiments were repeated at least 3 times.
3.5. Sunitinib induces IGF-1R degradation and ubiquitination
HEK293 cells were treated as above. Sunitinib (5 nM) also down regulated the IGF-1R expression, but its effects was postpone in comparison with IGF-1 and there was no obvious degradation until 12 h later. For the ubiquitination, cells were treated with 100 ng/ml IGF-1 or 5 nM sunitinib for 10 min after 24 h serum starvation, and then immunoprecipitation and immunobloting as above. Sunitinib could induce the IGF-1R ubiquitination after 10 min stimulation as we can see in Fig. 4. However, ubiquitination induced by sunitinib was weaker than that induced by IGF-1, which maybe due to different types of ubiquitination.
3.6. Sunitinib induces IGF-1R ubiquitination dependent on MDM2
Considering sunitinib mediates IGF-1R ubiquitination, we were interested in if it was dependent on MDM2 as a ligand. To perform these experiments, firstly, HEK293 cells were immunoprecipitated with IGF-1R antibody and detected with MDM2 antibody after sunitinib stimulation, then cells were transiently transfected with human HA-MDM2 (+MDM2) or siRNA to MDM2 (−MDM2). Following 24 h serum starvation, cells were stimulated with 10 nM sunitinib for 10 min. IGF-1R was immunoprecipitated as above and analyzed for ubiquitination. As shown in Fig. 4b, MDM2 combined to IGF-1R with sunitinib stimulation. In over-expressed MDM2 cells IGF-1R was ubiquitinated more than that in mock-transfected cells (control), in response to 10 nM sunitinib. Furthermore, there was nearly no ubiquination in –MDM2 cells. These results mean that sunitinib mediates IGF-1R dependent on MDM2. The ubiquitination of IGF-1R in normal, +MDM2 and −MDM2 cells were shown in Fig. 4d. The MDM2 transfection efficiency was also presented in Fig. 4c.
4. Discussion
The IGF-1R is activated by the binding of the ligands IGF-1 and IGF-2, which has a nearly 20-fold higher affinity for IGF-1 than for IGF-2. After the binding of these ligands to the receptor α-subunit, structural changes in the IGF-1R result in the phosphorylation and activation of tyrosine kinases. IGF-1R activation initiates lots of downstream cascade. These reactions mainly involve bellow pathways including PI3K (Phosphatidylinositol-3-Kinase), Akt and mTOR; and Ras, Raf and MAPK [21,22]. As we showed, IGF-1 binding to IGF-1R results in phosphorylation of tyrosine residues, and further induces ERK and Akt phosphorylation which are the major proteins involving in IGF-1R signaling transduction.
The activation of IGF-1R pathways led to oncogenic transformation, growth and survival of cancer cells [23]. Therefore, inhibition of this signaling transduction will contribute to cancer therapy. Recently, there are lots of researches focused on the IGF-1R, such as several drugs targeting to IGF-1R have been approved to clinical trials. Sunitinib as a receptor tyrosine kinases inhibitor, however, has never been used to inhibit the IGF-1R signaling. In the present experiments, sunitinib obviously reduced the IGF-1R tyrosine phosphorylation. Further, it also inhibits the IGF-1R signaling transduction such as inhibiting phosphorylation of Akt and ERK. Take these data together, we conclude that sunitinib not only inhibit the VEGFR and PDGFR, but also IGF-1R, which means sunitinib is a very powerful multi-targets drug for cancer therapy. Our research broadens the applied area of sunitinib and benefits the cancer patients.
Ubiquitin is not only for labeling of proteins destined for degradation but also for activation of many other enzymes and signal proteins. More and more research shed light on ubiquitination, especially on IGF-1R [8–11]. Our data shows that both IGF-1 and sunitinib mediate IGF-1R degradation and ubiquitination. IGF-1 and sunitinib obviously down-regulate the IGF-1R expression according to stimulation time; however IGF-1 is stronger than sunitinib. We also found that IGF-1 and sunitinib induced the ubiquitination.
Ubiquitination induced by sunitinib was weaker than that induced by IGF-1, which maybe due to different types of ubiquitination.
Several E3 ligases, such as Mdm2 [9], Nedd 4 [11] and c-cbl [24], have been demonstrated to mediate ubiquitins binding to lysine residues in IGF-1R. Different ligands binding to IGF-1R will active different downsteam reactions. On IGF-1 binding, both Mdm2 and c-Cbl are involving in IGF-IR ubiquitination, which mediate in variable pathway [9,24]. In Mdm2-mediated ubiquitination, reactions occur after stimulation with a low concentration of IGF-I and β-arrestins as an adapter [10], whereas c-Cbl required high concentrations. Our study demonstrated sunitinib mediated ubiquitination dependent on E3 ligase MDM2. The transfection experiments supported our findings. In +MDM2 cells IGF-1R was ubiquitinated more than that in mock-transfected cells (control), whereas nearly no ubiquination in −MDM2 cells. The results of IGF-1R ubiquitination in normal, +MDM2 and −MDM2 cells were shown in Fig. 4d.
In summary, we find that sunitinib not only inhibit the cell signaling transduction of IGF-1R, but also induce receptor ubiquitination which dependent on MDM2. These shed lights on sunitinib could be used for IGF-1R over-expressed cancers such as prostate, breast, colorectal and NSCLC cancers and so on.
Acknowledgements
This work was supported by National High-Tech Research and Development Program of China (863 Program, Grant 2007AA021802) and Natural Science foundation of Shandong Province (ZR2010HM067 and ZR2011HM077). There is no conflict of interest for all authors.
Contributor Information
Qi Liu, Email: liuqi66@sdu.edu.cn.
Jiajun Du, Email: dujiajun@sdu.edu.cn.
References
- 1.Ouban A., Muraca P., Yeatman T., Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum. Pathol. 2003;34(8):803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 2.Parker A.S., Cheville J.C., Janney C.A., Cerhan J.R. High expression levels of insulin-like growth factor-I receptor predict poor survival among women with clear-cell renal cell carcinomas. Hum. Pathol. 2002;33:801–805. doi: 10.1053/hupa.2002.126186. [DOI] [PubMed] [Google Scholar]
- 3.Spentzos D., Cannistra S.A., Grall F., Levine D.A., Pillay K., Libermann T.A., Mantzoros C.S. IGF axis gene expression patterns are prognostic of survival in epithelial ovarian cancer. Endocr. Relat. Cancer. 2007;14:781–790. doi: 10.1677/ERC-06-0073. [DOI] [PubMed] [Google Scholar]
- 4.Hailey J., Maxwell E., Koukouras K., Bishop W.R., Pachter J.A., Wang Y. Neutralizing anti-insulin-like growth factor receptor 1 antibodies inhibit receptor function and induce receptor degradation in tumor cells. Mol. Cancer Ther. 2002;1:1349–1353. [PubMed] [Google Scholar]
- 5.Sachdev D., Li S.L., Hartell J.S., Fujita-Yamaguchi Y., Miller J.S., Yee D. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 2003;63:627–635. [PubMed] [Google Scholar]
- 6.Goetsch L., Gonzalez A., Leger O., Beck A., Pauwels P.J., Haeuw J.F., Corvaia N. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int. J. Cancer. 2005;113:316–328. doi: 10.1002/ijc.20543. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P. Overview of the IGF-1 system. Horm. Res. 2006;65(Suppl. 1):3–8. doi: 10.1159/000090640. [DOI] [PubMed] [Google Scholar]
- 8.Girnita A., Girnita L., del Prete F., Bartolazzi A., Larsson O., Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 9.Girnita L., Girnita A., Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl. Acad. Sci. 2003;100:8247–8252. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girnita L., Shenoy S.K., Sehat B., Vasilcanu R., Girnita A., Lefkowitz R.J., Larsson O. {beta}-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J. Biol. Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 11.Vecchione A., Marchese A., Henry P., Rotin D., Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol. Cell. Biol. 2003;23:3363–3372. doi: 10.1128/MCB.23.9.3363-3372.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendel D.B., Laird A.D., Xin X. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 13.Abrams T.J., Lee L.B., Murray L.J., Pryer N.K., Cherrington J.M. SU11248 inhibits KIT and platelet-derived growth factor receptor β in preclinical models of human small cell lung cancer. Mol. Cancer Ther. 2003;2:471–478. [PubMed] [Google Scholar]
- 14.Kim D.W., Jo Y.S., Jung H.S. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J. Clin. Endocrinol. Metab. 2006;91:4070–4076. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 15.Homsi J., Daud A.I. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14:285–294. doi: 10.1177/107327480701400312. [DOI] [PubMed] [Google Scholar]
- 16.Pietras K., Sjoblom T., Rubin K., Heldin C.H., Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003;3:439–443. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 17.Murray L.J., Abrams T.J., Long K.R. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin. Exp. Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 18.O’Farrell A.M., Abrams T.J., Yuen H.A. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 19.Kim K.J., Li B., Winer J. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 20.Faivre S., Demetri G., Sargent W., Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 21.Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 22.LeRoith D., Werner H., Beitner-Johnson D. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 23.Baserga R., Hongo A., Rubini M. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim. Biophys. Acta. 1997;1332:F105–26. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 24.Sehat B., Andersson S., Girnita L., Larsson O. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 2008;68:5669–5677. doi: 10.1158/0008-5472.CAN-07-6364. [DOI] [PubMed] [Google Scholar]


