Abstract
The role of mesenchymal stromal cells (MSCs) in regulating immune responses in the thymus is currently unclear. Here we report the existence and role of a MSC population in the thymus that expresses the pericyte and MSC marker CD248 (endosialin). We show using a CD248-deficient mouse model, that CD248 expression on these cells is required for full post-natal thymus development and regeneration post-Salmonella infection. In CD248−/− mice the thymus is hypocellular and regeneration is poorer, with significant loss of all thymocyte populations. This identifies the requirement of CD248 to maintain optimal thymic cellularity post-partum and infection.
Keywords: CD248, Endosialin, MSC, Thymus
Highlights
▸ The mesenchymal stromal cell marker CD248 is expressed in the thymus on pericytes. ▸ Genetic deletion of CD248 prevents full post-natal development of the thymus. ▸ Genetic deletion of CD248 prevents infection-dependent regeneration following thymic atrophy. ▸ CD248 regulates blood endothelial vessel formation in the thymus.
1. Introduction
The thymus is a specialised microenvironment for the generation and selection of self-tolerant T-cells. Several studies have shown that mesenchymal-derived stromal cells (MSCs) play multiple roles in thymus development and function. While MSCs provide essential signals directly to the developing T-cell precursors [1], evidence suggests that MSCs can also influence thymic epithelial cell (TEC) populations. During embryonic thymus development, interactions between MSCs and TEC progenitors are required for the expansion of epithelial microenvironments, providing a finite number of niches that directly controls the efficacy of intrathymic T-cell production [2]. Without MSC support, an ineffective TEC network forms, naïve T-cell maturation is severely reduced and with it peripheral immunity [3,4]. While MSCs, including those of neural crest origin, clearly reside in the adult thymus, their function remains poorly characterised [5,6]. As well as dynamic changes in thymus volume during development, adult thymus commonly involutes during injurious threats such as infection, regenerating during resolution [7]. Therefore, understanding the biology of thymic MSCs and their role in the post-natal thymus is likely to help our understanding of thymic homeostasis and regeneration following involution.
CD248 (endosialin) is a member of a family of proteins involved in tissue repair and remodelling [8,9]. Expression of CD248 is restricted to cells of mesenchymal origin [10]. In humans, CD248 is expressed on bone marrow MSCs [11], interstitial fibroblasts and perivascular cells [12-14] a niche where MSCs have been suggested to reside after birth [15]. We have demonstrated that murine CD248 is spatially and temporally expressed in lymph nodes where it regulates tissue expansion following immunisation [16]. In addition, high CD248 expression is observed during lymphoid tissue development. Expression decreases post-natally, but re-expression occurs during inflammation in adult tissue [17].
Given the requirement for MSCs in the formation of embryonic thymic microenvironments through their impact on TEC progenitors, and the dynamic expression pattern of CD248 on MSCs during development, we explored the possible role of CD248-expressing MSCs in adult thymic regeneration following involution. Here we show that CD248 is expressed on cells of the thymic capsule, and pericytes within the thymus. In addition, using the CD248−/− mouse model we show that CD248 expression is required for optimal post-natal thymic growth and regeneration following infection-dependent thymic atrophy.
2. Materials and methods
2.1. Mice, histology and confocal microscopy
Wild-type (WT) C57Bl/6 mice were obtained from Harlan UK Limited, Oxford, UK. eYFP C57Bl/6 mice from BMSU, Birmingham University, UK. Generation of 129Sv CD248−/− mice has been previously described [18]. 129Sv CD248−/− mice were backcrossed onto C57Bl/6 mice >5 generations in-house generating CD248−/− mice used throughout. Frozen tissue sections were prepared, stained by indirect immunohistochemistry and analysed by confocal microscopy as described previously [16,17]. All experiments were performed in accordance with UK laws with approval of Local Ethics Committees.
2.2. Tissue digestion and flow cytometry
Thymuses were enzymatically digested as previously described [19]. Mature thymocytes were defined as CD45+CD4+CD8+ – double positive (DP) – CD45+CD4+CD8− – CD4+ single positive (SP) – CD45+CD4−CD8+ – CD8+ SP – or CD45+CD4−CD8− – double negative (DN). DN subpopulations were defined as previously described [20].
2.3. Salmonella infections
Attenuated Salmonella enterica serovar Typhimurium SL3261 (5 × 105) were used to immunize adult (9- to 10-week-old) WT or CD248−/− mice via I.P. injection as previously described [21].
2.4. Determination of vessel length
Complete thymic lobes were stained with antibodies to CD31 and whole tissue analysed using a Leica DM6000. Four nonoverlapping areas of 1 × 1.7 mm were chosen at random and using AF6000 Image Processing software, vessel length was measured. Data were tabulated and expressed as a percentage of the total number of vessels counted.
2.5. Statistics
Data are mean (±SEM) from three experiments, each with at least three replicates. P-values calculated by Student's non-paired t-test. * P < 0.05, ** P < 0.01, *** P < 0.001.
3. Results
3.1. Location of thymic CD248
We have previously reported that thymic CD248 expression is downregulated post-natally, with little/no expression in the adult thymus [17]. During ontogeny CD248 expression is observed in the perithymic mesenchyme that forms the thymic capsule, co-expressed PDGFRα confirming their mesenchymal origin (Fig. 1A). In addition, CD248 is detected on perivascular cells which invaginate from the capsule to surround the CD31+ vasculature (Fig. 1B).
Fig. 1.
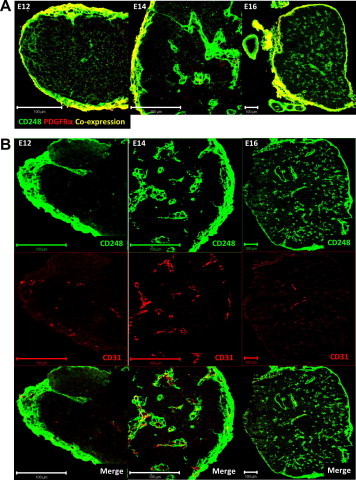
Location of thymic CD248 expressing cells. Frozen sections of E12, 14 and 16 thymuses were co-stained with CD248 (green) and either PDGFRα (A) or CD31 (B) (red). Bar = 100 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. CD248 is required for optimal post-natal thymus growth
During post-natal development the thymus first undergoes rapid logarithmic expansion in size and T-cell output. Growth reaches homeostasis in adulthood, followed by age-related involution. CD248 expression decreases post-natally in the thymus but is re-expressed in lymphoid tissue during infection [16,17]. In addition, CD248 plays a role in regulating tumour expansion and metastasis [18], therefore we explored the role of CD248 during thymic development in WT and CD248−/− mice.
WT and CD248−/− thymuses from young (1–2 weeks old), weaning (3–4 weeks old) and adult (9–10 weeks old) mice were weighed and their cellularity enumerated. All data shown are expressed normalised to body weight, to compensate for any starting differences in animal size. Thymuses from CD248−/− mice were significantly smaller and contained significantly fewer thymocytes by the time of weaning compared to WT thymuses (Fig. 2A). This difference continued into adulthood, although reduced.
Fig. 2.
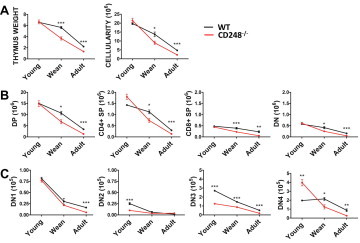
CD248 is required for efficient post-natal thymic development. Thymic weight and cellularity are reduced in young (1–2 weeks old), weaning (3–4 weeks old) and adult (9–10 weeks old) CD248−/− mice (A). Flow cytometry revealed a decrease in mature (B) and DN thymocyte populations (C) in CD248−/− mice.
Flow cytometric analysis revealed these differences were predominantly due to a significant decrease in the number of double positive (DP) thymocytes, with significant decreases in total CD4+ single positive (SP), total CD8+ SP and double negative (DN) thymocytes also observed (Fig. 2B). Analysis of DN thymocyte subsets (DN1–4) revealed significant differences in all subsets at different times (Fig. 2C). It is interesting to note, that only DN3 thymocytes showed a consistent decrease in CD248−/− mice irrespective of age. These cells undergo rapid proliferation in the sub-capsular zone which has been suggested to regulate thymic growth [22] and requires adhesion of the maturing thymocyte to extra-cellular matrix generated by MSCs [23].
3.3. CD248 is required for infection-dependent thymic regeneration
Adult thymus involutes during injurious threats such as infection, regenerating when that threat subsides [7]. Having observed that CD248 was required for optimal thymic growth during post-natal development, we reasoned the process of thymic regeneration following infection may also be linked to CD248 expression. Using a model of systemic Salmonella infection, thymic atrophy is observed, which by day 21 is accompanied by approximately 95% loss of DP thymocytes (Ross et al., submitted for publication [24]). This loss is followed by regeneration, with thymic cellularity and function re-established by 35 days post-infection.
Using this model, we assessed the role of CD248 during infection-dependent thymic regeneration using CD248−/− mice. The systemic response to Salmonella infection in CD248−/− mice is similar to that in WT mice, as assessed by splenic weight and Salmonella accumulation in the spleen (Fig. 3A). In contrast, thymic weight and cellularity were both significantly reduced in CD248−/− mice during infection-dependent thymic regeneration (Fig. 3B). Assessment of CD4, CD8 thymocyte subsets during this regenerative-phase revealed a decrease in numbers of all subpopulations in CD248−/− mice (Fig. 3C).
Fig. 3.
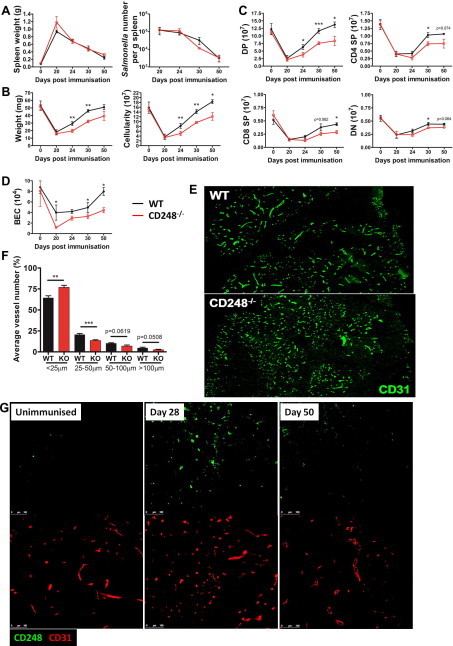
CD248 is required for infection-dependent thymic regeneration. Salmonella infection in CD248−/− mice, as assessed by splenomegaly and the number of bacteria per spleen, is similar to that of WT (A). Thymic weight and cellularity are reduced during Salmonella infection-dependent regeneration in CD248−/− mice (B). Significant loss of thymocyte populations was observed in CD248−/− mice (C). Re-vascularisation of regenerating thymuses was dysregulated in CD248−/− mice as assessed by flow cytometry of BEC number (D), and endothelial vessel length in thymuses taken 30 days post-Salmonella infection (E and F). CD248 (green) is upregulated temporally during thymic regeneration (G). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It has already been reported that tumour growth and metastasis is reduced in CD248−/− mice due to dysregulation of neo-vascularisation; a significant increase in the number of small vessels was observed with a concomitant decrease in larger, more mature vessels in CD248−/− mice [18]. Therefore, we analysed the number of blood endothelial cells (BECs) during infection-dependent thymic regeneration in WT and CD248−/− mice to quantify vascularisation of the regenerating thymus. Thymuses were enzymatically digested and BECs enumerated following flow cytometric analysis (defined by CD45−EpCAM− gp38−CD31+). Prior to infection, the numbers of thymic BECs were not significantly different when comparing WT to CD248−/− mice. However, following thymic atrophy and during regeneration the numbers of BECs were significantly and consistently lower in thymuses lacking CD248 (Fig. 3D). Moreover, when vascular structures were stained with CD31 and their length measured 30 days post-Salmonella infection, a significant increase in small vessels (<25 µm) was observed in CD248−/− thymuses (Fig. 3E and F). In addition, a significant decrease in 25–50 µm vessels was observed in CD248−/− thymuses; a trend that was consistent in larger more mature vessels. Importantly, in WT thymuses perivascular CD248 expression increases during regeneration (Day 28) but is down-regulated again once infection is cleared (Day 50) (Fig. 3G).
4. Discussion
Previously we identified a novel CD248+ cell population required for efficient popliteal lymph node expansion following NP-CGG immunisation [16]. Here we show expression of CD248 on a population of thymic MSCs is involved in controlling thymic growth during post-natal development and infection-dependent thymic regeneration.
We have shown that in CD248−/− thymuses, vascularisation during infection-dependent regeneration is disrupted and that CD248 expressed on the perivascular cells plays an important role in controlling the size of the thymus and the number of mature thymocytes within the tissue. Interestingly, the reduced size of CD248−/− thymuses during regeneration correlates to growth of tumours transplanted into the abdomen of CD248−/− mice [18]. Therefore, these data suggest that CD248 regulates both infection and tumorigenic dependent re-vascularisation. It is interesting that in subcutaneous tumours no difference in tumour growth was observed between WT and CD248−/− [18] and during infection-dependent lymph node expansion the delayed growth of CD248−/− lymph nodes normalised over time [16]. This may reveal important site-specific differences in tissue re-vascularisation.
The finding that all thymocyte populations remain reduced in CD248−/− thymuses, even 50 days post-infection when WT thymuses have re-established function and cellularity, is an intriguing one. This raises the possibility that CD248 may be important when considering how thymic structure and function is re-established; not only in terms of therapies to boost immunity post-infection or treatment with chemotherapeutic agents (both of which can cause thymic involution), but also on age-related thymic atrophy. Therefore, CD248 may represent a novel molecule that links thymic vascularisation, function, and size.
Questions still remain, especially when considering possible mechanisms of how CD248 elicits its effect in thymic MSCs. We and others have shown that CD248 expression on MSCs confers a proliferative and migratory advantage [16,25,26], possibly via PDGFR signalling [27]. Having observed an increase in smaller, less mature vessels in CD248−/− thymuses during tissue regeneration, it is possible that optimal proliferation and migration of CD248+ pericytes surrounding the developing vasculature promotes vessel stabilisation. Without sufficient perictye support, vascularisation is stunted reducing the size of epithelial cell niches which permits optimal progenitor T-cell differentiation. One contributing factor that cannot be ruled out however, is possible differences in recruitment of Early Thymic Progenitor to the thymus of CD248−/− mice during development and regeneration, which may influence thymic size and cellularity.
Taken together these findings suggest CD248 plays a role in re-vascularising thymuses during infection-dependent regeneration, which may influence tissue size. Isolating these cells and examining their ability to alter thymic growth/regeneration and peripheral immunity when transferred into deficient environments will be of interest to investigate the contribution of MSCs in immune responses.
Acknowledgement
This work was funded by a Wellcome Trust Project Grant.
References
- 1.Anderson G., Jenkinson E.J. Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 2.Jenkinson W.E., Rossi S.W., Parnell S.M., Jenkinson E.J., Anderson G. PDGFRα-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood. 2007;109:954–960. doi: 10.1182/blood-2006-05-023143. [DOI] [PubMed] [Google Scholar]
- 3.Odaka C. Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J. Histochem. Cytochem. 2009;57:373–382. doi: 10.1369/jhc.2008.952895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suniara R.K., Jenkinson E.J., Owen J.J. An essential role for the thymic mesenchyme in early T cell development. J. Exp. Med. 2000;191:1051–1056. doi: 10.1084/jem.191.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster K., Sheridan J., Veiga-Fernandas H., Roderick K., Pachnis V., Adams R., Blackburn C., Kioussis D., Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- 6.Muller S.M., Stolt C.C., Terszowski G., Blum C., Amagai T., Kessaris N., Iannarelli P., Richardson W.D., Wegner W., Roderwald H.R. Neural crest origin of perivascular mesenchyme in the adult thymus. J. Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 7.Wilson S., Dardenne M. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc. Nutr. Soc. 2010;69:636–643. doi: 10.1017/S0029665110002545. [DOI] [PubMed] [Google Scholar]
- 8.Norsworthy P.J., Fossati-Jimack L., Cortes-Hernandez J., Taylor P.R., Bygrave A.E., Thompson R.D., Nourshargh S., Walport M.J., Botto M. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J. Immunol. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 9.Conway E.M., Van de Wouwer M., Pollefeyt S., Jurk K., Van Aken H., De Vriese A., Weitz J.I., Weiler H., Hellings P.W., Schaeffer P., Herbert J-M., Collen D., Theilmeier G. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor κB and mitogen-activated protein kinase pathways. J. Exp. Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers S.M., Boles N.C., Lin K-Y.K., Tierney M.P., Bowman T.V., Bradfute S.B., Chen A.J., Merchant A.A., Sirin O., Weksberg D.C., Merchant M.G., Fisk C.J., Shaw C.A., Goodell M.A. Hematopoietic fingerprints: an expression database of stem cells and their progeny. Cell Stem Cell. 2007;1:578–591. doi: 10.1016/j.stem.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagley R.G., Weber W., Rouleau M.Y., Honma N., Kataoka S., Ishida I., Roberts B.L., Teicher B.A. Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers. Int. J. Oncol. 2009;34:619–627. doi: 10.3892/ijo_00000187. [DOI] [PubMed] [Google Scholar]
- 12.MacFadyen J., Savage K., Wienke D., Isacke C.M. Endosialin is expressed on stromal fibroblasts and CNS pericytes in mouse embryos and is downregulated during development. Gene Expr. Patterns. 2007;7:363–369. doi: 10.1016/j.modgep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.MacFadyen J.R., Haworth O., Robertson D., Hardie D., Webster M-T., Morris H.R., Panico M., Sutton-Smith M., Dell A., van der Geer P., Wienke D., Buckley C.D., Isacke C.M. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579:2569–2575. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 14.Simonavicius N., Robertson D., Bax D.A., Jones C., Huijbers I.J., Isacke C.M. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod. Pathol. 2008;21:308–315. doi: 10.1038/modpathol.3801006. [DOI] [PubMed] [Google Scholar]
- 15.Meirellesd. L.S., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 16.Lax S., Hardie D.L., Wilson A., Douglas M.R., Anderson G., Huso D., Isacke C.M., Buckley C.D. The pericyte and stromal cell marker CD248 (endosialin) is required for efficient lymph node expansion. Eur. J. Immunol. 2010;40:1884–1889. doi: 10.1002/eji.200939877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lax S., Hou T.Z., Jenkinson E., Salmon M., MacFadyen J.R., Isacke C.M., Anderson G., Cunningham A.F., Buckley C.D. CD248/Endosialin is dynamically expressed on a subset of stromal cells during lymphoid tissue development, splenic remodeling and repair. FEBS Lett. 2007;581:3550–3556. doi: 10.1016/j.febslet.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 18.Nanda A., Karim B., Peng Z., Liu G., Qiu W., Gan C., Vogelstein B., St Croix B., Kinzler K.W., Huso D.L. Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc. Natl. Acad. Sci. USA. 2006;103:3351–3356. doi: 10.1073/pnas.0511306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link A., Vogt T.K., Favre S., Britschgi M.R., Acha-Orbea H., Hinz B., Cyster J.G., Luther S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 20.Godfrey D., Kennedy J., Suda T., Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 2003;150:4244–4252. [PubMed] [Google Scholar]
- 21.Cunningham A.F., Gaspal F., Serre K., Mohr E., Henderson I.R., Scott-Tucker A., Kenny S.M., Khan M., Toellner K-M., Lane P.J.L., MacLennan I.C.M. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 22.Prockop S.E., Petrie H.T. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J. Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 23.Rossi S.W., Jeker L.T., Ueno T., Kuse S., Keller M.P., Zuklys S., Gudkov A.V., Takahama Y., Krenger W., Blazar B.R., Hollander G.A. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.E.A. Ross, R.E. Coughlan, A. Flores-Langarica, S. Lax, J. Nicholson, G.E. Desanti, J.E. Marshall, S. Bobat, J. Hitchcock, A. White, W.E. Jenkinson, M. Khan, I.R. Henderson, G.G. Lavery, C.D. Buckley, G. Anderson, A.F. Cunngham. Thymic function is maintained during Salmonella-induced atrophy and recovery. J Immunol, Submitted. [DOI] [PMC free article] [PubMed]
- 25.Christian S., Winkler R., Helfrich I., Boos A.M., Besemfelder E., Schadendorf D., Augustin H.G. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am. J. Pathol. 2008;172:486–494. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomkowicz B., Rybinski K., Foley B., Ebel W., Kline B., Routhier E., Sass P., Nicolaides N.C., Grasso L., Zhou Y. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc. Natl. Acad. Sci. USA. 2007;104:17965–17970. doi: 10.1073/pnas.0705647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomkowicz B., Rybinski K., Sebeck D., Sass P., Nicolaides N.C., Grasso L., Zhou Y. Endosialin/TEM-1/CD248 regulates pericyte proliferation through PDGF receptor signaling. Cancer Biol. Ther. 2010;9:908–915. doi: 10.4161/cbt.9.11.11731. [DOI] [PubMed] [Google Scholar]


