Highlights
► An early-life dietary intervention increasing the risk of obesity enhances adipocyte Pdk4 expression. ► Adenosine increases adipocyte PDK4 expression. ► An early-life dietary intervention augments PPARγ expression and enhances lipolytic stimulation. ► Enhanced lipolysis could supply PPARγ ligands enhancing adipocyte PDK4 via PPARγ activation.
Keywords: Pyruvate dehydrogenase complex, Programming, Lipogenesis, Adipose tissue
Abbreviations: ADO, adenosine; BSA, bovine serum albumin; CON, control; ISO, isoproterenol; HSL, hormone-sensitive lipase; KRHB, Krebs–Ringer HEPES buffer; MLP, maternal low protein; PC, pyruvate carboxylase; PDC, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase; PEPCK, phosphoenolpyruvate carboxykinase; PPAR, peroxisome proliferator-activated receptor; SCD, stearoyl-CoA desaturase; NEFA, non-esterified fatty acid; TAG, triacylglycerol; WAT, white adipose tissue
Abstract
We studied adipocytes from 8-week-old control rat offspring (CON) or rat offspring subjected to maternal low (8%) protein (MLP) feeding during pregnancy/lactation, a procedure predisposing to obesity. Acute exposure to isoproterenol or adenosine enhanced PDK4 and PPARγ mRNA gene expression in CON and MLP adipocytes. Enhanced adipocyte Pdk4 expression correlated with increased PPARγ expression. Higher levels of PDK4 and PPARγ were observed in MLP adipocytes. SCD1 is a PPARγ target. Isoproterenol enhanced adipocyte PDK4 and SCD1 gene expression in parallel. This could reflect augmented PPARγ expression together with enhanced lipolytic stimulation to supply endogenous PPARγ ligands, allowing enhanced adipocyte PDK4 and SCD1 expression via PPARγ activation. In contrast, the effect of adenosine to increase PDK4 expression is independent of stimulation of lipolysis and, as SCD1 expression was unaffected by adenosine, unlikely to reflect PPARγ activation. Increased adipocyte expression of both PDK4 and SCD1 in the MLP model could participate as components of a “thrifty” phenotype, favouring the development of obesity.
1. Introduction
Obesity, increased energy storage as triacylglycerol (TAG) in white adipose tissue (WAT), is an expanding problem in Western society because it increases the risk of developing type 2 diabetes, insulin resistance, and dyslipidaemia. In WAT, both lipolytic and re-esterification pathways are active, and both participate in the control of TAG storage as well as in non-esterified fatty acid (NEFA) release. There is net TAG deposition when the rate of FA (re)esterification exceeds that of TAG breakdown. The glycerol 3-phosphate used for FA (re)esterification can originate from glucose or from lactate and pyruvate through glyceroneogenesis [1]. While glucose is traditionally viewed as the main precursor of the glycerol backbone used for TAG, new data point to the indirect pathway (glucose to lactate to glycerol 3-phosphate) being key for fat deposition in adipose tissue under physiological conditions when blood glucose increases because of inhibition of hexokinase II by glucose 6-phosphate [2]. Glyceroneogenesis from pyruvate occurs via pyruvate carboxylase (PC), which carboxylates pyruvate to oxaloacetate, and the glyceroneogenic enzyme cytosolic phosphoenolpyruvate carboxykinase (PEPCK) 1. Activation of the lipogenic transcription factor peroxisome proliferator-activated receptor (PPAR)γ, essential for preadipocyte differentiation and TAG storage in mature adipocytes [3–5], increases gene expression of PCK1 (encoding PEPCK-1) and thereby facilitates adipocyte glyceroneogenesis and TAG storage [6]. An alternative, competing fate of pyruvate is its decarboxylation to acetyl-CoA by the mitochondrial pyruvate dehydrogenase complex (PDC), which facilitates pyruvate utilisation in fatty acid synthesis and incorporation into TAG [7]. Pyruvate flux through glyceroneogenesis in WAT is therefore negatively linked to PDC activity and suppression of PDC allows increased use of lactate and pyruvate for glyceroneogenesis [6].
Stimulation of glyceroneogenesis and inhibition of PDC in WAT is achieved by enhanced expression of pyruvate dehydrogenase kinase 4 (PDK4) [6], one of several PDKs that inhibit PDC by phosphorylation [7]. It is therefore important to evaluate factors that regulate PDK4 expression in WAT in relation to the potential to predispose to obesity. PPARγ activation rapidly (within 4 h) increases expression of Pdk4 in WAT, but not in liver or skeletal muscle, in conjunction with increased gene expression of PCK1 [6]. The key participation of PDK4 in facilitating glyceroneogenesis through pyruvate sparing in WAT has been demonstrated by its inhibition or knockdown, which blocks increases in glyceroneogenesis induced by PPARγ activation [6,8]. Acute (2 h) exposure to epinephrine, as occurs during exercise, also increases adipose-tissue PDK4 mRNA expression [8] but, unlike PPARγ, epinephrine promotes lipolysis (predominantly through the activation of cAMP formation by adenylate cyclase, secondary to activation of β-adrenergic receptors and the G protein Gs) rather than lipogenesis (reviewed in Ref. [9]). Increased adipocyte Pdk4 expression in response to epinephrine stimulation is nevertheless prevented by PPARγ antagonism [8]. The seemingly paradoxical rapid increase in PDK4 expression seen in response to epinephrine may arise because the rate of TAG/FA cycling in rat adipocytes is also markedly increased by β-adrenergic stimulation [10]. TAG/FA cycling implies that β-adrenergic stimulation increases the rate of FA re-esterification as well as lipolysis. Addition of adenosine deaminase, which degrades adenosine, also stimulates TAG/FA cycling [10], but effects of adenosine on adipocyte PDK4 expression is not known.
The present study analysed effects of the β-adrenergic agent isoproterenol (ISO) and of adenosine, alone and in combination, on PDK4 expression in isolated white adipocytes in relation to their effects on lipolysis. In addition, since it is known that gene expression of stearoyl-CoA desaturase (SCD1), the rate limiting enzyme in the desaturation of cellular lipids into monounsaturated fatty acids, is augmented by PPARγ [11], we examined whether changes in Pdk4 expression paralleled those of Scd1, implying a mechanism involving PPARγ activation. Finally, we hypothesised that enhanced PDK4 expression in adipocytes might function as a component of a “thrifty” phenotype in WAT so as to maximise glyceroneogenesis to facilitate FA re-esterification. In the longer-term, this might also predispose to obesity. To test this hypothesis, we utilised a rat model which is established to increase susceptibility to the later development of obesity (reviewed in Ref. [12]), namely maternal protein restriction (an isocaloric low (8%) protein diet or control diet (20% protein) during pregnancy and lactation (Maternal Low Protein, MLP).
We identify a striking parallel between increased mRNA expression of PDK4, PPARγ and SCD1, and increased rates of lipolysis stimulated by the β-adrenergic agonist isoproterenol in MLP adipocytes. In the light of recent studies showing that adipocytes from mice deficient in hormone-sensitive lipase (HSL−/− mice) display attenuated activation of PPARγ, with no change following lipolytic stimulation [13], we propose that enhanced lipolysis could supply endogenous PPARγ ligands allowing enhanced adipocyte mRNA expression of PDK4 and SCD1 via PPARγ activation. In contrast, the effect of adenosine to increase adipocyte Pdk4 expression is independent of stimulation of lipolysis and, as Scd1 expression is unaffected by adenosine, unlikely to reflect PPARγ activation. Irrespective of the mechanisms involved, increased adipocyte gene expression of both PDK4 and SCD1 in the MLP model could participate as components of a “thrifty” phenotype, favouring the development of obesity.
2. Materials and methods
2.1. Materials
Reverse transcriptase real-time PCR reagents were from Invitrogen (Paisley, UK) and Applied Biosystems (Paisley, UK). Laboratory reagents were from Roche Diagnostics (Lewes, East Sussex, UK), Sigma (Poole, Dorset, UK) or Fisher Scientific (Loughborough, UK). Type I collagenase was from Worthington Biochemical (Twyford, Berks, UK).
2.2. Animal model
Studies were conducted in adherence to the regulations of the UK Animal Scientific Procedures Act (1986). All animals were maintained on a 12 h light/12 h dark cycle (light from 07:00). Pregnant female Wistar rats (250–300 g; Charles River, Kent, UK) were randomly assigned to either control (CON; 20% protein) or an isocaloric maternal low protein (MLP; 8%) diets (Hope Farms BV, Woerden, Netherlands) and maintained on the respective diet throughout pregnancy and lactation, as described in previous studies of the effects of early protein restriction on adult metabolism (see e.g. Refs. [14,15]). Pregnant dams fed the low-protein diet did not exhibit any differences in caloric intake compared to controls. Despite this, MLP offspring weighed significantly less at 3 days of age than CON offspring (28%; P < 0.05). Male offspring were weaned at 24 days onto standard rodent diet and continued to be maintained on standard rodent diet for 5 weeks, at which time white adipocytes were prepared and analysed for lipolytic responses and gene expression.
2.3. Adipocyte preparation and incubation
Epididymal fat pads were collected in Krebs-Ringer HEPES buffer (KRHB) containing 3% insulin-free bovine serum albumin (BSA) and 5 mM glucose (KRHB pH 7.4) and adipocytes isolated and incubated as previously described in Ref. [16], with minor modifications as described in Ref. [17]. Adipocyte numbers were determined by counting under phase-contrast microscopy. Isoproterenol (ISO) or adenosine was added as indicated. Medium was collected at the end of the 2-h treatment, and glycerol release into the media was determined using a Free Glycerol Kit Sigma (Poole, Dorset, UK). The coefficient of variation for these assays in our laboratory is <10%.
2.4. Quantitative RT-PCR
RNA was isolated from adipocytes following incubation as indicated, cDNA was synthesised and RT-PCR analysis was performed using Taqman or Sybr green methodology. Relative differences in gene expression between groups were determined using the 2−ΔΔCT method. The amplification efficiencies of the gene of interest and the housekeeping gene (18S) were equivalent, and there was no effect of the experimental manipulations on the expression of housekeeping gene.
2.5. Statistical analysis
Data are presented as means ± S.E. Statistical analyses were performed by ANOVA followed by Fisher’s post-hoc tests for individual comparisons or unpaired (in vivo) or paired (in vitro) Student’s t-tests as appropriate using StatView (Abacus Concepts, Inc., Berkeley, CA, USA). Statistical significance was set at P < 0.05.
3. Results
3.1. Pdk4 expression is enhanced by isoproterenol in isolated rat adipocytes in conjunction with stimulation of lipolysis
Pdk4 gene expression in adipose-tissue explants is increased by acute (2 h) exposure to epinephrine or the PPARγ activator rosiglitazone [8]. We first evaluated effects of the β-adrenergic agonist isoproterenol (ISO, 0.1 μM) on Pdk4 expression is isolated white adipocytes from control (CON) offspring maintained on standard diet. The acute (2 h) addition of ISO significantly increased adipocyte Pdk4 expression (by 33%; P < 0.001) (Fig. 1A). This effect was observed in conjunction with marked stimulation of lipolysis, assessed by glycerol release (13.4-fold; P < 0.01) (Fig. 1B).
Fig. 1.
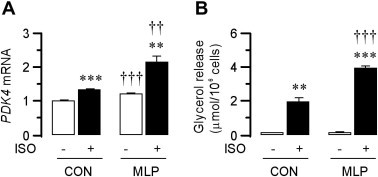
PDK4 mRNA expression (panel A) in control (CON) adipocytes is enhanced by isoproterenol (ISO, 0.1 μM) (open bars) in conjunction with stimulation of lipolysis (panel B). MLP augments PDK4 mRNA expression in the absence or presence of ISO and increases ISO-stimulated lipolysis. Data are presented as means ± S.E. for six adipocyte preparations from control rats and six adipocyte preparations from MLP rats. Statistically significant effects of ISO are indicated by: ∗∗P < 0.01; ∗∗∗P < 0.001. Statistically significant effects of MLP are indicated by: ††P < 0.01; †††P < 0.001.
3.2. MLP increases adipocyte Pdk4 expression and exaggerates effects of isoproterenol to enhance adipocyte Pdk4 expression and lipolysis in isolated rat adipocytes
We studied adipocytes from young MLP offspring at 8 weeks of age before the development of obesity, 5 weeks after their transfer to standard (20% protein) diet. Adipocytes isolated from MLP offspring showed higher (22%; P < 0.001) Pdk4 expression than adipocytes from CON offspring (Fig. 1A). Acute (2 h) exposure to ISO further increased (76%; P < 0.01) Pdk4 expression in adipocytes from MLP offspring (Fig. 1A). Pdk4 expression in ISO-stimulated MLP adipocytes were therefore significantly (61%; P < 0.01) higher than those of ISO-stimulated CON adipocytes (Fig. 1A). ISO-stimulated lipolytic rates with adipocytes from MLP offspring were also significantly higher (98%; P < 0.001) than those of CON offspring (Fig. 1B).
3.3. Adenosine enhances adipocyte Pdk4 expression
Addition of adenosine deaminase, which removes adenosine, increases TAG/FA cycling in rat adipocytes [10]. We therefore examined whether adenosine addition (200 nM) either suppressed Pdk4 expression or could oppose effects of ISO to increase Pdk4 expression. We found, however, that adenosine addition to CON adipocytes significantly increased adipocyte Pdk4 expression in the absence of ISO (72%; P < 0.05), and elicited a modest, but non-significant, increase in the presence of ISO (23%) (Fig. 2A). With adipocytes from CON offspring, the effect of adenosine to increase adipocyte Pdk4 expression was more marked than that of ISO. An effect of adenosine to increase Pdk4 expression was also seen with adipocytes from MLP offspring. In the absence of ISO, PDK4 mRNA expression showed markedly greater responsiveness to adenosine in MLP adipocytes (114%; P < 0.05), than CON adipocytes (72%; P < 0.05) (compare Fig. 2A and B), despite already increased PDK4 mRNA expression. An effect of adenosine to increase Pdk4 expression was also seen in ISO-stimulated MLP adipocytes (59%; P < 0.01) (Fig. 2B). Although adenosine is thought to inhibit lipolysis through activation of its A1 receptors in mature adipocytes, we were unable to detect any anti-lipolytic effect of adenosine under the conditions of our experiments, either in the absence or presence of ISO (Fig. 2C and D). However, rates of lipolysis were greatest with adipocytes from MLP offspring stimulated with or without adenosine (Fig. 2D).
Fig. 2.
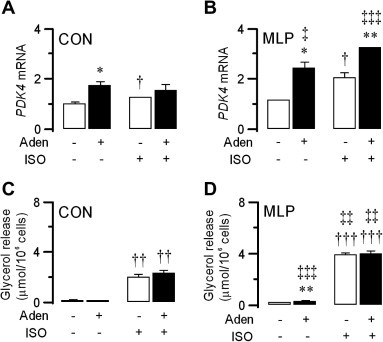
Adenosine addition (ADO, 200 nM) increases PDK4 mRNA expression in CON (panel A) and MLP (panel B) adipocytes in the absence and presence of ISO (0.1 μM). Adenosine addition (200 nM) also increased PDK4 mRNA expression in CON and MLP adipocytes in the presence of ISO (0.1 μM) (B). Rates of lipolysis were greater with adipocytes from MLP offspring (panel D) stimulated with ISO and adenosine in combination (C). Data are presented as means ± S.E. for six adipocyte preparations from control rats and six adipocyte preparations from MLP rats. Statistically significant effects of adenosine are indicated by: ∗P < 0.05; ∗∗P < 0.01. Statistically significant effects of ISO are indicated by: †P < 0.05; †††P < 0.001. Statistically significant effects of MLP are indicated by: ‡P < 0.05; ‡‡P < 0.01; ‡‡‡P < 0.001.
3.4. Adenosine and MLP increase PPARγ gene expression in isolated adipocytes
The effects of rosiglitazone and epinephrine to increase adipocyte Pdk4 expression are both mediated via the lipogenic transcription factor PPARγ [8]. Acute (2 h) exposure to ISO did not significantly affect PPARγ mRNA expression with adipocytes from CON offspring, although there was a non-significant trend towards increased PPARγ mRNA expression. In contrast, the addition of adenosine significantly increased (53%; P < 0.01) PPARγ mRNA expression with adipocytes from CON offspring (Fig. 3A). PPARγ mRNA expression was significantly greater (270%; P < 0.01) in untreated adipocytes from MLP offspring compared to adipocytes from CON offspring (Fig. 3B), and was significantly increased by ISO in adipocytes from MLP offspring (62%; P < 0.01). Adenosine also increased PPARγ mRNA expression with adipocytes from MLP offspring (19%) (Fig. 3B), particularly when added in combination with ISO. Thus the highest PPARγ mRNA expression levels were seen in adipocytes from MLP offspring treated with ISO and adenosine in combination (Fig. 3B).
Fig. 3.
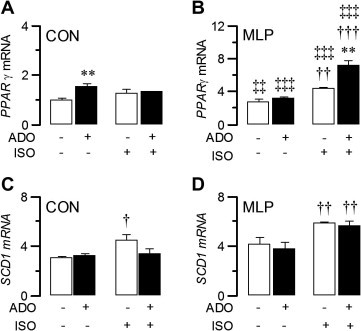
PPARγ mRNA expression is stimulated by adenosine and augmented in adipocytes from MLP offspring (panel B) compared to adipocytes from CON offspring (panel A). SCD1 mRNA expression is augmented in adipocytes from MLP offspring (panel D) compared to adipocytes from CON offspring (panel C), particularly under conditions of ISO stimulation. Data are presented as means ± S.E. for six adipocyte preparations from control rats and six adipocyte preparations from MLP rats. Statistically significant effects of adenosine are indicated by: ∗∗P < 0.01. Statistically significant effects of ISO are indicated by: †P < 0.05; ††P < 0.01; †††P < 0.001. Statistically significant effects of MLP are indicated by: ‡‡P < 0.01; ‡‡‡P < 0.001.
3.5. Scd1 expression is enhanced by isoproterenol and MLP, but not by adenosine, in isolated adipocytes
SCD1 is highly expressed by adipose tissue, predominantly in adipocytes [11] and is rate limiting in the desaturation of cellular lipids into monounsaturated fatty acids. A PPARγ response element is located on the SCD1 promoter [18], and SCD1 expression in human adipose tissue and isolated adipocytes is increased after treatment with pioglitazone, a PPARγ agonist [11]. If increased PDK4 gene expression reflects PPARγ activation, it would be anticipated that Pdk4 and Scd1 expression would change in parallel. Acute (2 h) exposure to adenosine did not significantly affect SCD1 mRNA expression with adipocytes from CON offspring, whereas there was a significant increase in SCD1 mRNA expression in response to ISO in the absence but not the presence of adenosine (Fig. 3C). SCD1 mRNA expression tended to be higher in untreated adipocytes from MLP offspring compared to adipocytes from CON offspring (Fig. 3D), and was significantly increased by ISO in adipocytes from MLP offspring, both in the absence and presence of adenosine (Fig. 3D). In contrast, adenosine was without effect of SCD1 mRNA expression with adipocytes from MLP offspring (Fig. 3B and D).
4. Discussion
The present study with isolated white adipocytes supports the previous finding that epinephrine, presumably via β-adrenergic stimulation, increases Pdk4 expression in white adipose tissue [8]. In addition, we identify a novel acute effect of adenosine to increase adipocyte Pdk4 expression. The highest levels of PDK4 mRNA expression were observed in MLP adipocytes challenged by ISO and adenosine in combination. Changes in adipocyte Pdk4 expression in response to ISO and/or adenosine were paralleled by increased PPARγ expression, irrespective of offspring group, with higher levels of Pdk4 expression in adipocytes from MLP offspring which were characterised by enhanced PPARγ expression in adipocytes suggesting a bias towards a lipogenic phenotype. Finally, we identify striking parallels between augmented PDK4 mRNA expression, increased ISO-stimulated rates of lipolysis, and gene expression of SCD1, an established gene target of PPARγ. Increased PDK4 expression may promote scavenging of pyruvate for glyceroneogenesis to provide a backbone for fatty acid re-esterification under conditions of stimulated lipolysis, whilst increased SCD1 expression favours the desaturation of available fatty acids, which favours their incorporation into neutral lipid. Finally, we demonstrate that protein restriction in early life (maternal protein restriction during pregnancy and lactation, MLP) increases adipocyte Pdk4 and Scd1 expression in later life, even after the offspring have been weaned and subsequently maintained for 5 weeks on a diet containing the normal amount of protein. Increased adipocyte gene expression of both PDK4 and SCD1 in the MLP model could participate as components of a “thrifty” phenotype, favouring the development of obesity by promoting storage of fatty acids as TAG.
A close relationship between adipocyte SCD1, an established gene target of PPARγ, and PDK4 mRNA expression is consistent with the concept that adipocyte PDK4 is a gene target of PPARγ. Augmented gene expression of both SCD1 and PDK4 in response to ISO parallel increases in ISO-stimulated rates of lipolysis, being greatest in adipocytes form MLP offspring stimulated by ISO. HSL contributes to the lipolytic degradation of adipocyte TAG stores, being rate limiting for diacylglycerol and cholesteryl ester hydrolysis, and can also provide PPARγ ligands for normal preadipocyte differentiation to mature adipocytes [13]. Because of the striking parallels between augmented adipocyte PDK4 and SCD1 mRNA expression and increased ISO-stimulated rates of lipolysis, it is possible that PPARγ ligands may also be generated from TAG (via diacylglycerol) on β-adrenergic stimulation. The ISO concentration used in our experiments, 0.1 μM, is suboptimal for stimulation of glycerol release by adipocytes prepared from MLP offspring and corresponding age-matched controls (see Ref. [19]). It would therefore be interesting to perform further experiments to establish the dose-response relationships between increased Pdk4 and SCD1 gene expression and stimulation of lipolysis, whether increased Pdk4 and Scd1 expression is observed solely when lipolysis is stimulated by β-adrenergic stimulation, or whether other agents that stimulate lipolysis but do not act via a β-adrenergic receptor are also effective. Alternatively, it may be that the effects of β-adrenergic stimulation are not necessarily a consequence of activation of lipolysis, but reflect an alternative action linked to increased intracellular cAMP.
We anticipated that adenosine would suppress adipocyte Pdk4 expression in concert with an anti-lipolytic action through lowering cAMP via effects on its A1R receptors (see e.g. Ref. [20]). However, we did not observe adenosine-mediated inhibition of ISO-stimulated lipolysis (assessed from glycerol release) under the conditions of our experiments. Furthermore, in the absence of ISO, adenosine did not affect glycerol release, yet nevertheless increased Pdk4 expression. As the highest levels of PDK4 mRNA expression were observed in MLP adipocytes challenged by ISO and adenosine in combination, which were characterised by greatly augmented mRNA expression of PPARγ, our data support the suggestion from previous work that adipocyte PDK4 mRNA expression is regulated by PPARγ signalling [8], but the question remains as to the mechanism and physiological significance of the effect of adenosine to acutely augment adipocyte PDK4 mRNA expression. Adenosine’s action to increase adipocyte PPARγ and PDK4 mRNA expression can be seen in the absence of altered lipolysis; nevertheless, the response to adenosine is particularly prominent when there is concomitant β-adrenergic stimulation, suggesting a distinct mechanism. It is emerging that, whilst the role of adenosine in adipocytes has been perceived to be the regulation of lipolysis, extracellular adenosine can also act as a stress signal, in particular in relation to adaptation to limited oxygen availability [21]. Cellular hypoxia may be a key factor in adipocyte physiology in obesity [22]. Furthermore, diverting pyruvate away from mitochondrial oxidation achieved by activation of PDK gene expression is known to be an important alteration induced by hypoxia in other cell systems (reviewed in Ref. [23]). Irrespective of the mechanism(s) involved, the present study demonstrates that adipocytes from MLP offspring show an exaggerated response of Pdk4 expression to adenosine, which may indicate that the adipocyte signalling response to adenosine and/or the uptake and metabolism of adenosine is affected by MLP or early-life interventions that predispose to the later development of obesity.
Re-esterification of TAG when there is concomitant lipolysis creates a substrate cycle, whereby the products of TAG hydrolysis can be recycled into TAG within the cell. A change in the bias of flux towards FA re-esterification when there is a high rate of substrate cycling can exert a major effect on FA release under lipolytic conditions. As the MLP model is associated with the later development of obesity, our data suggest that an increased risk of obesity in MLP programmed offspring may be facilitated by an early adaptation which anticipates that adipocyte TAG storage might be compromised in later life because of poor early nutrition. We therefore suggest that, by sensitising the adipocyte to stimuli that increase PPARγ expression, MLP allows an increased amount of glucose-derived carbon to be trapped in the glycerol backbone of TAG via increased glyceroneogenesis from lactate in vivo facilitated by increased adipocyte Pdk4 expression. At the same time, increased SCD1 expression favours the desaturation of available fatty acids, which favours their (re)esterification to TAG. Thus, adipose tissue could be adapted to accelerate TAG turnover in MLP offspring, facilitating rapid adipocyte TAG repletion and/or expansion of the TAG pool when nutrients are available, and ensuring a supply of FA and glycerol when nutrients are scarce.
Acknowledgements
We thank Professor S. Greenwald for setting up the MLP model. This research was supported by grants from Diabetes UK (BDA:RD03/0002725 and BDA:RD04/0002863). M.G. Zariwala was a recipient of a research studentship from Diabetes UK (BDA:RD03/0002725).
References
- 1.Nye C., Kim J., Kalhan S.C., Hanson R.W. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol. Metab. 2008;19:356–361. doi: 10.1016/j.tem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Munoz S., Franckhauser S., Elias I., Ferre T., Hidalgo A., Monteys A.M., Molas M., Cerdan S., Pujol A., Ruberte J., Bosch F. Chronically increased glucose uptake by adipose tissue leads to lactate production and improved insulin sensitivity rather than obesity in the mouse. Diabetologia. 2010;53:2417–2430. doi: 10.1007/s00125-010-1840-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosen E.D., Spiegelman B.M. Peroxisome proliferator-activated receptor gamma ligands and atherosclerosis: ending the heartache. J. Clin. Invest. 2000;106:629–631. doi: 10.1172/JCI10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., Puigserver P., Spiegelman B.M. Transcriptional activation of adipogenesis. Curr. Opin. Cell Biol. 1999;11:689–694. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 5.Imai T., Takakuwa R., Marchand S., Dentz E., Bornert J.M., Messaddeq N., Wendling O., Mark M., Desvergne B., Wahli W., Chambon P., Metzger D. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadoudal T., Distel E., Durant S., Fouque F., Blouin J.M., Collinet M., Bortoli S., Forest C., Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes. 2008;57:2272–2279. doi: 10.2337/db08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugden M.C., Holness M.J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 2006;112:139–149. doi: 10.1080/13813450600935263. [DOI] [PubMed] [Google Scholar]
- 8.Wan Z., Thrush A.B., Legare M., Frier B.C., Sutherland L.N., Williams D.B., Wright D.C. Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am. J. Physiol. Cell Physiol. 2010;299:C1162–C1170. doi: 10.1152/ajpcell.00188.2010. [DOI] [PubMed] [Google Scholar]
- 9.Lafontan M., Barbe P., Galitzky J., Tavernier G., Langin D., Carpene C., Bousquet-Melou A., Berlan M. Adrenergic regulation of adipocyte metabolism. Hum. Reprod. 1997;12(Suppl 1):6–20. doi: 10.1093/humrep/12.suppl_1.6. [DOI] [PubMed] [Google Scholar]
- 10.Brooks B., Arch J.R., Newsholme E.A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 1982;146:327–330. doi: 10.1016/0014-5793(82)80945-9. [DOI] [PubMed] [Google Scholar]
- 11.Yao-Borengasser A., Rassouli N., Varma V., Bodles A.M., Rasouli N., Unal R., Phanavanh B., Ranganathan G., McGehee R.E., Jr., Kern P.A. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J. Clin. Endocrinol. Metab. 2008;93:4431–4439. doi: 10.1210/jc.2008-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottrell E.C., Ozanne S.E. Early life programming of obesity and metabolic disease. Physiol. Behav. 2008;94:17–28. doi: 10.1016/j.physbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Shen W.J., Yu Z., Patel S., Jue D., Liu L.F., Kraemer F.B. Hormone-sensitive lipase modulates adipose metabolism through PPARgamma. Biochim. Biophys. Acta. 2011;1811:9–16. doi: 10.1016/j.bbalip.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahri S., Snoeck A., Reusens-Billen B., Remacle C., Hoet J.J. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(Suppl 2):115–120. doi: 10.2337/diab.40.2.s115. [DOI] [PubMed] [Google Scholar]
- 15.Holness M.J., Sugden M.C. Antecedent protein restriction exacerbates development of impaired insulin action after high-fat feeding. Am. J. Physiol. 1999;276:E85–E93. doi: 10.1152/ajpendo.1999.276.1.E85. [DOI] [PubMed] [Google Scholar]
- 16.Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 17.Walker C.G., Bryson J.M., Hancock D.P., Caterson I.D. Leptin secretion is related to glucose-derived lipogenesis in isolated adipocytes. Int. J. Obes. (Lond) 2007;31:723–729. doi: 10.1038/sj.ijo.0803462. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Ge L., Tran T., Stenn K., Prouty S.M. Isolation and characterization of the human stearoyl-CoA desaturase gene promoter: requirement of a conserved CCAAT cis-element. Biochem. J. 2001;357:183–193. doi: 10.1042/0264-6021:3570183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozanne S.E., Wang C.L., Dorling M.W., Petry C.J. Dissection of the metabolic actions of insulin in adipocytes from early growth-retarded male rats. J. Endocrinol. 1999;162:313–319. doi: 10.1677/joe.0.1620313. [DOI] [PubMed] [Google Scholar]
- 20.Johansson S.M., Lindgren E., Yang J.N., Herling A.W., Fredholm B.B. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur. J. Pharmacol. 2008;597:92–101. doi: 10.1016/j.ejphar.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Koeppen M., Eckle T., Eltzschig H.K. Interplay of Hypoxia and A(2B) Adenosine Receptors in Tissue Protection. Adv. Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 22.Wood I.S., de Heredia F.P., Wang B., Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 23.Coleman M.L., Ratcliffe P.J. Oxygen sensing and hypoxia-induced responses. Essays Biochem. 2007;43:1–15. doi: 10.1042/BSE0430001. [DOI] [PubMed] [Google Scholar]


