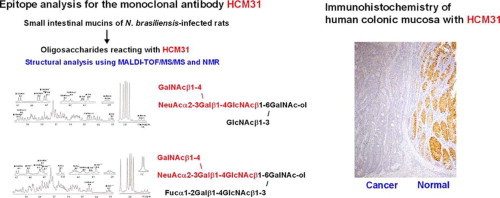
Abstract
Rat small intestinal goblet cell mucins reacting with monoclonal antibody HCM31 increase significantly during regeneration from experimental mucosal damage and at the period of expulsion of parasitic nematode, Nippostrongylus brasiliensis (N.b). The reduction in reactivity of HCM31 with mucin upon neuraminidase treatment, suggested that HCM31 recognizes sialylated oligosaccharide on mucin. HCM31-reactive sialomucins are therefore considered to play an important role in the physiological and pathological changes in the gastrointestinal mucosa. To determine the epitope for HCM31, oligosaccharide–alditols reacted with HCM31 were obtained from the small intestinal mucins of N.b-infected rats and purified by ion-exchange chromatography followed by normal-phase HPLC. Two HCM31-reactive oligosaccharide-alditols were obtained. Analyses using tandem mass spectrometry and NMR spectroscopy showed that these oligosaccharides were core 4 mucin-type oligosaccharides having a common tetrasaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ- (Sda blood group antigen). These structures were not found in the small intestinal mucin oligosaccharides from uninfected rats. This epitope specificity of HCM31 was also confirmed using previously established anti-GM2 and anti-Sda antibodies. Taken together, these results strongly suggest that HCM31 specifically recognizes mucin-type oligosaccharides with the Sda tetrasaccharide sequence. Immunohistochemical examination of human gastrointestinal tracts showed that HCM31 site-specifically stained the goblet cells in normal sigmoid colon and normal rectum, but the goblet cells stained with HCM31 were reduced in the corresponding cancer tissues. HCM31 seems to be useful for diagnosis of colonic cancer and for examining the function of secretory-type mucin with Sda antigen.
Keywords: Mucin, Monoclonal antibody, Nippostrongylus brasiliensis, Sda antigen, Sialylated oligosaccharide, Colon cancer, MALDI-TOF/MS, NMR
Abbreviations: mAb, monoclonal antibody; MALDI-TOF/MS, matrix-assisted laser desorption/ionization-time of flight mass spectrometry; MS/MS, tandem mass spectrometry; GalNAc-ol, N-acetylgalactosaminitol; N.b, Nippostrongylus brasiliensis
Graphical abstract
Highlights
▸ We analyzed the epitope for the monoclonal antibody HCM31. ▸ HCM31 recognizes core 4 oligosaccharides with the Sda antigen obtained from small intestinal mucins of N. brasiliensis-infected rats. ▸ We propose that the Sda tetrasaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ-, is the epitope for HCM31. ▸ Goblet cells stained with HCM31 decrease with malignant change in human colon tissues. ▸ HCM31 might be useful for the diagnosis of colonic cancer and for examining the function of mucin with Sda tetrasaccharide.
1. Introduction
The gastrointestinal mucus covering the mucosal surface is considered a major factor in the gastrointestinal defense mechanism against various infectants such as microorganisms, viruses and parasites [1]. Mucin, a highly O-glycosylated glycoprotein with a high molecular mass, is a major component of the gastrointestinal mucus and plays an important role in the mucus barrier covering mucosal surfaces. Mucins are located site-specifically in the gastrointestinal mucosa according to the distinct type of core protein [2–4] and attached carbohydrates [5,6]. For the precise characterization of individual mucins on a biochemical and physiological basis, many anti-mucin monoclonal antibodies (mAbs) reacting with specific types of carbohydrate chains of gastrointestinal mucins have been developed in our laboratory, and their properties have been characterized histochemically and biochemically. Histochemical studies show that distinct types of mucins have specific stainability with mAbs [7–9]. The epitope of an mAb, HIK1083, which stains mucins from mucus neck cells and pyloric gland cells, has been determined as a peripheral α-linked GlcNAc on the mucin oligosaccharides [9]. Recently, gastric mucins with this epitope on their oligosaccharides have been shown to act as a natural antibiotic protecting the host from Helicobacter pylori [10] and to play an essential role in preventing gastric cancer [11]. Thus, epitope analysis of an mAb that reacts with a specific oligosaccharide chain bound to the mucin molecules is needed to clarify the biological function of the particular oligosaccharides.
The sialomucins with specific sialylated oligosaccharide determinants are considered to have multiple biological functions. The sialyl 6-sulfo Lewis x determinant on cell-surface-associated sialomucin is expressed on the high endothelial venules in lymph nodes as a major ligand for L-selectin in order to allow lymphocyte homing [12]. Increasing serum levels of sialomucin having the sialyl Lewis A determinant are correlated with increasing tumor metastasis [1]. In gastrointestinal mucosa, sialomucins as mucus components are secreted by the epithelial cells and have diverse sialylated oligosaccharide structures. However, the distribution and the function of individual gastrointestinal sialomucins having distinct types of sialylated oligosaccharides are poorly understood. Appropriate mAbs are needed to distinguish the specific types of sialomucins.
Recently, we developed an mAb, HCM31, which reacts with sialylated oligosaccharides of rat small intestinal mucins [13]. Although HCM31 only partially stains the jejunal goblet cells in normal rat, HCM31-positive goblet cells increased remarkably during the processes of regeneration from mucosal damage caused by the administration of an antineoplastic chemotherapy drug [14] and nonsteroidal anti-inflammatory drugs [15]. Furthermore, HCM31-positive goblet cells were found to increase remarkably after infection with the intestinal nematode Nippostrongylus brasiliensis (N.b) [16]. HCM31-reactive sialomucins are therefore considered to play an important role in the physiological and pathological changes in the gastrointestinal mucosa.
In this study, to characterize the epitope recognized by HCM31, the oligosaccharides reacting with this mAb were obtained from small intestinal mucins of N.b-infected rats and their structures were analyzed by tandem mass spectrometry (MS/MS) and NMR spectroscopy. The oligosaccharides obtained from uninfected rats were also analyzed to verify specific expression of the epitope of HCM31 after N.b infection. In this paper, the unique epitope sequence containing a sialic acid residue and the histochemical distribution of the sialomucins recognized by this mAb in human normal and cancer gastrointestinal tract are presented.
2. Results
2.1. Studies of antigenic determinant of HCM31 by the modification of mucin
To characterize the epitope of HCM31, an mAb developed using human colonic mucin as an antigen, periodate oxidation and trypsin digestion of the purified rat mucin were performed to degrade the carbohydrate and peptide moieties, respectively, and then the residual antigenic activity was tested by ELISA. Periodate oxidation reduced the antigenic activity to HCM31, whereas trypsin digestion did not affect the reactivity of this mAb (data not shown). These results indicate that the carbohydrate moieties of the mucin were involved in the epitope of HCM31.
Fig. 1 shows the immunohistochemical observations of rat jejunal mucosa stained with HCM31. Only a small number of goblet cells were stained on uninfected rat jejunum (Fig. 1a). In contrast, HCM31-reactive goblet cells increased on day 14 of N.b infection (Fig. 1b), the time when the worms were expelled from the rats. Staining was conserved during de-O-acetylation treatment of sialic acid (Fig. 1c) but was significantly reduced after a neuraminidase treatment, which removes the sialic acid residue from mucin oligosaccharide (Fig. 1d). These observations indicate that HCM31 reacts with oligosaccharides that have sialic acid that is not O-acetylated.
Fig. 1.
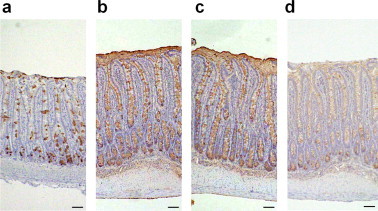
Immunohistochemistry for the rat jejunal mucosa with HCM31. Immunostaining of the jejunal mucosal specimens of uninfected (a) and N.b-infected (b)–(d) rats was performed as described in Section 4. The specimens were stained with HCM31 without additional treatment (a, b), and after de-O-acetylation (c) or α2-3,6,8-sialidase (d) treatment. Original magnification 25×. Bars = 50 μm.
2.2. Characterization of HCM31-reactive oligosaccharides from small intestinal mucin of N.b-infected rats
The oligosaccharides were prepared from rat small intestinal mucin with or without N.b infection by alkaline borohydride treatment, fractionated by anion exchange chromatography on a TOYOPEARL QAE-550C column and then tested for reactivity with HCM31. From the uninfected rats, one neutral fraction, UN, eluted with distilled water, and two acidic fractions, UA1 and UA2, eluted from the column with a gradient of 0.1–0.5 M NaOAc, were obtained (Fig. 2a). Similarly, one neutral fraction, IN, and two acidic fractions, IA1 and IA2, were obtained from the infected rats (Fig. 2b). The inhibition assay indicated that IA1 and IA2 significantly reacted with HCM31 (Fig. 2d), whereas UA1 did not react with HCM31, but UA2 did (Fig. 2c). The reactivity of IA2 was higher than that of UA2. These findings indicated that the oligosaccharides reacting with HCM31 were acidic; this result is consistent with the immunohistochemical examination using neuraminidase treatment (Fig. 1).
Fig. 2.
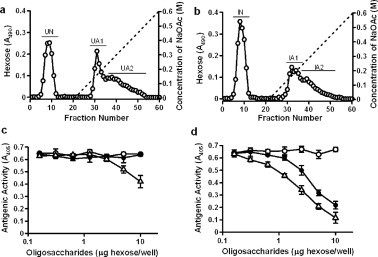
TOYOPEARL QAE-550C anion exchange chromatography of the small intestinal mucin oligosaccharides and the reactivity of oligosaccharides with HCM31. The same amount of oligosaccharides obtained from uninfected (a) and N.b-infected (b) rats was loaded onto the column and eluted with water followed by a linear gradient of 0–0.6 M NaOAc (dashed line). The hexose content (○) of each fraction was assessed by the phenol-sulfuric acid method. The antigenic activities of various amounts of the oligosaccharides from the pooled fractions, UN (○), UA1 (•) and UA2 (Δ) in uninfected rats (c), and IN (○), IA1 (•) and IA2 (Δ) in infected rats (d), were examined by competitive ELISA as described in Section 4. Data are expressed as mean ± SD from three experiments.
UA1 and IA1 were chosen for further analyses because IA1 reacted with HCM31 whereas UA1 did not (Fig. 2). UA1 and IA1 were further purified by two steps of normal-phase HPLC using TSK-Gel Amide-80 columns. By the first-step HPLC, eight major fractions, designated as UA1-1 to -8 and IA1-1 to -8, were obtained from uninfected (Fig. 3a; upper panel) and infected rats (Fig. 3a; lower panel), respectively. The inhibition assay showed that fractions IA1-5 and IA1-8 (Fig. 3b) and IA1-6 and IA1-7 (data not shown) significantly reacted with HCM31, whereas fractions IA1-2 and IA1-3 (Fig. 3b) and IA1-1 and IA1-4 (data not shown) did not. None of UA1-1 to -8 reacted with this mAb (data not shown).
Fig. 3.
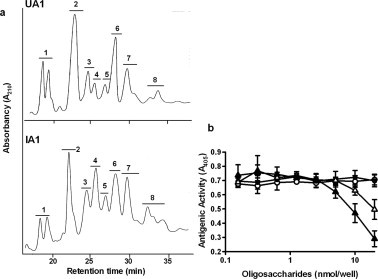
First-step HPLC of oligosaccharide fractions of uninfected and N.b-infected rats using Tskgel Amide-80 columns and the reactivity of oligosaccharides with HCM31. Fractions UA1 (upper panel) and IA1 (lower panel) were loaded on the column and then eluted with a 30 min linear gradient of acetonitrile (68–55%) (a). Absorbance was monitored at 210 nm. The antigenic activities of various amounts of the oligosaccharides from purified oligosaccharide fractions, IA1-2 (○), IA1-3(•), IA1-5 (Δ) and IA1-8 (▴), in infected rats were examined by competitive ELISA (b), as described in Section 4. Data are expressed as mean ± SD from three experiments.
The oligosaccharides fractionated by the first-step HPLC were analyzed by MALDI-TOF/MS in negative mode (Table 1). The masses of the oligosaccharides were distributed from 675 to 1746, corresponding to tri- to nona-saccharides. The sugar compositions of all the tested oligosaccharides were assigned to the appropriate acidic oligosaccharide–alditols, bearing either a sulfate or a sialic acid residue, as well as having GalNAc-ol at the reducing terminus on the basis of their masses. Because all HCM31-reactive fractions (IA1-5, -6, -7 and -8 in infected rat) contained sialylated oligosaccharides, these data support the idea that HCM31 reacted with sialylated carbohydrate sequence (Fig. 1). Fig. 4a shows MS spectra of UA1-5 and IA1-5. Two oligosaccharides ( m/z [M−H]− of 1097 and 1284) were detected in IA1-5, but not in UA1-5, as also shown in Table 1. Similarly, three oligosaccharides ( m/z [M−H]− of 1486, 1535 and 1592) were detected in IA1-8, but not in UA1-8 (Fig. 4b, Table 1).
Table 1.
Differences of mucin oligosaccharide separated by the first-step HPLC between uninfected and N.b-infected rats: identified by MALDI-TOF/MS. Oligosaccharides detected only in the infected rats are noted in bold text.
| Fraction | [M−H]- (m/z) | Expected composition of oligosaccharide-alditols | Uninfected (U) | Infected (I) |
|---|---|---|---|---|
| A1-1 | 675 | (NeuAc)(Hex)GalNAc-ol | + | + |
| 813 | (SO3H)(dHex)(Hex)(HexNAc)GalNAc-ol | + | + | |
| A1-2 | 675 | (NeuAc)(Hex)GalNAc-ol | + | − |
| 878 | (NeuAc)(Hex)(HexNAc)GalNAc-ol | + | + | |
| 894 | (NeuGc)(Hex)(HexNAc)GalNAc-ol | − | + | |
| 975 | (SO3H)(dHex)(Hex)2(HexNAc)GalNAc-ol | − | + | |
| 1016 | (SO3H)(dHex)(Hex)(HexNAc)2GalNAc-ol | + | + | |
| 1073 | (SO3H)(Hex)(HexNAc)3GalNAc-ol | − | + | |
| 1081* | (NeuAc)(Hex)(HexNAc)2GalNAc-ol | − | + | |
| A1-3 | 878 | (NeuAc)(Hex)(HexNAc)GalNAc-ol | + | + |
| 894 | (NeuGc)(Hex)(HexNAc)GalNAc-ol | + | + | |
| 975 | (SO3H)(dHex)(Hex)2(HexNAc)GalNAc-ol | + | + | |
| 1016 | (SO3H)(dHex)(Hex)(HexNAc)2GalNAc-ol | + | + | |
| 1081* | (NeuAc)(Hex)(HexNAc)2GalNAc-ol | − | + | |
| 1097 | (NeuGc)(Hex)(HexNAc)2GalNAc-ol | − | + | |
| 1121 | (SO3H)(dHex)2(Hex)2(HexNAc)GalNAc-ol | + | + | |
| 1178 | (SO3H)(dHex)(Hex)2(HexNAc)2GalNAc-ol | − | + | |
| A1-4 | 975 | (SO3H)(dHex)(Hex)2(HexNAc)GalNAc-ol | + | + |
| 1121 | (SO3H)(dHex)2(Hex)2(HexNAc)GalNAc-ol | + | + | |
| 1178 | (SO3H)(dHex)(Hex)2(HexNAc)2GalNAc-ol | − | + | |
| A1-5 | 1097 | (NeuGc)(Hex)(HexNAc)2GalNAc-ol | − | + |
| 1121 | (SO3H)(dHex)2(Hex)2(HexNAc)GalNAc-ol | + | + | |
| 1178 | (SO3H)(dHex)(Hex)2(HexNAc)2GalNAc-ol | + | + | |
| 1186* | (NeuAc)(dHex)(Hex)2(HexNAc)GalNAc-ol | + | + | |
| 1284* | (NeuAc)(Hex)(HexNAc)3GalNAc-ol | − | + | |
| 1324 | (SO3H)(dHex)2(Hex)2(HexNAc)2GalNAc-ol | + | − | |
| A1-6 | 1186* | (NeuAc)(dHex)(Hex)2(HexNAc)GalNAc-ol | + | + |
| 1324 | (SO3H)(dHex)2(Hex)2(HexNAc)2GalNAc-ol | + | + | |
| 1381 | (SO3H)(dHex)(Hex)2(HexNAc)3GalNAc-ol | + | + | |
| 1389* | (NeuAc)(dHex)(Hex)2(HexNAc)2GalNAc-ol | − | + | |
| A1-7 | 1178 | (SO3H)(dHex)(Hex)2(HexNAc)2GalNAc-ol | + | + |
| 1202 | (NeuGc)(dHex)(Hex)2(HexNAc)GalNAc-ol | + | − | |
| 1324 | (SO3H)(dHex)2(Hex)2(HexNAc)2GalNAc-ol | + | + | |
| 1340 | (SO3H)(dHex)(Hex)3(HexNAc)2GalNAc-ol | + | − | |
| 1381 | (SO3H)(dHex)(Hex)2(HexNAc)3GalNAc-ol | − | + | |
| 1389* | (NeuAc)(dHex)(Hex)2(HexNAc)2GalNAc-ol | + | + | |
| 1486 | (SO3H)(dHex)2(Hex)3(HexNAc)2GalNAc-ol | + | + | |
| A1-8 | 1486 | (SO3H)(dHex)2(Hex)3(HexNAc)2GalNAc-ol | − | + |
| 1535* | (NeuAc)(dHex)2(Hex)2(HexNAc)2GalNAc-ol | − | + | |
| 1551* | (NeuAc)(dHex)(Hex)3(HexNAc)2GalNAc-ol | + | + | |
| 1592* | (NeuAc)(dHex)(Hex)2(HexNAc)3GalNAc-ol | − | + | |
| 1689 | (SO3H)(dHex)2(Hex)3(HexNAc)3GalNAc-ol | + | + | |
| 1746 | (SO3H)(dHex)(Hex)3(HexNAc)4GalNAc-ol | + | + |
The compositions were determined by MS/MS analysis in terms of whether they contained NeuAc or NeuGc.
Fig. 4.
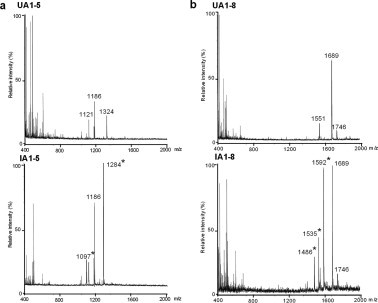
Mass spectra of oligosaccharide fractions obtained by the first-step HPLC. The oligosaccharide fractions, UA1-5 (upper panel) and IA1-5 (lower panel), obtained from the uninfected and N.b-infected rats, respectively, were analyzed by MALDI-TOF/MS (a). The oligosaccharide fractions, UA1-8 (upper panel) and IA1-8 (lower panel), obtained from the uninfected and infected rats were also analyzed (b). Masses detected in infected but not uninfected rats are indicated by asterisks.
Because it was expected that HCM31-reactive oligosaccharides were induced by N.b infection, each of IA1-5 and IA1-8 was further purified by the second-step HPLC and characterized. Fraction IA1-5 separated into five fractions, designated IA1-5a, -5b, -5c, -5d and -5e (Fig. 5a; lower panel), corresponding to m/z [M−H]− of 1121, 1097, 1186, 1284 and 1186, respectively (Table 2). Fraction IA1-8 separated into three fractions, designated IA1-8a, -8b and -8c (Fig. 5b; lower panel), corresponding to m/z [M−H]− of 1486, 1592 and 1535, respectively (Table 2). IA1-5d and IA1-8b were major fractions among IA1-5 and IA1-8, respectively, and their amounts were sufficient for inhibition assay with HCM31. The inhibition assay showed that IA1-5d and IA1-8b significantly reacted with HCM31 (Fig. 6). Because UA1-5 and UA1-8 did not contain the oligosaccharides assigned as IA1-5d and IA1-8b, respectively (Fig. 5), this result confirmed that the two HCM31-reactive oligosaccharides are abundantly expressed by N.b infection.
Fig. 5.
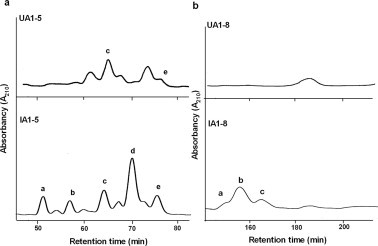
Second-step HPLC of oligosaccharide fractions of uninfected and N.b-infected rats using TSKgel Amide-80 columns. Fractions, UA1-5 (a; upper panel) and UA1-8 (b; upper panel) from uninfected rats, and IA1-5 (a; lower panel) and IA1-8 (b; lower panel) from N.b-infected rats, were eluted with 74% acetonitrile under isocratic conditions. The absorbance was monitored at 210 nm.
Table 2.
Oligosaccharides separated by the second-step HPLC from small intestine in N.b-infected rats: identified by MALDI-TOF/MS.
| Fraction | [M-H]-(m/z) | Expected composition of oligosaccharide-alditols |
|---|---|---|
| IA1-5a | 1121 | (SO3H)(dHex)2(Hex)2(HexNAc)GalNAc-ol |
| IA1-5b | 1097 | (NeuGc)(Hex)(HexNAc)2GalNAc-ol |
| IA1-5c | 1186 | (NeuAc)(dHex)(Hex)2(HexNAc)GalNAc-ol |
| IA1-5d | 1284 | (NeuAc)(Hex)(HexNAc)3GalNAc-ol |
| IA1-5e | 1186 | (NeuAc)(dHex)(Hex)2(HexNAc)GalNAc-ol |
| IA1-8a | 1486 | (SO3H)(dHex)2(Hex)3(HexNAc)2GalNAc-ol |
| IA1-8b | 1592 | (NeuAc)(dHex)(Hex)2(HexNAc)3GalNAc-ol |
| IA1-8c | 1535 | (SO3H)(dHex)2(Hex)3(HexNAc)2GalNAc-ol |
Fig. 6.
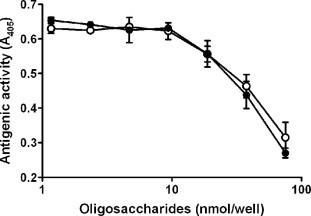
The reactivity of purified oligosaccharides after second-step HPLC with HCM31. The antigenic activities of various amounts of purified oligosaccharides, IA1-5d (○) and IA1-8b (•), in infected rats were examined by competitive ELISA as described in Section 4. Data are expressed as mean ± SD from two experiments.
2.3. Structural analysis of sialylated oligosaccharides recognized by HCM31
To obtain further structural information for the epitope of HCM31, purified HCM31-reactive oligosaccharides, IA1-5d and IA1-8b, were analyzed by MS/MS, amino sugar analysis and NMR spectroscopy. MS/MS analyses of the oligosaccharides were performed using positive mode after methylesterification of the sialic acid residue.
2.3.1. Structural analysis of IA1-5d
Amino sugar analysis of IA1-5d showed that the molar ratio of GalNAc-ol, GalNAc and GlcNAc was 1.0:1.0:2.1. Fig. 7a shows the fragmentation spectrum of IA1-5d ( m/z 1322 as [M+Na]+) by MS/MS analysis. In this spectrum, the core 4 structure was identified by the diagnostic ion Y2β at m/z 652. The series of Bi ions at m/z 591 (HexNAc-Hex-HexNAc), 693 (NeuAc-Hex-HexNAc or NeuAc-[HexNAc-]Hex) and 896 (NeuAc-[HexNAc-]Hex-HexNAc) and Y1α/Y3β” ion at m/z 916 (NeuAc-Hex-HexNAc-GalNAc-ol) allow reconstruction of the sequence as follows: NeuAc-(GalNAc-)Gal-GlcNAc-[GlcNAc-]GalNAc-ol illustrated in Fig. 7a. Fig. 8a shows the one-dimensional1H NMR spectrum of IA1-5d (≈0.15 mg). In the spectrum, β-anomeric resonances (4.69, 4.56, 4.52 and 4.51 ppm) were recognized as one residue of the β-linked GalNAc, two residues of the β-linked GlcNAc, and one residue of the β-linked Gal, respectively, by their coupling to a high-field H-2 resonance and the pattern of the cross-peaks in the TOCSY spectrum (data not shown). The carbohydrate composition of IA1-5d obtained from the NMR spectrum agreed with that expected by the data obtained from the molecular mass and amino sugar analyses. From the13C chemical shifts of the heteronuclear multiple-quantum coherence spectroscopy (HMQC) spectra of IA1-5d (Table 3), no substitution could be observed on one of the two β-linked GlcNAc residues, indicating that this GlcNAc residue is present at the non-reducing terminal in this structure. Another β-linked GlcNAc is attached at position 4 (3.73 ppm), which could be proved by the lower field changes in the HMQC spectrum (+4.0 ppm) of GlcNAc compared with that of the standard β-methylated GlcNAc [9]. The residues of the β-linked Gal and GalNAc-ol at the reducing terminus showed the glycosylation shift at positions 3 and 4, and positions 3 and 6, respectively, on the basis of13C chemical shift assessment. The existence of residues of NeuAc and β-linked GalNAc, and the substitutions at positions 3 and 4 of the β-linked Gal indicated that the oligosaccharide has a terminal sequence, NeuAcα2-3(GalNAcβ1-4)Galβ- [17]. To elucidate whether NeuAc-containing branch attached at position C3 or C6 of GalNAc-ol, the C–C bond of the GalNAc-ol at the reducing terminal was cleaved at positions C4 and C5 by mild periodate oxidation. The molecular masses of the fragments were estimated by MALDI-TOF/MS. Two fragments, corresponding to NeuAc-(GalNAc)Gal-GlcNAc-O–CH2–CHO and GlcNAc-O–CH(CHO)–CH(NHCOCH3)–CH2–CHO, were obtained. This indicated that the NeuAc-containing branch presented the C6 side of the reducing-end GalNAc-ol residue. Finally, it was proposed that oligosaccharide IA1-5d had the following structure:
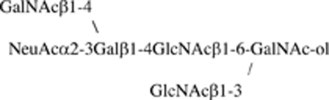
Fig. 7.
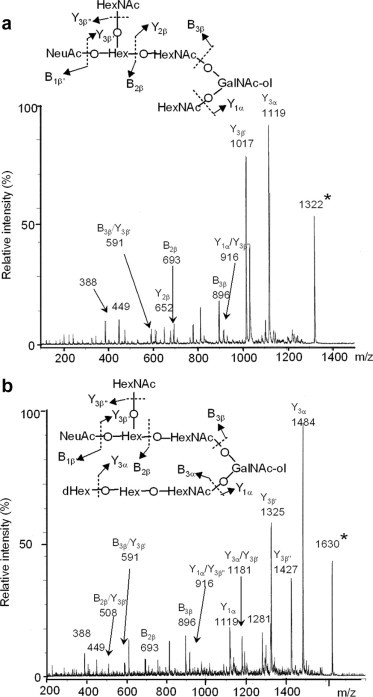
MS/MS spectra of oligosaccharides, IA1-5d (a) and IA1-8b (b). Precursor ions are indicated by an asterisk. Fragmentation uses the Domon and Costello nomenclature [37].
Fig. 8.
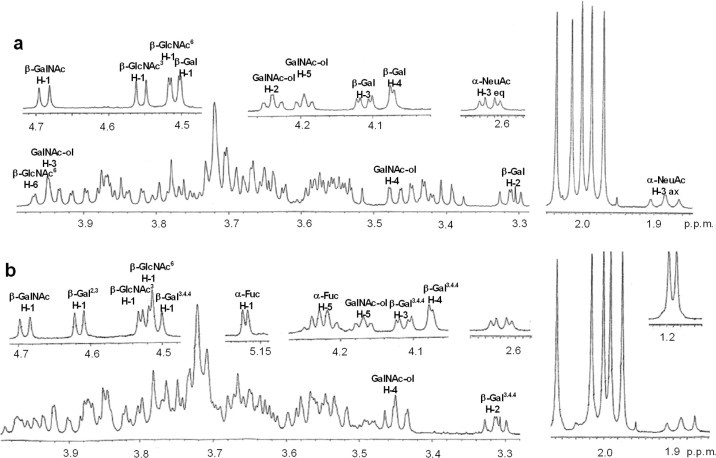
One-dimensional 1H NMR spectra of oligosaccharides, IA1-5d (a) and IA1-8b (b).
Table 3.
Chemical shifts of each sugar component. Chemical shifts labeled with an asterisk indicate occurrence of glycosylation shift.
| IA1-5d | IA1-8b | Standardsb | |||
|---|---|---|---|---|---|
| Sugar | 1H | 13C | 1H | 13C | 13C |
| GalNAc-ol | |||||
| Position 1 | 3.58/3.84 | 62.7 | 3.58/3.83 | 62.7 | 61.5 |
| Position 2 | 4.24 | 51.4 | 4.24 | 51.3 | 51.5 |
| Position 3 | 3.94* | 76.6* | 3.93* | 77.0* | 68.4 |
| Position 4 | 3.47 | 69.1 | 3.45 | 69.3 | 69.4 |
| Position 5 | 4.20 | 67.7 | 4.17 | 67.9 | 69.8 |
| Position 6 | 3.66/3.86* | 71.0* | 3.66/3.86* | 71.1* | 63.2 |
| Ac-CH3 | 2.04 | 22.2 | 2.06 | 22.2 | 21.7 |
| β-GlcNAc3a | |||||
| Position 1 | 4.56 | 102.3 | 4.53 | 101.5 | 101.9 |
| Position 2 | 3.71 | 55.8 | 3.76 | 55.1 | 55.4 |
| Position 3 | 3.55 | 73.4 | 3.63 | 72.3 | 73.9 |
| Position 4 | 3.44 | 69.9 | 3.73* | 73.9* | 69.9 |
| Position 5 | 3.42 | 75.6 | 3.44 | 75.0 | 75.8 |
| Position 6 | 3.71/3.91 | 61.0 | 3.76 /3.97 | 61.0 | 60.7 |
| Ac-CH3 | 2.00 | 22.0 | 2.01 | 21.9 | 21.9 |
| β-GlcNAc6a | |||||
| Position 1 | 4.52 | 101.5 | 4.52 | 102.7 | 101.9 |
| Position 2 | 3.72 | 54.8 | 3.71 | 54.8 | 55.4 |
| Position 3 | 3.66 | 74.6 | 3.67 | 74.6 | 73.9 |
| Position 4 | 3.73* | 73.9* | 3.72* | 73.9* | 69.9 |
| Position 5 | 3.56 | 74.7 | 3.55 | 74.9 | 75.8 |
| Position 6 | 3.76/3/96 | 60.4 | 3.73 /3.95 | 60.4 | 60.7 |
| Ac-CH3 | 2.02 | 22.1 | 2.02 | 22.2 | 21.9 |
| β-Gal4,3a | |||||
| Position 1 | 4.62 | 100.1 | 103.7 | ||
| Position 2 | 3.57* | 76.3* | 70.7 | ||
| Position 3 | 3.81 | 73.4 | 72.7 | ||
| Position 4 | 3.86 | 69.0 | 68.6 | ||
| β-Gal4,6a | |||||
| Position 1 | 4.51 | 102.5 | 4.51 | 102.5 | 103.7 |
| Position 2 | 3.31 | 70.0 | 3.31 | 69.9 | 70.7 |
| Position 3 | 4.11* | 74.2* | 4.12* | 74.2* | 72.7 |
| Position 4 | 4.07* | 77.1* | 4.08* | 77.1* | 68.6 |
| α-Fuc2,4a | |||||
| Position 1 | 5.17 | 99.3 | 99.4 | ||
| Position 2 | 3.74 | 67.9 | 67.8 | ||
| Position 5 | 4.22 | 66.4 | 66.4 | ||
| CH3 | 1.19 | 15.2 | 15.2 | ||
| β-GalNAc | |||||
| Position 1 | 4.69 | 102.7 | 4.69 | 102.6 | 99.0 |
| Position 2 | 3.87 | 52.2 | 3.88 | 52.2 | 52.0 |
| Position 3 | 3.64 | 71.3 | 3.64 | 71.1 | 70.5 |
| Position 4 | 3.86 | 67.9 | 3.86 | 67.7 | 67.5 |
| Ac-CH3 | 1.97 | 22.5 | 1.97 | 22.5 | 22.3 |
| α-NeuAc | |||||
| Position 1 | 174.3 | 174.2 | 171.6 | ||
| Position 2 | 101.6 | 101.6 | 99.4 | ||
| Position 3(ax) | 1.89 | 36.8 | 1.89 | 36.8 | 39.1 |
| Position 3(eq) | 2.62 | 36.8 | 2.62 | 36.8 | 39.1 |
| Position 4 | 3.73 | 68.1 | 3.73 | 68.1 | 67.5 |
| Ac-CH3 | 1.99 | 22.3 | 1.99 | 22.3 | 22.2 |
A superscript at a monosaccharide residue indicates to which position of the adjacent monosaccharide it is glycosidically linked. Two superscripts map out the pathway from the residue toward the GalNAc-ol residue.
Standards are α- and β-methyl derivatives of each component sugar except GalNAc-ol.
2.3.2. Structural analysis of IA1-8b
Fig. 7b shows the fragmentation spectrum of IA1-8b ( m/z 1630 as [M+Na]+). This oligosaccharide has the same branch having NeuAc as IA1-5d because the series of Bi ions at m/z 591, 693 and 896 and Y1α/Y3β” ion at m/z 916 were also detected. The existence of Y1α/Y2β or Y2α/Y1β ion at 449 (HexNAc-GalNAc-ol), but not at 408 (Hex-GalNAc-ol), indicated that this structure is based on a core 3 or 4 structure. The B3α ion at 534 indicated a fragment of (dHex)Hex-HexNAc. From these fragment ions and data of amino sugar analysis (GalNAc-ol:GalNAc:GlcNAc, 1.0:1.0:2.0), the structure was estimated to be NeuAc-(GalNAc-)Gal-GlcNAc-[(Fuc-)Gal-GlcNAc-]GalNAc-ol. Fig. 8b shows the one-dimensional1H NMR spectrum of IA1-8b (≈0.2 mg). β-anomeric resonances (4.69, 4.62, 4.53, 4.52 and 4.51 ppm) were recognized as one residue of the β-linked GalNAc, one of two residues of the β-linked Gal, two residues of the β-linked GlcNAc, and one residue of another β-linked Gal, respectively, by their coupling to a high-field H-2 resonance and the TOCSY spectrum. One lower-field α-anomeric resonance was also recognized as one residue of the α-linked Fuc by a method similar to that described above. The Fuc residue attached to the one β-linked Gal residue at position 2 (3.57 ppm), which could be proved by the lower-field changes in the HMQC spectrum (+5.6 ppm) of Gal [9]. The glycosylation shifts were observed at positions 3 and 6, positions 3 and 4, and position 4 of the GalNAc-ol, another β-linked Gal and two residues of the β-linked GlcNAc, respectively, on the basis of the13C chemical shift assessment (Table 3). The structure was confirmed by the mild periodate oxidation: two fragments, corresponding to NeuAc-(GalNAc)Gal-GlcNAc-O–CH2–CHO and Fuc-Gal-GlcNAc-O–CH(CHO)–CH(NHCOCH3)–CH2–CHO, were obtained from IA1-8b. The structure of IA1-8b was estimated as follows:
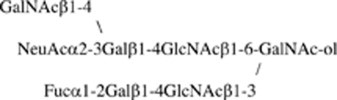
2.4. Structural analysis of other sialylated oligosaccharides
Because IA1-6 and IA1-7 also reacted with HCM31, MS/MS analyses of sialylated oligosaccharides contained in these fractions were achieved after the first-step HPLC. The fragmentation spectrum of IA1-6 ( m/z 1427 as [M+Na]+) included the series of Bi ions at m/z 591, 693 and 896 and Yi ion at m/z 916 (data not shown), which indicated the presence of the sequence, NeuAc-(HexNAc)Gal-GlcNAc-GalNAc-ol. Y ions at m/z 408 and 449 indicated that this oligosaccharide had a core 2 structure. Therefore, the IA1-6 may include the oligosaccharide Fuc-Gal-[NeuAc-(HexNAc-)Gal-GlcNAc-]GalNAc-ol, having core 2 and a terminal sequence, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ-. Although IA1-7 reacted with HCM31, the MS/MS data did not allow reconstruction of the sequence owing to the low intensity of the fragment ions.
MS/MS analyses of sialylated oligosaccharides in UA1-1 to -8 and IA1-1 to -4, none of which reacts with HCM31, were also achieved (Table S1). The fragmentation spectra for the sialylated oligosaccharides showed that all oligosaccharides contained Y ion at m/z 408, which indicated the presence of a core 1 or 2 structure, but not at 591 (HexNAc-Hex-HexNAc) and 896 (NeuAc-(HexNAc-)Hex-HexNAc). These results suggest that the HCM31-unreactive sialylated oligosaccharide fractions contain neither the core 4 structure nor NeuAc-(GalNAc-)Gal-GlcNAc- sequence. This result strongly suggests that the core 4 oligosaccharides having a tetrasaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ- (Sda antigen), include the epitope of HCM31.
2.5. Comparison of antigenic specificities of HCM31 with anti-Sda and anti-GM2 monoclonal antibodies
Because the Sda antigen, NeuAcα2-3(GalNAcβ1-4)Galβ1-[4GlcNAcβ]-, has a common terminal trisaccharide sequence with GM2 ganglioside, NeuAcα2-3(GalNAcβ1-4)Galβ1-[4Glcβ-Cer], the antigenic specificity of HCM31 was compared with previously established mAbs, GMB28 [18] and KM694 [19,20]. GMB28 specifically reacts with GM2 ganglioside. KM694 has been shown to detect Sda active carbohydrate epitopes, in addition to GM2 ganglioside, efficiently. We found that KM694 bound to the mucin obtained from N.b-infected rats as well as HCM31 did, and the reactivity of KM694 to infected mucin was higher than that obtained from non-infected rats (Fig. S1a). A commercial GM2 ganglioside was recognized by GMB28 and KM694, but not by HCM31 (Fig. S1b). The antigenic specificities of these mAbs are summarized in Table S2.
2.6. Immunohistochemical study of human gastrointestinal tract with HCM31
Fig. 9 shows the immunohistochemical reactivity of HCM31 with various sections of human gastrointestinal mucosa. There were no epithelial cells stained with HCM31 in the epithelia of the esophagus, gastric fundus (data not shown), gastric pylorus (Fig. 9a) and duodenum (Fig. 9b). The bronchial mucosa was also negative with this mAb (data not shown). In the upper part of crypt of the right-side colon (cecum), the goblet cells were only partly stained with HCM31 (Fig. 9c). The change of stainability was less apparent on the cancer tissues of cecum. In the left-sided colon (sigmoid colon, Fig. 9d; and rectum, data not shown), most goblet cells showed immunoreactivity with HCM31. A deeper upper gradient with enhanced upper expression was evident. HCM31-reactive goblet cells decreased with malignant change in the sigmoid colon (Fig. 10) and the rectum (data not shown). The results obtained in normal and cancer human gastrointestinal tracts are summarized in Table S3.
Fig. 9.
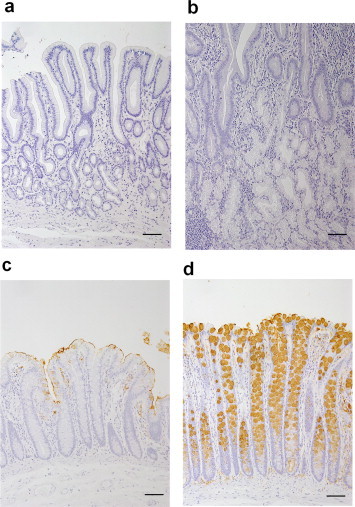
Immunostaining of the human gastrointestinal mucosae with HCM31. Immunostaining of the normal tissues [pylorus (a), duodenum (b), cecum (c) and sigmoid colon (d)]. Original magnification 50×. Bars = 50 μm.
Fig. 10.
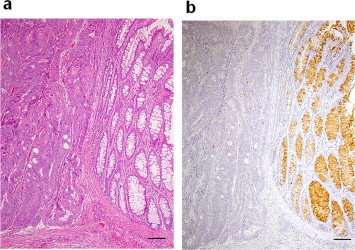
Histochemical observation of the human colonic mucosa with malignant change. H&E staining (a) and immunostaining with HCM31 (b) for a sigmoid colon cancer specimen. The malignant change is seen on the left side of the picture, while normal mucosal structure is seen on the right side. Original magnification 50×. Bars = 50 μm.
3. Discussion
In the present study, two HCM31-reactive sialylated oligosaccharides were obtained from the small intestinal mucin of N.b-infected rats. These were completely characterized as core 4 hexa- and octa-saccharides both having Sda blood group antigen, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ-. To our knowledge, the core 4 oligosaccharides having the Sda antigen have not been described previously. This study indicates that the Sda tetrasaccharide sequence is necessary as an epitope determinant of HCM31 for the following reasons. (a) The two HCM31-reactive oligosaccharides, IA1-5d and IA1-8b, contain this common tetrasaccharide sequence. (b) The reduction in the reactivity with HCM31 of the mucin by the neuraminidase treatment showed that HCM31 recognized the sialylated oligosaccharides (Fig. 1). (c) Because HCM31 did not react with GM2 ganglioside, NeuAcα2-3(GalNAcβ1-4)Galβ1-4Glcβ-Cer (Fig. S1), the GlcNAcβ residue on the Sda antigen is a necessary part of the epitope. This idea is also supported by the fact that HCM31 did not react with IA1-2 and -3 (Fig. 3b), which seemed to include the Sda-like trisaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ-, without the GlcNAcβ residue (Table S1) [21]. (d) HCM31 did not react with the oligosaccharide fractions IA1-1 to -4 obtained from N.b-infected animals, and all the UA1 fractions, UA1-1 to -8, obtained from the uninfected animals, fractionated by the first-step HPLC. The MS/MS analysis showed that the fragment ions derived from the Sda antigen could not be detected in these fractions (Table S1). (e) The sialylated oligosaccharide, Fuc-Gal-[NeuAc-Gal-GlcNAc-]GalNAc-ol, in UA1-5 of uninfected rat mucin seemed to include NeuAcα2-3Galβ1-4GlcNAcβ- sequence (Table S1). Thus, the GalNAc residue linked to Gal in the NeuAcα2-3Galβ1-4GlcNAc sequence via β1-4 linkage could be required for sufficient reaction with HCM31.
Both IA1-5d and IA1-8b have a core 4 structure. However, the core 4 structure may be unnecessary as an epitope determinant of HCM31. Although HCM31 was developed using human colonic mucin as an antigen [13], the Sda antigens are expressed on core 3 oligosaccharides, but not on core 4 oligosaccharides, in human colonic mucin [22]. Therefore, HCM31 may also recognize core 3 oligosaccharide having Sda antigen. Furthermore, HCM31 may recognize core 2 oligosaccharide having the Sda antigen in fraction IA1-6 from the infected rat mucin. Thus, the type of core structure seems not to relate to the reactivity with HCM31. In conclusion, we propose that HCM31 recognizes the tetrasaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ-.
Some mAbs against Sda antigen, CT1 and CT2, were developed by Conzelmann and Lefrancois [23], which react with a terminal trisaccharide sequence, NeuAcα2-3(GalNAcβ1-4)Galβ-. Previously reported KM694 [19] and KM531 [24] react with both GM2 ganglioside and a neolacto-series Sda glycolipid, which share oligosaccharide NeuAcα2-3(GalNAcβ1-4)Galβ structures at their non-reducing terminals. Therefore, the epitope determinant of KM694 and KM531 seems to be the trisaccharide sequence, the same as that of CT1 and CT2. In the present study, we showed that KM694 also recognized the mucins (Fig. S1). On the other hand, HCM31, developed using human colonic mucin as an immunogen, reacted with rat goblet cell mucins having Sda, but not GM2. The difference of antigenic activities is assumed to be attributable to their epitope saccharide sequences. HCM31 and KM694 probably recognize the terminal tetrasaccharide and trisaccharide without inner GlcNAcβ, respectively, of the Sda tetrasaccharide attached to the mucin. As an anti-GM2 mAb, GMB28, did not react with the mucins (Fig. S1), the Glcβ residue on GM2 seems to be a necessary part of the epitope for this mAb. Although the biological function of Sda tetrasaccharide on mucin remains unknown, HCM31 will contribute to clarify this issue.
The Sda antigen expressed as a glycolipid on chief cells disappeared along with the malignant changes in human gastric mucosa [20]. Kawamura et al. [19] reported that metastasis of human gastrointestinal cancer cells was reduced by expression of the Sda antigen on their surface. In the present study, HCM31 did not stain the epithelial cells on the human gastric mucosa. The tissue fixation method used in our study might not fix the membrane glycolipids including Sda antigen. Meanwhile, HCM31 extensively stained the secretory granules of goblet cells in sigmoid colon and rectum (Fig. 9). Furthermore, the stainability with HCM31 disappeared in the cancerous state of these tissues. Thus, HCM31 might be useful as a negative marker for colonic cancer. This is consistent with the data that Sda antigen on core 3-type mucin oligosaccharide [22] and β1-4-N-acetylgalactosamine-transferase (β1-4GalNAcT), responsible for the Sda antigen biosynthesis, are co-expressed on human colonic mucosa, but this expression declines along with malignant changes [25]. However, it is still unknown how the secretory-type mucin having this antigen acts on the gastrointestinal mucosa. HCM31 seems to be useful for examining the function of secretory-type mucin with Sda antigen.
Recently, we reported that goblet cell sialomucins that reacted with HCM31 in the rat jejunal mucosa increased up to 16 days after N.b infection, the time when the worms were expelled from the rats [16]. No similar change could be observed by another mAb, PGM34, which extensively stains goblet cells in the rat small intestine, and recognizes sulfated oligosaccharide [26]. Karlsson et al. [21] reported that sialylated tetra- and penta-saccharides having Sda-like trisaccharide, NeuAc/NeuGcα2-3(GalNAcβ1-4)Galβ-, were expressed in small intestinal mucin of N.b-infected rats. In the present study, NeuAc/NeuGc-(HexNAc-)Gal-[GlcNAc-]GalNAc-ol appeared in A1 fraction by N.b infection in addition to Sda-containing oligosaccharides. Thus, sialomucins with Sda antigen-related oligosaccharides are associated with worm expulsion. The present study also showed that the core 4 mucin-type oligosaccharides having sialic acid with or without Sda sequence are expressed upon infection (Fig. 5 and Table S1). β1-3GlcNAc-transferase (β1-3GlcNAcT) and β1-6GlcNAc-transferase (β1-6GlcNAcT) are needed for the synthesis of core 4 glycan [27]. Therefore, expression of these transferases may increase with infection, but further studies are needed to confirm these changes.
In summary, HCM31 is a very useful tool for recognizing the characteristic sialomucin bearing the Sda blood group antigen in immunochemical and immunohistochemical methods. Goblet cells producing the specific sialomucins increased during process of expulsion of parasitic nematode in rat small intestine and decreased with malignant change in human colon. Further research using this mAb could elucidate the biological significance of sialomucins containing the Sda tetrasaccharide in health and disease.
4. Materials and methods
4.1. Reagents
Bio-Gel A-1.5m was obtained from Bio-Rad Laboratories (Richmond, CA, USA). Dowex-50 was purchased from Dow Chemical Company (Midland, MI, USA). TOYOPEARL QAE-550C resin and TSKgel Amide-80 column were purchased from Tosoh Co. Inc. (Tokyo, Japan). A graphitized carbon column (GL-Pak Carbograph, 150 mg/3 ml) was obtained from GL-Science (Tokyo, Japan).
The mAb, HCM31 (antibody subclass IgG1 κ), was prepared as previously described [13]. The anti-GM2 mAb, GMB28, was kindly provided by Drs. I. Kawashima and K. Ogura (Tokyo Metropolitan Institute of Medical Science). The anti-Sda mAb, KM694, was kindly provided by Tokyo Research Park, Kyowa Hakko Kirin Co. Ltd. (Machida, Japan).
4.2. Preparation of small intestinal mucin from uninfected and N.b-infected rats
N.b was maintained in our laboratory as previously described [16]. Eight-week-old male Wistar rats (weighing 200–250 g, SLC, Shizuoka, Japan) were used. The rats were inoculated subcutaneously with the third-stage larvae (L3) of N.b (2000 L3/rat). The rats were sacrificed after 24 h of fasting at 16 days after the inoculation, and their small intestines were removed. The small intestines were also obtained from uninfected rats. The small intestines were successively cut open, washed with saline and then lyophilized followed by weighing. Mucins were extracted from the pulverized small intestine using 2% Triton X-100 in 50 mM Tris–HCl (pH 7.2), isolated with a Bio-Gel A-1.5 m column and precipitated with ethanol as described previously [28]. This study was conducted in accordance with the guidelines of the Animal Laboratory Center of Kitasato University School of Medicine.
4.3. Preparation and purification of oligosaccharides from the rat intestinal mucin
Alkaline borohydride treatment of the purified rat small intestinal mucin was carried out according to the method of Carlson [29] with 0.05 M NaOH in 1.0 M NaBH4 at 50 °C for 24 h. After acidification, the reaction mixture was applied onto a column of Dowex-50 (H+-form) to remove sodium and the peptides. The passed fractions were evaporated and borate was removed by repeated evaporation with acidified methanol. After being redissolved in water, the oligosaccharide solution was subsequently applied onto an anion-exchange column of TOYOPEARL QAE-550C. The column was washed with distilled water and eluted with a linear gradient of 0–0.6 M NaOAc. The effluent was monitored by hexose content [30]. The fractions were collected and desalted on a graphitized carbon column using 25% acetonitrile for the neutral oligosaccharides or 25% acetonitrile containing 0.05% trifluoroacetic acid for the acidic ones as eluting solvent [31].
4.4. Normal-phase HPLC
Two-step normal-phase HPLC using TSKgel Amide-80 columns was employed. In the first step, the columns (7.8 mm × 300 mm × 2 columns) were equilibrated with 68% (v/v) CH3CN in 2.5 mM NaH2PO4, and the gradient was initiated after injection and decreased to 55% CH3CN over 30 min at a flow rate of 2.0 ml/min. In the second step (4.6 mm × 250 mm × 2 columns), the fractions obtained from the first-step HPLC were rechromatographed under isocratic conditions of 74% CH3CN for 1 h at a flow rate of 1.0 ml/min. UV absorption of the effluent solution was monitored at 210 nm. For the removal of NaH2PO4, the fractions were chromatographed using a graphitized carbon column as described above.
4.5. Amino sugar analysis
Amino sugar analysis was performed as previously described [32] after the oligosaccharides were hydrolyzed with 6 M HCl at 98 °C for 4 h using the Waters’ Pico-Tag Workstation.
4.6. Mass spectrometry
The molecular masses of the oligosaccharides were analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF/MS) using an autoflex III TOF/TOF instrument (Bruker Daltonik GmbH, Bremen, Germany) in reflector mode by summarizing 1000 signal spectra (5 × 200 shots) with a 50 Hz laser applying the following instrument settings: ion source 1: 19.00 kV, ion source 2: 16.60 kV, lens: 8.55 kV, reflector 1: 21.00 kV, reflector 2: 9.70 kV, reflector detector: 1400 V, suppression up to 400 Da by deflection. Then, MS/MS spectra were further measured with an Autoflex III TOF/TOF instrument in LIFT (MS/MS) mode using the following instrument settings: ion source 1: 6.00 kV, ion source 2: 5.30 kV, lens: 3.00 kV, reflector 1: 27.00 kV, reflector 2: 11.65 kV, lift 1: 19.00 kV, lift 2: 4.20 kV, reflector detector: 1400 V. Each sample was mixed with an equal volume of 2,5-dihydroxy benzoic acid dissolved in distilled water/CH3CN (1:1, v/v) at 10 mg/ml as the matrix solutions [33]. Two μl of this mixture was then applied to a stainless steel target plate and air-dried at room temperature before the target was introduced into the spectrometer.
4.7. Methylesterification of sialylated oligosaccharides
Sialylated oligosaccharides were methylesterified for MS/MS analysis in accordance with the procedure by Powell and Harvey [34]. The oligosaccharides were dissolved in 1 μl of dimethyl sulfoxide, mixed with 1 μl of methyl iodide and allowed to react for 2 h at room temperature. Unreacted methyl iodide was removed and dried under a gentle stream of nitrogen.
4.8. NMR spectroscopy
The NMR spectra were obtained using Varian INOVA 600 or Varian NMR System 600 NMR spectrometer (Varian Associates, Palo Alto, CA, USA) equipped with a1H{15N–31P} pulse field gradient (PFG) indirect-detecting probe. Standard pulse sequences were used throughout. The proton NMR spectrum was assigned through PFG multiple-quantum-correlation spectroscopy and one-dimensional HOHAHA spectroscopy. The13C assignments were made from an HMQC spectrum obtained with carbon decoupling. The lyophilized powder of the purified oligosaccharides was dissolved in deuterium oxide (2H2O) and evaporated to exchange the unstable1H with2H. The evaporation and dissolution were repeated five times and the sample was finally dissolved in 0.04 or 0.15 ml of2H2O and then subjected to NMR spectroscopy using nano-probe or Shigemi tube, respectively. Chemical shifts of the reduced oligosaccharide structures were identified with reference to those described by Kamerling and Vliegenthart [17]. NMR spectra of α-methyl-N-acetylneuraminic acid (Nagara Science, Gifu, Japan) and β-para-nitrophenyl-N-acetylgalactosamine (Glycosynth, Cheshire, England) were utilized as references for chemical shift assignment. The NMR spectral data of the standard α and β methylated monosaccharides as well as those of GalNAc-ol reported by Ishihara et al. [9] were also utilized as references for the chemical shift assignment.
4.9. Mild periodate oxidation of oligosaccharides
To cleave the C–C bond of the reducing terminal GalNAc-ol at positions C4 and C5, mild periodate oxidation of oligosaccharides was performed according to the methods of Chai et al. [35]. The oligosaccharides (approx. 10 μg) were oxidized with sodium periodate in imidazole buffer, pH 6.5, at 0 °C for 5 min. After excess periodate was destroyed by incubation with butane-2,3-diol at 0˚C for 40 min, the oligosaccharides were purified with a column of graphitized carbon.
4.10. ELISA and competitive ELISA
The microtiter plates were coated with 2–200 ng of the purified mucin and kept overnight at 4 °C followed by blocking with 2% skimmed milk [8]. In case of using 2–200 ng of GM2 ganglioside (Alexis Corporation, Lausen, Switzerland) as antigen, the coated wells were blocked with 1% BSA for overnight. After the wells were washed, a definite amount of HCM31, KM694 or GMB28 was added to each well followed by incubation at ambient temperature for 1 h. The wells were successively incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulins (Dako, Kyoto, Japan) and 2,2′-azino bis-[3-ethylbenzthiazoline-6-sulfonate] (ABTS)/H2O2 solution (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA), and the color was allowed to develop. The wells were washed three times with PBS containing 0.05% Tween-20 for mucin or PBS for GM2 between each process. The UV absorption was measured at 405 nm (reference at 492 nm) at 30 min thereafter using a Bio-Rad Model 550 microplate reader.
A competitive ELISA was applied to detect the reactivity of HCM31 with the oligosaccharide fractions. The microtiter plates coated with 100 ng of the purified mucin followed by blocking with 2% skim milk were prepared as previously mentioned. At the same time, PBS solutions of the oligosaccharide fraction, each containing 0.15–10 μg or 0.15–75 nmol per well as the hexose basis or the GalNAc-ol basis, respectively, were pre-incubated with a definite amount of HCM31 for 2 h at ambient temperature. Instead of the sugar-containing solution, PBS was pre-incubated with HCM31 as the negative control. The pre-incubated mixtures were then added to the antigen-coated wells and incubated for 1 h. The remaining ELISA steps were the same as already described.
4.11. Periodate treatment and trypsin digestion of mucins
Periodate treatment was performed by exposing the mucin coated on the microtiter wells to 0.1–2.5 mM NaIO4 in 50 mM sodium acetate, pH 4.5, for 1 h at room temperature. Trypsin digestion was performed by exposing the mucin coated on the microtiter wells to trypsin for 1 h at 37 °C. Trypsin at 2.5 mg/ml in 10 mM Tris–HCl, pH 8.0, containing 2 mM CaCl2 was used with two-fold serial dilution. Each of the remaining ELISA steps was the same as already described.
4.12. Immunohistochemistry
Human tissues (20% formalin-fixed, routinely processed, and paraffin-embedded) were selected from the surgical pathology files of the Department of Laboratory Medicine, Shinshu University Hospital, Matsumoto, Japan. The following histologically normal tissues were selected: bronchi (n = 6), esophagus (n = 5), gastric fundic mucosa (n = 6), gastric pyloric mucosa (n = 6), duodenum (n = 6), cecum (n = 4), sigmoid colon (n = 4) and rectum (n = 1). The cancer tissues of cecum (n = 5), sigmoid colon (n = 4) and rectum (n = 1) were also selected. In addition, the proximal half of rat small intestine, defined as the jejunum, was resected and immediately fixed for 3 h in freshly prepared Carnoy's solution according to the method described previously [36]. After fixation, the jejunum was routinely processed and embedded in paraffin.
From these specimens, 3 μm paraffin sections were prepared for immunostaining with HCM31. Immunohistochemical staining was performed using the immuno-enzyme polymer method (Histofine Simple Stain MAX PO Multi, Nichirei Biosciences, Tokyo, Japan) with 3,3-diaminobenzidine as the chromogen. Briefly, endogenous peroxidase activity was blocked with 0.3% H2O2, then the specimens were sequentially incubated with the mAb, HRP-conjugated immuno-enzyme polymer, and 0.02% 3,3-diaminobenzidine in 50 mM Tris–HCl, pH 7.6, containing 0.005% H2O2. Counterstaining was performed with hematoxylin.
4.13. Sialidase treatment and de-O-acetylation for the specimens
Enzymatic treatment was performed by exposing the specimens for immunostaining to 0.1 units of α2-3,6,8-sialidase from Arthrobacter ureafaciens (Nacalai Tesque, Kyoto, Japan) in 10 mM sodium phosphate, pH 5.0, for 4 h at 37 °C.
De-O-acetylation treatment of sialic acid was performed by exposing the specimens to 0.2 M NaOH at 37 °C for 2 h.
Acknowledgements
We would like to express our sincere appreciation to Ms. S. Sugawara and Ms. F. Mikami for their valuable technical assistance. We also thank Drs. I. Kawashima and K. Ogura for kindly providing us with the anti-GM2 antibody. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (to K.I.), and by grants from the Kitasato University Integrative Research Program of the Graduate School of Medical Sciences (to D.T. and to Y.G.) and School of Allied Health Sciences (to K.I. No. 2010-1016).
Supplementary Material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2012.07.006.
Appendix. Supplementary material
The reactivity of small intestinal mucins or GM2 ganglioside with anti-GM2 and anti-Sda monoclonal antibodies and HCM31. The antigenic activities of various amounts of small intestinal mucins (a), obtained from non-infected (open symbols) or N.b-infected (solid symbols) rats, or GM2 (b: open symbols) with three mAbs, GMB28 (circle), KM694 (triangle) and HCM31 (square), were examined by ELISA as described in “Experimental procedures”. Data are expressed as relative absorbance value compared to the highest absorbance in the experiment expressed as 1.0, and as mean ± SD from three experiments.
Sialylated oligosaccharide structures of rat small intestinal mucins after the first-step HPLC: identified by MALDI-TOF/MS/MS
Antigenic specificities of monoclonal antibodies.
Reactivity of HCM31 with gastrointestinal tissues obtained from human.
References
- 1.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.De Bolós C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 3.Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka Y, Pascall JC, Brown KD. Quantitative analysis reveals differential expression of mucin (MUC2) and intestinal trefoil factor mRNAs along the longitudinal axis of rat intestine. Biochim. Biophys. Acta. 1999;1489:336–344. doi: 10.1016/s0167-4781(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 5.Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y. A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem. J. 1991;23:22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- 6.Robbe C, Capon C, Coddeville B, Michalski JC. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004;384:307–316. doi: 10.1042/BJ20040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara K, Kurihara M, Eto H, Kasai K, Shimauchi S, Hotta K. A monoclonal antibody against carbohydrate moiety of rat gastric surface epithelial cell-derived mucin. Hybridoma. 1993;12:609–620. doi: 10.1089/hyb.1993.12.609. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara K, Kurihara M, Goso Y, Ota H, Katsuyama T, Hotta K. Establishment of monoclonal antibodies against carbohydrate moiety of gastric mucins distributed in the different sites and layers of rat gastric mucosa. Glycoconj. J. 1996;13:857–864. doi: 10.1007/BF00702350. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K. Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem. J. 1996;318:409–416. doi: 10.1042/bj3180409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- 11.Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J. Essential role of gastric gland mucin in preventing gastric cancer in mice. J. Clin. Invest. 2012;122:923–934. doi: 10.1172/JCI59087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 13.Ikezawa T, Goso Y, Ichikawa T, Hayashida H, Nakamura T, Kurihara M, Okayasu I, Saigenji K, Ishihara K. Immunohistochemical localization in rat gastrointestinal tract of a sialomucin species recognized by HCM31, a new anti-mucin monoclonal antibody. Biomed. Res. 2002;23:63–68. [Google Scholar]
- 14.Saegusa Y, Ichikawa T, Iwai T, Goso Y, Okayasu I, Ikezawa T, Shikama N, Saigenji K, Ishihara K. Changes in the mucus barrier of the rat during 5-fluorouracil-induced gastrointestinal mucositis. Scand. J. Gastroenterol. 2008;43:59–65. doi: 10.1080/00365520701579662. [DOI] [PubMed] [Google Scholar]
- 15.Iwai T, Ichikawa T, Goso Y, Ikezawa T, Saegusa Y, Okayasu I, Saigenji K, Ishihara K. Effects of indomethacin on the rat small intestinal mucosa: immunohistochemical and biochemical studies using anti-mucin monoclonal antibodies. J Gastroenterol. 2009;44:277–284. doi: 10.1007/s00535-009-0007-0. [DOI] [PubMed] [Google Scholar]
- 16.Tsubokawa D, Nakamura T, Goso Y, Takano Y, Kurihara M, Ishihara K. Nippostrongylus brasiliensis: increase of sialomucins reacting with anti-mucin monoclonal antibody HCM31 in rat small intestinal mucosa with primary infection and reinfection. Exp. Parasitol. 2009;123:319–325. doi: 10.1016/j.exppara.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Kamerling JP, Vliegenthart JFG. High-resolution1H-nuclear magnetic resonance spectroscopy of oligosaccharides released from mucin-type O-glycoproteins. Biol. Magn. Reson. 1992;10:1–287. [Google Scholar]
- 18.Kotani M, Ozawa H, Kawashima I, Ando S, Tai T. Generation of one set of monoclonal antibodies specific for a-pathway ganglio-series gangliosides. Biochim. Biophys. Acta. 1992;1117:97–103. doi: 10.1016/0304-4165(92)90168-t. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura YI, Kawashima R, Fukunaga R, Hirai K, Toyama-Sorimachi N, Tokuhara M, Shimizu T, Dohi T. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005;65:6220–6227. doi: 10.1158/0008-5472.CAN-05-0639. [DOI] [PubMed] [Google Scholar]
- 20.Dohi T, Kawamura YI. Incomplete synthesis of the Sda/Cad blood group carbohydrate in gastrointestinal cancer. Biochim. Biophys. Acta. 2008;1780:467–471. doi: 10.1016/j.bbagen.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson NG, Olson FJ, Jovall PA, Andersch Y, Enerbäck L, Hansson GC. Identification of transient glycosylation alterations of sialylated mucin oligosaccharides during infection by the rat intestinal parasite Nippostrongylus brasiliensis. Biochem. J. 2000;350:805–814. [PMC free article] [PubMed] [Google Scholar]
- 22.Capon C, Maes E, Michalski JC, Leffler H, Kim YS. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J. 2001;358:657–664. doi: 10.1042/bj3580657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conzelmann A, Lefrancois L. Monoclonal antibodies specific for T cell-associated carbohydrate determinants react with human blood group antigens CAD and SDA. J Exp Med. 1988;167:119–131. doi: 10.1084/jem.167.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohi T, Ohta S, Hanai N, Yamaguchi K, Oshima M. Sialylpentaosylceramide detected with anti-GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosa. J. Biol. Chem. 1990;265:7880–7885. [PubMed] [Google Scholar]
- 25.Malagolini N, Dall'Olio F, Di Stefano G, Minni F, Marrano D, Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc alpha 2,3Gal beta-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466–6470. [PubMed] [Google Scholar]
- 26.Tsubokawa D, Goso Y, Sawaguchi A, Kurihara M, Ichikawa T, Sato N, Suganuma T, Hotta K, Ishihara K. A monoclonal antibody, PGM34, against 6-sulfated blood-group H type 2 antigen, on the carbohydrate moiety of mucin. FEBS J. 2007;274:1833–1848. doi: 10.1111/j.1742-4658.2007.05731.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwientek T, Nomoto M, Levery SB, Merkx G, van Kessel AG, Bennett EP, Hollingsworth MA, Clausen H. Control of O-glycan branch formation. Molecular cloning of human cDNA encoding a novel beta1,6-N-acetylglucosaminyltransferase forming core 2 and core 4. J. Biol. Chem. 1999;274:4504–4512. doi: 10.1074/jbc.274.8.4504. [DOI] [PubMed] [Google Scholar]
- 28.Azuumi Y, Ichikawa T, Ishihara K, Hotta K. The validity of the ethanol precipitation method for the measurement of mucin content in human gastric juices and its possible relationship to gastroduodenal diseases. Clin. Chim. Acta. 1993;221:219–225. doi: 10.1016/0009-8981(93)90037-5. [DOI] [PubMed] [Google Scholar]
- 29.Carlson DM. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J. Biol. Chem. 1968;243:616–626. [PubMed] [Google Scholar]
- 30.Dubois M, Gilles KA, Hamilton JK, Roebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 31.Packer NH, Lawson MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 1998;15:737–747. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara K, Kameyama J, Hotta K. Development of an HPLC method to estimate hexosamines and its application to determine mucin content in rat and human gastric mucosa. Comp. Biochem. Physiol. B. 1993;104:781–786. doi: 10.1016/0305-0491(93)90213-o. [DOI] [PubMed] [Google Scholar]
- 33.Harvey DJ. Quantitative aspects of the matrix-assisted laser desorption mass spectrometry of complex oligosaccharides. Rapid Commun. Mass Spectrom. 1993;7:614–619. doi: 10.1002/rcm.1290070712. [DOI] [PubMed] [Google Scholar]
- 34.Powell AK, Harvey DJ. Stabilization of sialic acids in N-linked oligosaccharides and gangliosides for analysis by positive ion matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1996;10:1027–1032. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1027::AID-RCM634>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Chai W, Stoll MS, Galustian C, Lawson AM, Feizi T. Neoglycolipid technology: deciphering information content of glycome. Methods Enzymol. 2003;362:160–195. doi: 10.1016/S0076-6879(03)01012-7. [DOI] [PubMed] [Google Scholar]
- 36.Ota H, Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem. J. 1992;24:86–92. doi: 10.1007/BF01082444. [DOI] [PubMed] [Google Scholar]
- 37.Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988;5:397–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The reactivity of small intestinal mucins or GM2 ganglioside with anti-GM2 and anti-Sda monoclonal antibodies and HCM31. The antigenic activities of various amounts of small intestinal mucins (a), obtained from non-infected (open symbols) or N.b-infected (solid symbols) rats, or GM2 (b: open symbols) with three mAbs, GMB28 (circle), KM694 (triangle) and HCM31 (square), were examined by ELISA as described in “Experimental procedures”. Data are expressed as relative absorbance value compared to the highest absorbance in the experiment expressed as 1.0, and as mean ± SD from three experiments.
Sialylated oligosaccharide structures of rat small intestinal mucins after the first-step HPLC: identified by MALDI-TOF/MS/MS
Antigenic specificities of monoclonal antibodies.
Reactivity of HCM31 with gastrointestinal tissues obtained from human.


