Highlights
► 17-Allylamino-17-demethoxygeldanamycin (17-AAG) binds to Hsp90 and inhibits its function. ► Inhibition of Hsp90 downregulates differentiation marker cytokeratin 1 and cytokeratin 10. ► Phospho-p38 was upregulated by 17-AAG in a concentration-dependent manner. ► Involucrin expression was increased by upregulation of p38 MAPK phosphorylation. ► Inhibition of Hsp90 represses cell cycle arrest.
Keywords: Cytokeratin 1 and 10, Involucrin, Hsp90, 17-AAG, Keratinocyte differentiation, HaCaT
Abstract
Hsp90 is essential for maintaining the activity of numerous signaling factors, and plays a key role in cellular signal transduction networks. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is an ansamycin antibiotic that binds to Hsp90 and inhibits its function. HaCaT human keratinocytes were used to investigate the cellular and molecular functions of Hsp90 in keratinocyte differentiation. Inhibition of Hsp90 by 17-AAG leads to downregulation of the differentiation markers cytokeratin 1 and cytokeratin 10 at the protein and mRNA levels.
1. Introduction
Heat shock protein 90 (Hsp90) in a molecular chaperone originally identified as one of several conserved proteins expression of which is upregulated in response to heat stress. Hsp90 is distinguished from other chaperones in that most of its known substrates are signal transduction proteins, the classical examples of which are steroid hormone receptors and signaling kinases [1,2]. As Hsp90 is essential for maintaining the activity of numerous signaling factors, it plays a key role in cellular signal transduction networks [3]. The ansamycin antibiotic 17-allylamino-17-demethoxygeldanamycin (17-AAG) has been shown to bind to Hsp90, inhibiting its function and thus leading to disruption of signal transduction. This results in induction of apoptosis and differentiation in leukemic blasts [4]. The epidermis, a stratified squamous epithelium that forms the protective covering of the skin, is composed of four layers: the basal layer, stratum spinosum, granular layer, and stratum corneum. Basal layer keratinocytes, including epidermal stem cells and transit-amplifying cells, are cuboidal, express cytokeratins (CKs) 5 and 14, and have high proliferative potential [5]. The stratum spinosum is characterized by a switch in keratin expression from CK5 and CK14 to CK1 and CK10 [6]. Involucrin, a marker of early terminal differentiation, is also synthesized in the upper part of this layer [7]. The spinous cells continue differentiation and maturation to form the granular layer, which is characterized by expression of the late terminal differentiation markers loricrin and filaggrin [8,9]. Terminal differentiation gives rise to the stratum corneum. Many groups have been engaged in investigating the mechanisms of keratinocyte differentiation. For example, PI3K/pAkt signaling has been shown to regulate early keratinocyte differentiation, and differentiation marker expression levels are reduced by Akt or PI3K inhibition [10]. The TGFβ-Smad2/3 signaling pathway in keratinocyte differentiation is independent of Smad4, and controls cell cycle withdrawal during keratinocyte terminal differentiation [11,12]. Hsp27 expression and its phosphorylation by p38 MAPK are required for keratinocyte differentiation and for the formation of a regularly stratified epidermis [13]. However, the detailed mechanisms underlying the differentiation of these cells have not been elucidated. We showed that the Hsp40 regulates keratin expression, and Hsp40 knockdown was suggested to induce expression of the differentiation maker CK10 in HaCaT cells [14]. Although Hsp27 is known as a keratinocyte differentiation marker, its role in keratinocyte differentiation is not yet clearly understood. Here, we report the effects of Hsp90 inhibition by 17-AAG on keratinocyte differentiation.
2. Materials and methods
2.1. Cell culture
The HaCaT cell line was originally derived from normal human adult skin, and is non-tumorigenic [15]. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco Co. Ltd., Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) in which the calcium had been chelated using Chelex beads (Bio-Rad, Hercules, CA), and antibiotics (100 μg/ml penicillin, 100 μg/ml streptomycin, and 2.5 mg/ml amphotericin B), and CaCl2 was added at a concentration of 0.03 or 6 mM. Cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere.
2.2. Antibodies and reagents
Anti-pAkt antibody was purchased from Acris (San Diego, CA). Anti-Akt antibody was purchased from Anaspec (San Jose, CA). Anti-involucrin antibody was purchased from Thermo (Fremont, CA). Anti-cytokeratin 14 antibody was purchased from Progen (Heidelberg, Germany). Anti-cytokeratin 1 and anti-Ki67 antibodies were purchased from Abnova Corp. (Taipei, Taiwan). Anti-cytokeratin 5 antibody was purchased from Sanbio B.V. (Uden, The Netherlands). Anti-cytokeratin 10 antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The Hsp90 inhibitor 17-AAG was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
2.3. Real-time RT-PCR
Total RNA was purified using an RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse transcribed with a PrimeScript™ RT reagent kit (Takara Bio Inc., Shiga, Japan). An iQ SYBR Green RT-PCR kit (Bio-Rad) was used for real-time PCR analysis using the following gene-specific primers: GAPDH, 5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-TGGGATTTCCATTGATGACA-3’; K1, 5′-ATTTCTGAGCTGAATCGTGTGATC-3′ and 5′-CTTGGCATCCTTGAGGGCATT-3′; K5, 5′-TCTCGCCAGTCAAGTGTGTC-3′ and 5′-ATAGCCACCCACTCCACAAG-3′; K10, 5′-CATCCTGCTTCAGATCGACA-3′ and 5′-TCATTTCCTCCTCGTGGTTC-3′; K14, 5′-TTCTGAACGAGATGCGTGAC-3′ and 5′-GCAGCTCAATCTCCAGGTTC-3′; filaggrin, 5′-CAATCAGGCACTCATCACAC-3′ and 5′-ACTGTTAGTGACCTGACTACC-3′; involucrin, 5′-TAGAGGAGCAGGAGGGACAA-3′ and 5′-AGGGCTGGTTGAATGTCTTG-3′; Hsp90, 5′-TCCAATAGGCTTGTGTCTTCCCCC-3′ and 5′-AATCCGTTCCATGTTGGCTGTCC-3′. Relative differences in gene expression level between groups were expressed using cycle time (Ct) values, which were first normalized relative to that of GAPDH in the same sample and then expressed as fold change compared to control (100%). Real-time fluorescence detection was carried out using an Opticon Real-Time PCR Detection System (Bio-Rad).
2.4. Western blotting analysis
Proteins were extracted from several cultured cell lines using cell extraction buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitors (2 mM N-ethylmaleimide, 50 mg/ml aprotinin, 50 mg/ml leupeptin and pepstatin) at 48 °C. After centrifugation, the soluble protein in the extract was quantified according to the method described by Bradford [16]. Proteins were separated by SDS–PAGE using 8–10% gels and blotted onto polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked in 1% non-fat dry milk TBST [10 mM Tris (pH 7.8), 150 mM NaCl, and 0.05% Tween 20 at room temperature overnight. Membranes were incubated with primary antibodies (dilution 1:500–1:1000) at room temperature for 1 h, washed with TBST, and incubated with horseradish peroxidase-conjugated anti-mouse or rabbit IgG antibody (dilution 1:10 000) at room temperature for 1 h. The bands were detected with ECL Plus Western blotting detection system (GE Healthcare, Piscataway, NJ).
2.5. Laser scanning confocal microscopy
Cells were grown in flat-bottomed dishes (Iwaki Glass Co. Ltd., Tokyo, Japan), fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100. To monitor Ki67 expression, the cells were incubated with anti-Ki67 antibody and Alexa568-conjugated secondary antibody (Molecular Probes-Invitrogen, Eugene, OR) and then nuclear counterstained with DAPI. Fluorescence microscopy images were obtained using a laser scanning confocal microscope (FV-1000; Olympus Co., Tokyo, Japan).
3. Results
3.1. Effects of Hsp90 inhibition by 17-AAG on keratinocyte differentiation marker expression
To determine whether 17-AAG affects the differentiation of keratinocytes, we performed real-time RT-PCR assays to examine mRNA expression of the markers of keratinocyte differentiation CK1, CK10, involucrin, and filaggrin, and the basal layer keratinocyte markers CK5 and CK14. Pretreatment of cells with 17-AAG before changing to high-calcium medium (6 mM CaCl2) resulted in suppression of CK1 and CK10 expression and upregulation of CK5 expression. However, 17-AAG had no effects on the expression of involucrin or filaggrin (Fig. 1). Next, we performed Western blotting analysis to examine CK1, CK5, CK10, CK14, involucrin, and filaggrin expression at the protein level. Hsp90 inhibition was confirmed by Akt phosphorylation, and Akt phosphorylation was blocked by 17-AAG (Fig. 2A). Western blotting analysis also showed that the levels of CK1 and CK10 protein expression were reduced and that of CK14 protein expression was increased by 17-AAG-pretreatment. No changes were observed in Hsp90 protein expression level (Fig. 2B).
Fig. 1.
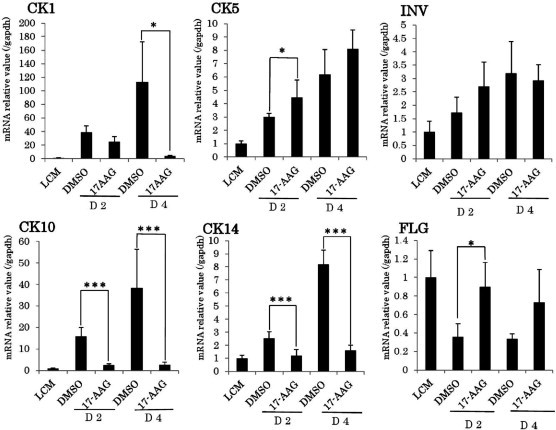
Real-time RT-PCR analysis of K1, K5, K10, K14, involucrin, and filaggrin expression under normal proliferation conditions (0.03 mM CaCl2) for 24 h, with DMSO or 17-AAG (0.5 μM) for 24 h, and under high-Ca2+ conditions (6 mM CaCl2) for 2 or 4 days (D2, D4, respectively) in HaCaT. ∗P < 0.05, ∗∗∗P < 0.001.
Fig. 2.
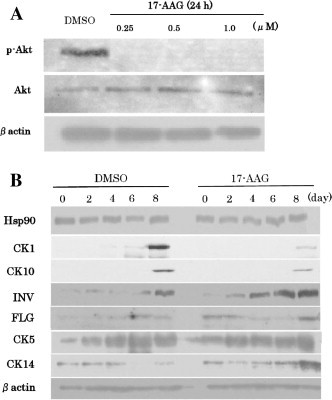
HaCaT cell differentiation was inhibited by 17-AAG pretreatment. Western blot of phospho-Akt and Akt in cells grown in medium without calcium chelate processes and incubation with DMSO or 17-AAG (0.1, 0.5, and 1.0 μM) for 24 h (A). Western blot of Hsp90, K1, K10, K5, K14, filaggrin, and involucrin in HaCaT cells grown under normal proliferation conditions (0.03 mM, CaCl2) for 24 h, with DMSO or 17-AAG (0.5 μM) for 8 h, and under high-Ca2+ conditions (6 mM CaCl2) for 2, 4, 6, and 8 days. β-Actin was used as a loading control (B).
3.2. Effects of Hsp90 inhibition by 17-AAG on signaling protein expression
Western blotting analysis was performed to examine phosphorylation of Akt, Smad2/3, and p38, all of which are related to keratinocyte differentiation. Phosphorylation of Akt was significantly inhibited by 17-AAG at all concentrations examined. However, 17-AAG did not affect phosphorylation of Smad2/3 or p38. In contrast, phospho-p38 level was upregulated by 17-AAG in a concentration-dependent manner (Fig. 3).
Fig. 3.
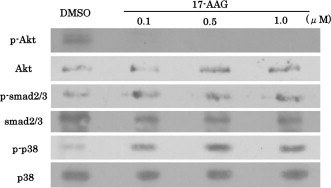
Effects of 17-AAG on phosphorylation of signaling proteins. Western blot of phospho-Akt, Akt, phospho-Smad2/3, Smad2/3, phospho-p38, and p38 in cells grown under normal proliferation conditions (0.03 mM CaCl2) for 24 h or under high-Ca2+ conditions (6 mM CaCl2) for 24 h followed by incubation with DMSO or 17-AAG (0.1, 0.5, and 1.0 μM).
3.3. Effects of Hsp90 inhibition by 17-AAG on the cell cycle
To investigate the effects of 17-AAG on the cell cycle, cells were stained for the proliferation marker Ki67. Laser scanning confocal microscopy revealed that Ki67 expression was upregulated by 17-AAG under conditions of proliferation and differentiation (Fig. 4).
Fig. 4.
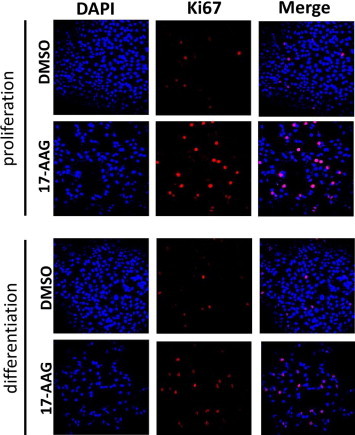
Hsp90 inhibition increased expression of the proliferation marker Ki67. Immunofluorescence analysis of Ki67 (red) in cells grown under normal proliferation conditions (0.03 mM, CaCl2) for 24 h (proliferation) or under high-Ca2+ conditions (6 mM CaCl2) for 24 h (differentiation) followed by incubation with DMSO or 17-AAG (0.5 μM).
4. Discussion
The details of keratinocyte differentiation mechanisms have been examined in previous studies. The HSP family is known to play important roles in the cellular homeostasis of many cell types, contributing to protein folding, stability, and degradation [16]. However, the role of Hsp90 in keratinocyte differentiation is not clearly understood. In the present study, we showed that inhibition of Hsp90 by 17-AAG blocked expression of the differentiation markers CK1 and CK10 at both mRNA and protein levels. On the other hand, Hsp90 inhibition had no effect on another differentiation marker, filaggrin. In contrast, involucrin expression was increased at the protein level. These results indicated that inhibition of Hsp90 only affects switching of keratin expression from CK5 and CK14 to CK1 and CK10 in differentiation from basal layer to stratum spinosum. Extracellular calcium concentration-dependent keratinocyte differentiation is induced by formation of the E-cadherin complex. Thus, extracellular calcium promotes Akt phosphorylation and promotes early differentiation [10]. Phospho-Akt is stabilized by Hsp90 [18]. Therefore, inhibition of Hsp90 represses CK1 and CK10 expression. CK10 is essential for late differentiation and has been shown to inactivate Akt/PKB kinase activity and to inhibit cell proliferation [19]. The mechanism involved in induction of CK1 and CK10 expression is different from those of involucrin or filaggrin expression. Therefore, 17-AAG influenced only CK1 and CK10 expression. In addition, Hsp90 is involved in stabilizing various proteins involved in keratinocyte differentiation, including TGF receptor, EGF receptor, MEK, Src, etc. [20–22]. Therefore, we investigated the phosphorylation of Smad2/3 and p38 MAPK signaling proteins and phosphorylation of Akt, related to keratinocyte differentiation [10–12]. As noted above, phosphorylation of Akt is necessary for CK1 and CK10 expression. Phosphorylation of p38 MAPK is necessary for involucrin promoter activation, which depends on the AP-1 transcription factor. Apoptosis signal-regulating kinase 1 (ASK1) also activates the p38 MAPK cascade, but Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis [23,24]. Phosphorylation of Smad2/3 induces cell cycle arrest in keratinocytes, and Smad3 activity is suppressed by Akt [25]. The results of the present study suggest that inhibition of Hsp90 upregulates Smad2/3 phosphorylation. Cell cycle exit is one of the major processes involved in entering the terminally differentiated state [26,27], and is not dependent on high extracellular calcium but on confluency [28]. Ki67 is a nuclear protein and is expressed in the proliferating stage. Hence, the presence or absence of Ki67 is used to examine the cell cycle [29,30]. However, on laser scanning confocal microscopy, Ki67 expression was shown to be increased by 17-AAG. These results indicated that inhibition of cell cycle arrest by 17-AAG is independent of the Smad2/3 signaling pathway. In conclusion, inhibition of Hsp90 leads to suppression of Akt phosphorylation, which results in repression of CK1 and CK10 and upregulation of involucrin was via p38 MAPK phosphorylation. The results of the present study indicated that Hsp90 plays a key role in keratinocyte differentiation.
Acknowledgments
We thank the staff of the Gene Research Center and Center for Instrumental Analysis, Hirosaki University. This work was supported in part by a grant for the promotion of International Scientific Research (YK; 2008 C-1).
References
- 1.Picard D., Khursheed B., Garabedian M.J., Fortin M.G., Lindquist S., Yamamoto K.R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y., Lindquist S. Heat-shock protein hsp90 governs the activity of pp 60v-src kinase. Proc. Natl. Acad. Sci. USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter K., Buchner J. Hsp90: chaperoning signal transduction. J. Cell. Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 4.Nimmanapalli R., O’Bryan E., Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001;61:1799–1804. [PubMed] [Google Scholar]
- 5.Koster M.I., Roop D.R. Mechanisms regulating epithelial stratification. Annu. Rev. Cell. Dev. Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs E. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 1995;11:123–153. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 8.Murphy G.F., Flynn T.C., Rice R.H., Pinkus G.S. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J. Invest. Dermatol. 1984;82:453–457. doi: 10.1111/1523-1747.ep12260945. [DOI] [PubMed] [Google Scholar]
- 9.Candi E., Schmidt R., Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 10.Calautti E., Li J., Saoncella S., Brissette J.L., Goetinck P.F. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z., Bikle D.D. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin–catenin complex at the plasma membrane is required for calcium-induced phospholipase C-1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 12.Descargues P., Sil A.K., Sano Y., Korchynskyi O., Han G., Owens P., Wang X.-J., Karin M. IKKα is a critical coregulator of a Smad4-independent TGFβ-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc. Natl. Acad. Sci. USA. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonak C., Mildner M., Klosner G., Paulitschke V., Kunstfeld R., Pehamberger H., Tschachler E., Trautinger F. The hsp27 kD heat shock protein and p38-MAPK signaling are required for regular epidermal differentiation. J. Dermatol. Sci. 2011;61:32–37. doi: 10.1016/j.jdermsci.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki S., Uchiumi A., Katagata Y. Hsp40 regulates the amount of keratin proteins via ubiquitin–proteasome pathway in cultured human cells. Int. J. Mol. Med. 2012;29:165–168. doi: 10.3892/ijmm.2011.826. [DOI] [PubMed] [Google Scholar]
- 15.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asryants R.A., Duszenkova I.V., Nagradova N.K. Determination of Sepharose-bound protein with Coomassie brilliant blue G-250. Anal. Biochem. 1985;151:571–574. doi: 10.1016/0003-2697(85)90222-2. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., Chang S.M., Pennypacker S.D., Liao E.Y., Bikle D.D. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol. Biol. Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paramio J.M., Segrelles C., Ruiz S., Jorcano J.L. Inhibition of protein kinase B (PKB) and PKC-mediates keratin K10-induced cell cycle arrest. Mol. Cell. Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrighton K.H., Lin X., Feng X. Critical regulation of TGFβ signaling by Hsp90. Proc. Natl. Acad. Sci. USA. 2008;105:9244–9249. doi: 10.1073/pnas.0800163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso A.D., Solit D.B., Chiosis G., Giri B., Tsichlis P., Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 22.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 2002;8:55–61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R., Luo D., Miao R., Bai L., Ge Q., Sessa W.C. Min W Hsp90–Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 24.Efimova T., LaCelle P., Welter J.F., Eckert R.L. Regulation of human Involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- 25.Song K., Wang H., Krebs T.L., Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-b/ALK5-mediated Smad3 activation. EMBO J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missero C., Calautti E., Eckner R., Chin J., Tsai L.H., Livingston D.M., Dotto G.P. Involvement of the cell cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl. Acad. Sci. USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Missero C., Di Cunto F., Kiyokawa H., Koff A., Dotto G.P. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 28.Kolly C., Suter M.M., Müller E.J. Proliferation, cell cycle exit, and onset of terminal differentiation in cultured keratinocytes: pre-programmed pathways in control of C-Myc and Notch1 prevail over extracellular calcium signals. J. Invest. Dermatol. 2005;124:1014–1025. doi: 10.1111/j.0022-202X.2005.23655.x. [DOI] [PubMed] [Google Scholar]
- 29.Gerdes J., Lemke H., Baisch H., Wacker H.H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 30.Obarzanek-Fojt M., Favre B., Kypriotou M., Ryser S., Huber M., Hohl D. Homeodomain-only protein HOP is a novel modulator of late differentiation in keratinocytes. Eur. J. Cell Biol. 2011;90:279–290. doi: 10.1016/j.ejcb.2010.11.001. [DOI] [PubMed] [Google Scholar]


