Abstract
Antimalarial chloroquine is also used for the treatment of immune-mediated diseases. The interference of chloroquine with interferon-γ-induced tryptophan breakdown and neopterin production has been investigated in human peripheral blood mononuclear cells (PBMC) in vitro. Micromolar concentrations (2–50 μM) of chloroquine dose-dependently suppressed mitogen-induced tryptophan breakdown in PBMC but not in the myelomonocytic THP-1-Blue cell line, after 48 h of treatment. In stimulated PBMC, neopterin production was super-induced by 10 μM chloroquine, while it was significantly suppressed at a concentration of 50 μM. These anti-inflammatory effects may relate to the therapeutic benefit of chloroquine in inflammatory conditions and may widen the spectrum of its clinical applications.
Keywords: Chloroquine; Indoleamine (2,3)-dioxygenase (IDO); Tryptophan; Neopterin; PBMC
Highlights
▸ 2–50 μM chloroquine suppresses mitogen-induced tryptophan breakdown in human PBMCs. ▸ The same effect was not seen in the myelomonocytic THP-1-Blue cell line. ▸ The anti-inflammatory property of chloroquine targets T cells more than monocytes. ▸ This anti-inflammatory effect may explain the drug's therapeutic benefit for malaria. ▸ Chloroquine treatment could be of benefit for other chronic inflammatory conditions.
1. Introduction
Since the 1930s, chloroquine [(RS)-N′-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine] is a widely used antimalarial drug, mainly due to its relatively good tolerability and the cost-efficient synthesis [1,2]. Upon cellular uptake, chloroquine accumulates in acidic organelles such as endosomes, lysosomes and in Golgi vesicles, where it interferes with the activity of enzymes and posttranslational protein modification steps [3,4]. Furthermore, drug accumulation decreases the intracellular iron concentration and induces oxidative stress [5,6]. Accumulated in the digestive vacuoles of parasite affected cells, chloroquine inhibits the malaria parasite's digestive pathway for hemoglobin [7]. The development of resistant Plasmodium strains has now led to the replacement of the first-line treatment and prophylaxis with chloroquine and its derivatives with other therapeutics such as artesimin [8,9]. However, chloroquine and analogs became interesting as treatment options for other immune-related disorders, due to their immunomodulatory properties, e.g. by interfering with pro-inflammatory cytokine secretion [10,11]. On the basis of its anti-inflammatory properties, chloroquine and hydroxychloroquine are used for the treatment of rheumatoid arthritis, discoid lupus erythematosus, amebic hepatitits and chronic Q fever [2,12–15].
The pro-inflammatory cytokine interferon-γ (IFNγ) plays a central role in the cellular immune response, as it induces several immune-regulatory pathways and cellular responses [16,17]. Inflammation is further characterized through the activation of the redox-sensitive transcription factor nuclear factor-kappa B (NF-κB). NF-κB regulates a variety of genes that control immune responses such as the pro-inflammatory cytokines [18]. IFNγ stimulates also the production of neopterin by guanosine triphosphate (GTP)-cyclohydrolase-I (GTP-CH-I, EC 3.5.4.16) in macrophages [19]. Likewise, neopterin was found to support oxidation processes by reactive oxygen and chloride metabolites [20] as well as their formation in inflammatory cells like neutrophils [21]. In turn, in target cells redox-sensitive signaling cascades such as nuclear factor-κB are triggered by neopterin [22]. IFNγ signaling is also involved in the activation of indoleamine 2,3-dioxygenase (IDO, EC 1.13.11.52), the enzyme that catalyses the rate-limiting step in the conversion of tryptophan to kynurenine [23]. Neopterin levels, tryptophan concentrations and IDO activity have been successfully used to monitor cell-mediated immune activation and to reveal prognostic information in a variety of diseases, including rheumatoid arthritis [24–26].
Elevated concentrations of urine and serum neopterin have been detected in patients infected with Plasmodium falciparum and Plasmodium vivax from epidemiologically distinct populations [27–30]. Similarly, increased breakdown of tryptophan has been reported in a murine malaria model [31].
A more detailed analysis of potential interactions of chloroquine with interferon-γ-induced tryptophan breakdown and neopterin production might help to explain the beneficial effects for the treatment of rheumatic diseases and might introduce new therapeutic regimen for disorders that are associated with increased immune activation.
Therefore, the aim of this in vitro study was to investigate the immunomodulatory properties of chloroquine in human peripheral blood mononuclear cells (PBMC) and in THP-1-Blue cells. Mitogen-stimulated PBMC represent a widely used model to evaluate pro- and anti-inflammatory properties of compounds, where neopterin production and tryptophan degradation can be used as read-outs [32]. The THP-1-Blue cell line is transfected with a NF-κB/AP-1-inducible reporter system that allows the monitoring of NF-κB activity in cell supernatants. In this cell line, lipopolysaccharide (LPS)-induced NF-κB expression has been reported to correlate with neopterin production and IDO activity [33]. Further, the production of soluble interleukin 2 receptor alpha (sIL-2Rα) was used to monitor the influence of chloroquine on the inflammatory process [32,34].
2. Materials and methods
2.1. Chemicals
Lipopolysaccharide (LPS) of Escherichia coli, phytohemagglutinin (PHA) and chloroquine were obtained from Sigma–Aldrich (Vienna, Austria). The latter was dissolved in RPMI 1640 medium (MedPro, Vienna, Austria) before each experiment.
2.2. Isolation and culture of human PBMC
PBMC were isolated from whole blood obtained from healthy donors by density centrifugation (Lymphoprep, Nycomed Pharma AS, Oslo, Norway). After isolation, PBMC were washed three times in phosphate buffered saline containing 0.5 mM EDTA. Cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS, Biochrom, Berlin, Germany), 2 mM l-glutamine (Serva, Heidelberg, Germany) and 50 μg/ml gentamicin (Bio-Whittaker, Walkersville, MD) at 37 °C with 5% CO2.
2.3. THP-1-Blue cell culture
THP-1-Blue cells (Invivogen, San Diego, USA) were incubated at 37 °C with 5% CO2 in RPMI 1640 medium supplemented with 10% FCS and 200 μg/ml zeocin (Invivogen, San Diego, USA).
2.4. Cell treatment
PBMC were seeded at a density of 1.5 × 106 cells/ml in supplemented RPMI 1640, preincubated for 30 min with or without different concentrations of chloroquine (2.0–50 μM) and stimulated, or not, with 10 μg/ml PHA for another 48 h. THP-1-Blue cells were seeded at a density of 5 × 105 cells/ml in supplemented RPMI 1640. Cells were preincubated for 30 min with different concentrations of chloroquine (6.25–50 μM) and stimulated or not with 1 μg/ml LPS.
Supernatants for Kyn/Trp determination were collected after 48 h, after this period the accumulated tryptophan breakdown and neopterin formation reaches a plateau [32]. Supernatants from THP-1-Blue cells for NF-κB activity measurement were collected for both, 24 and 48 h treatment duration. However, in agreement with earlier observations, the read-out at 24 h was able to better discriminate results obtained with different concentrations of compounds [33].
2.5. Measurement of tryptophan, kynurenine, neopterin and sIL-2Rα concentrations
Tryptophan, kynurenine, neopterin and cytokine measurements were performed in centrifuged supernatants. Tryptophan and kynurenine concentrations were measured by high performance liquid chromatography (HPLC) using 3-nitro-l-tyrosine as an internal standard [35]. To estimate IDO activity, Kyn/Trp was calculated and expressed as μmol kynurenine/mmol tryptophan.
Neopterin concentrations were determined by ELISA (BRAHMS, Hennigsdorf, Berlin, Germany) with a detection limit of 2 nM, IL-2Rα concentrations were measured by ELISA obtained from R&D (Biomedica, Vienna, Austria), both according to the manufacturer's instructions.
2.6. Measurement of NF-κB activity and cell viability in THP-1-Blue cells
THP-1-Blue cells are stably transfected with a reporter plasmid expressing the secreted embryonic alkaline phosphatase (SEAP) under the control of a NF-κB/AP-1 inducible promoter. Upon NF-κB activation, SEAP is expressed and released into the media, where the enzyme activity has been measured in a colorimetric assay at 635 nm (Bio-Tek Instruments, Bad Friedrichshall, Germany) by incubating 10% (v/v) of cell supernatant with 90% (v/v) of Quanti-Blue reagent (Invivogen, San Diego, USA).
Cell viability was measured by CellTiter-Blue® assay (Promega, Vienna, Austria) according to the manufacturer's instructions.
2.7. Statistical analysis
For statistical analysis, the Statistical Package for the Social Sciences (version 19, SPSS, Chicago, Ill, USA) was used. Because not all data sets showed normal distribution, for comparison of grouped data non-parametric Friedman test and Wilcoxon signed-ranks test were applied. p-Values below 0.05 were considered to indicate significant differences.
3. Results
3.1. Effect of chloroquine on tryptophan metabolism and neopterin formation in PBMC
After an incubation period of 48 h, neopterin concentrations (±SEM) in culture supernatants of unstimulated PBMC were 3.8 ± 0.1 nM and the mean Kyn/Trp ratio was 99.2 ± 46.6 μmol/mmol. Upon stimulation of cells with the phytohemagglutinin (PHA), neopterin production increased to 20.1 ± 1.4 nM and the mean Kyn/Trp was increased approximately 100-fold. The concentrations of neopterin, tryptophan, kynurenine and IDO activity, indicated by Kyn/Trp, in unstimulated and PHA-stimulated PBMC are listed in Table 1.
Table 1.
Concentrations of tryptophan, kynurenine, kynurenine to tryptophan ratio (Kyn/Trp) and neopterin in the supernatant of unstimulated PBMC and in cells stimulated with 10 μg/ml PHA for 48 h. Results shown are the mean values ± SEM of three independent experiments run in duplicates.
| Tryptophan (μM) | Kynurenine (μM) | Kyn/Trp (μmol/mmol) | Neopterin (nM) | |
|---|---|---|---|---|
| Unstimulated | 29.9 ± 6.2 | 2.4 ± 0.9 | 99.2 ± 46.6 | 3.8 ± 0.1 |
| PHA (10 μg/ml) | 1.9 ± 1.1* | 14.3 ± 0.7* | 12408 ± 4379* | 20.1 ± 1.4* |
p < 0.05, compared to unstimulated cells.
In unstimulated cells, tryptophan concentrations were not affected by chloroquine addition (2.0–50 μM of chloroquine, Fig. 1A), but kynurenine levels decreased in a dose-dependent manner (Fig. 1B). In PHA-stimulated PBMC, chloroquine suppressed tryptophan breakdown in a dose-dependent manner (Fig. 1A) and in parallel kynurenine levels declined (Fig. 1B). A reduction of the Kyn/Trp upon chloroquine treatment was dose-dependent in both, unstimulated and PHA-stimulated PBMC, with a more pronounced effect in PHA-stimulated cells (Fig. 1C).
Fig. 1.
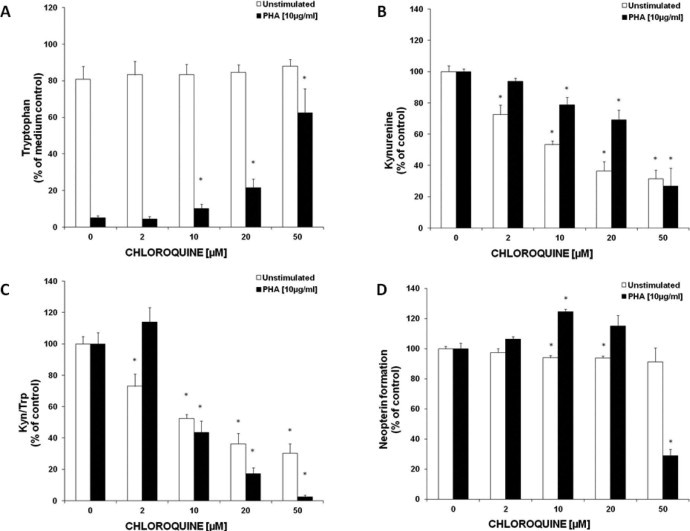
Effect of 48 h of chloroquine treatment on tryptophan (A) and kynurenine (B) concentrations, on IDO activity expressed as kynurenine to tryptophan ratio (Kyn/Trp) (C) and on neopterin production (D) in unstimulated (white bars) and PHA-stimulated PBMC (black bars), expressed as % of baseline (control cells treated with or without PHA, respectively). Results shown are the mean values ± SEM of three independent experiments run in duplicates (*p < 0.05, compared to cells without added chloroquine).
The addition of chloroquine to unstimulated PBMC resulted in an only slight suppression of neopterin concentrations in culture supernatants, e.g. 20 μM chloroquine suppressed neopterin production (±SD) to 93.8 ± 3.5% of baseline. In PHA-stimulated cells, the neopterin formation was significantly increased at a concentration of 10 μM chloroquine (124.5 ± 4.0%), while 50 μM of chloroquine resulted in a strong decrease of neopterin concentrations down to 29.0 ± 10.4% compared to PHA-stimulated control cells (Fig. 1D).
3.2. Effect of chloroquine on sIL2Rα secretion in PHA-stimulated PBMC
IL2Rα concentrations in cell culture supernatants were increased more than 100-fold in PHA-stimulated PBMC in comparison to unstimulated cells (p < 0.005), additional chloroquine treatment (10 and 50 μM) reduced this effect in a dose-dependent manner (p < 0.05 and 0.005 for 10 and 50 μM chloroquine- and PHA-stimulated PBMC in comparison to PHA-stimulated control, details not shown).
3.3. Effects of chloroquine in THP-1-Blue cells
Cytotoxicity of chloroquine was evaluated in THP-1-Blue cells treated with increasing doses for 24 h, with or without additional stimulation by lipopolysaccharide (LPS). Treatment resulted in a dose-dependent decrease of cell viability with IC50 values of 63.16 μM in unstimulated and 54.35 μM in stimulated cells.
After an incubation of 48 h, the Kyn/Trp (±SEM) was significantly increased in LPS-stimulated THP-1-Blue cells (96.4 ± 12.6 μmol/mmol) in comparison to unstimulated cells (20.4 ± 1.1 μmol/mmol). There was no effect on Kyn/Trp upon treatment of THP-1-Blue cells with chloroquine for both, LPS-stimulated and unstimulated cells (data not shown).
Upon 24 h of LPS stimulation, the activation of NF-κB according to SEAP activity was increased (9.60 ± 1.18 (SEM)-fold) in comparison to unstimulated cells (p < 0.005). The additional treatment with chloroquine decreased SEAP activity in a dose-dependent manner in LPS-stimulated cells (SEAP activity expressed as fold of unstimulated control: 12.5 μM = 6.77 ± 0.84, 50 μM = 4.24 ± 0.63, p < 0.005), while unstimulated cells were not affected (Fig. 2).
Fig. 2.
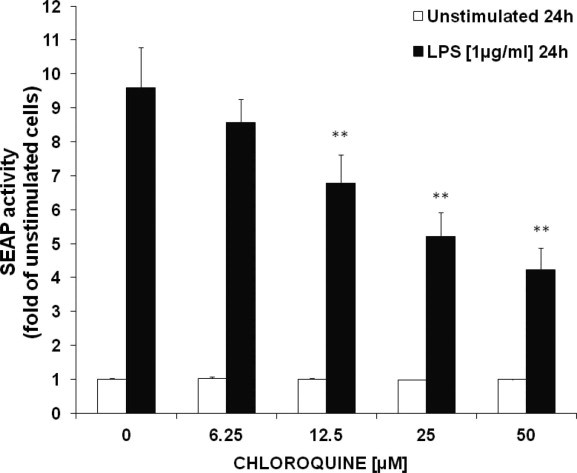
Effect of chloroquine on the enzyme secreted embryonic alkaline phosphatase (SEAP) as a measure of nuclear factor-kappa B (NF-κB) activation in unstimulated (white bars) and lipopolysaccharide (LPS)-stimulated (black bars) THP-1-Blue cells, after 24 h of treatment. Results shown are the mean values ± SEM of six independent experiments performed in duplicates (**p < 0.005, compared to baseline = control cells treated with or without LPS, respectively).
4. Discussion
In this study, the capacity of chloroquine to modulate immune responses in human PBMC and in myelomonocytic THP-1-Blue cells was investigated in vitro. In PBMC, both stimulated or not with the mitogen PHA, chloroquine reduced Kyn/Trp effectively and in a dose-dependent manner already at low concentrations. Also, IL-2Rα secretion was decreased in mitogen-stimulated cells upon chloroquine treatment.
In agreement with our study, a variety of effects of antimalarial drugs on immune-mediated mechanisms have been described such as the suppression of pro-inflammatory cytokine production. Chloroquine has been shown to decrease tumor necrosis factor-α (TNF-α), IFNγ, interleukin-1 (IL-1) and 6 (IL-6) secretion PBMC and malaria patient blood samples [9,11], to reduce monocyte receptor expression [36] and to regulate monocyte GTP-cyclohydrolase-1 expression [37].
Only recently, He et al. reported a new mechanism underlying the anti-inflammatory activity of chloroquine, whereby glucocorticoid signaling is activated and thereby promotes the transrepression of pro-inflammatory cytokines in a mouse collagen-induced arthritis model [38]. Our finding, that chloroquine interferes with T-helper cell type-1 immune response in vitro by repressing tryptophan breakdown in PHA-stimulated PBMC at low micromolar concentrations, represents an additional strategy to inhibit inflammation. Other nonsteroidal anti-inflammatory substances, such as acetylsalicylic acid, and corticosteroid drugs, such as prednisolone, have been reported to inhibit tryptophan breakdown and also neopterin production [39,40]. Interestingly, in our study, neopterin production decreased significantly only at the highest dose of 50 μM chloroquine in PHA-stimulated PBMC, while it was significantly increased with 10 μM.
Of note, chloroquine had no effects on the tryptophan metabolism in unstimulated as well as LPS-stimulated THP-1-Blue cells at the tested concentrations. In LPS-stimulated THP-1-Blue cells, chloroquine was able to reduce NF-κB/AP-1 driven reporter gene expression in a dose-dependent manner. The decrease of NF-κB activation goes in parallel with a decrease in cell viability. Cepika et al. reported that chloroquine had no effect at low micromolar concentrations (2 μM) in monocyte populations of PBMC upon LPS challenge, and suggested that chloroquine regulation of cytokine secretion in monocytes is exerted only at high toxic concentrations, while lower doses affect other signaling pathways and upregulation of costimulatory molecules [41]. On the contrary to monocytes, in human astroglial cells, chloroquine alone, without additional stimulus, was able to induce activation of NF-κB and subsequent expression of pro-inflammatory genes [42]. Therefore, the effect of chloroquine on NF-κB activation remains elusive.
In conclusion, our in vitro study shows that chloroquine treatment results in the suppression of distinct mechanisms in mitogen-stimulated PBMC in comparison to toll like receptor (TLR) stimulated monocytic THP1-Blue cells. Data indicates that chloroquine might have stronger influence on IDO activity by acting on cytokine secretion of T-cells than by acting on THP-1-Blue monocytes. Although THP-1-Blue cells are at an advanced stage of myelomonocytic development and their responsiveness to LPS has been extensively examined, they represent an undifferentiated phenotype [33,43]. The use of macrophages that have an exaggerated response to LPS and act differently than monocytes, might give more insight into chloroquine's mode of action.
However, our findings might be of importance for the discussion about the use of chloroquine for the treatment of other disorders associated with overwhelming immune response. The application of chloroquine and its analogs has already been proved efficient in the treatment studies of autoimmune disease, e.g. in systemic lupus erythomatosus [12,13]. Furthermore, there are clinical trials ongoing with chloroquine and its derivatives that explore their potential for the treatment of viral infections and cancer, with human immunodeficiency virus (HIV) infection being the most studied disease [44–47]. Our results support the view that a more detailed analysis of chloroquine activities in further in vitro and in vivo studies will be of central importance to explore additional potential therapeutic regimes in other chronic inflammatory conditions such as coronary heart disease or neurodegeneration.
Acknowledgments
This work was supported by a grant from the Austrian Research Promotion Agency (FFG, Project No. FFG834169). The content of this article does not necessarily reflect the views or policies of the funding sources.
References
- 1.Surrey A.R., Hammer H.F. Some 7-substituted 4-aminoquinoline derivatives. J. Am. Chem. Soc. 1946;68:113–116. doi: 10.1021/ja01205a036. [DOI] [PubMed] [Google Scholar]
- 2.Cooper R.G., Magwere T. Chloroquine: novel uses & manifestations. Indian J. Med. Res. 2008;127:305–316. [PubMed] [Google Scholar]
- 3.Thorens B., Vassalli P. Chloroquine and ammonium chloride prevent terminal glycosylation of immunoglobulins in plasma cells without affecting secretion. Nature. 1986;321:618–620. doi: 10.1038/321618a0. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Noriega A., Grubb J.H., Talkad V., Sly W.S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J. Cell Biol. 1980;85:839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magwere T., Naik Y.S., Hasler J.A. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic Biol Med. 1997;22:321–327. doi: 10.1016/s0891-5849(96)00285-7. [DOI] [PubMed] [Google Scholar]
- 6.Toler S.M., Noe D., Sharma A. Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme. Neurosurg. Focus. 2006;21:E10. doi: 10.3171/foc.2006.21.6.1. [DOI] [PubMed] [Google Scholar]
- 7.Krishna S., White N.J. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin. Pharmacokinet. 1996;30:263–299. doi: 10.2165/00003088-199630040-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lin J.T., Juliano J.J., Wongsrichanalai C. Drug-resistant malaria: the era of ACT. Curr. Infect. Dis. Rep. 2010;12:165–173. doi: 10.1007/s11908-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballal A., Saeed A., Rouina P., Jelkmann W. Effects of chloroquine treatment on circulating erythropoietin and inflammatory cytokines in acute Plasmodium falciparum malaria. Ann. Hematol. 2009;88:411–415. doi: 10.1007/s00277-008-0636-z. [DOI] [PubMed] [Google Scholar]
- 10.Weber S.M., Levitz S.M. Chloroquine interferes with lipopolysaccharide-induced TNF-alpha gene expression by a nonlysosomotropic mechanism. J. Immunol. 2000;165:1534–1540. doi: 10.4049/jimmunol.165.3.1534. [DOI] [PubMed] [Google Scholar]
- 11.van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 12.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozniacka A., Lesiak A., Narbutt J., McCauliffe D.P., Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus. 2006;15:268–275. doi: 10.1191/0961203306lu2299oa. [DOI] [PubMed] [Google Scholar]
- 14.Ornstein M.H., Sperber K. The antiinflammatory and antiviral effects of hydroxychloroquine in two patients with acquired immunodeficiency syndrome and active inflammatory arthritis. Arthritis Rheum. 1996;39:157–161. doi: 10.1002/art.1780390122. [DOI] [PubMed] [Google Scholar]
- 15.Fox R.I. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin. Arthritis Rheum. 1993;23:82–91. doi: 10.1016/s0049-0172(10)80012-5. [DOI] [PubMed] [Google Scholar]
- 16.Billiau A., Heremans H., Vermeire K., Matthys P. Immunomodulatory properties of interferon-gamma. An update. Ann. NY Acad. Sci. 1998;856:22–32. doi: 10.1111/j.1749-6632.1998.tb08309.x. [DOI] [PubMed] [Google Scholar]
- 17.Saha B., Jyothi Prasanna S., Chandrasekar B., Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Smale S.T. Hierarchies of NF-κB target-gene regulation. Nat. Immunol. 2011;12:689–694. doi: 10.1038/ni.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murr C., Widner B., Wirleitner B., Fuchs D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G., Fuchs D., Hausen A., Reibnegger G., Werner E.R., Werner-Felmayer G., Semenitz E., Dierich M.P, Wachter H. Neopterin modulates toxicity mediated by reactive oxygen and chloride species. FEBS Lett. 1993;321:89–92. doi: 10.1016/0014-5793(93)80627-7. [DOI] [PubMed] [Google Scholar]
- 21.Razumovitch J.A., Semenkova G.N., Fuchs D., Cherenkevich S.N. Influence of neopterin on the generation of reactive oxygen species in human neutrophils. FEBS Lett. 2003;549:83–86. doi: 10.1016/s0014-5793(03)00796-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann G., Schobersberger W., Frede S., Pelzer L., Fandrey J., Wachter H., Fuchs D., Grote J. Neopterin activates transcription factor nuclear factor-kappa B in vascular smooth muscle cells. FEBS Lett. 1996;391:181–184. doi: 10.1016/0014-5793(96)00729-6. [DOI] [PubMed] [Google Scholar]
- 23.Taylor M.W., Feng G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 24.Schröcksnadel K., Wirleitner B., Winkler C., Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Schroecksnadel K., Kaser S., Ledochowski M., Neurauter G., Mur E., Herold M., Fuchs D. Increased degradation of tryptophan in blood of patients with rheumatoid arthritis. J. Rheumatol. 2003;30:1935–1939. [PubMed] [Google Scholar]
- 26.Schroecksnadel K., Winkler C., Duftner C., Wirleitner B., Schirmer M., Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin. Rheumatol. 2006;25:334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 27.Reibnegger G., Boonpucknavig V., Fuchs D., Hausen A., Schmutzhard E., Wachter H. Urinary neopterin is elevated in patients with malaria. Trans. R. Soc. Trop. Med. Hyg. 1984;78:545–546. doi: 10.1016/0035-9203(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 28.Reibnegger G., Fuchs D., Hausen A., Schmutzhard E., Werner E.R., Wachter H. The dependence of cell-mediated immune activation in malaria on age and endemicity. Trans. R. Soc. Trop. Med. Hyg. 1987;81:729–733. doi: 10.1016/0035-9203(87)90009-5. [DOI] [PubMed] [Google Scholar]
- 29.Brown A.E., Webster H.K., Teja-Isavadharm P., Keeratithakul D. Macrophage activation in falciparum malaria as measured by neopterin and interferon-gamma. Clin. Exp. Immunol. 1990;82:97–101. doi: 10.1111/j.1365-2249.1990.tb05410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremsner P.G., Feldmeier H., Zotter G.M., Jansen-Rosseck R., Graninger W., Rocha R.M., Bienzle U. Immunological alterations in uncomplicated Plasmodium falciparum malaria. Relationship between parasitaemia and indicators of macrophage activation. Acta Trop. 1989;46:351–359. doi: 10.1016/0001-706x(89)90047-8. [DOI] [PubMed] [Google Scholar]
- 31.Tetsutani K., To H., Torii M., Hisaeda H., Himeno K. Malaria parasite induces tryptophan-related immune suppression in mice. Parasitology. 2007;134:923–930. doi: 10.1017/S0031182007002326. [DOI] [PubMed] [Google Scholar]
- 32.Jenny M., Klieber M., Zaknun D., Schroecksnadel S., Kurz K., Ledochowski M., Schennach H., Fuchs D. In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm. Res. 2011;60:127–135. doi: 10.1007/s00011-010-0244-y. [DOI] [PubMed] [Google Scholar]
- 33.Schroecksnadel S., Jenny M., Kurz K., Klein A., Ledochowski M., Uberall F., Fuchs D. LPS-induced NF-kappaB expression in THP-1 Blue cells correlates with neopterin production and activity of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2010;399:642–646. doi: 10.1016/j.bbrc.2010.07.134. [DOI] [PubMed] [Google Scholar]
- 34.Samsonov M.Y., Tilz G.P., Pisklakov V.P., Reibnegger G., Nassonov E.L., Nassonova V.A., Wachter H., Fuchs D. Serum-soluble receptors for tumor necrosis factor-alpha and interleukin-2, and neopterin in acute rheumatic fever. Clin. Immunol. Immunopathol. 1995;74:31–34. doi: 10.1006/clin.1995.1005. [DOI] [PubMed] [Google Scholar]
- 35.Widner B., Werner E.R., Schennach H., Wachter H., Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 36.Goldring J.P., Nemaorani S. Antimalarial drugs modulate the expression of monocyte receptors. Int. J. Immunopharmacol. 1999;21:599–607. doi: 10.1016/s0192-0561(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 37.Cumming B.M., Watson G.M., Goldring J.P. Plasmodium falciparum: effect of antimalarial drugs, malaria pigment (β-haematin) and Plasmodium falciparum lysate on monocyte GTP-cyclohydrolase 1 gene expression. Exp. Parasitol. 2011;129:312–317. doi: 10.1016/j.exppara.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 38.He Y., Xu Y., Zhang C., Gao X., Dykema K.J., Martin K.R., Ke J., Hudson E.A., Khoo S.K., Resau J.H., Alberts A.S., MacKeigan J.P., Furge K.A., Xu H.E. Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Sci. Signal. 2011;4:ra44. doi: 10.1126/scisignal.2001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroecksnadel K., Winkler C., Wirleitner B., Schennach H., Fuchs D. Aspirin down-regulates tryptophan degradation in stimulated human peripheral blood mononuclear cells in vitro. Clin. Exp. Immunol. 2005;140:41–45. doi: 10.1111/j.1365-2249.2005.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroecksnadel S., Sucher R., Kurz K., Fuchs D., Brandacher G. Influence of immunosuppressive agents on tryptophan degradation and neopterin production in human peripheral blood mononuclear cells. Transpl. Immunol. 2011;25:119–123. doi: 10.1016/j.trim.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Cepika A.M., Bendelja K., Vergles J.M., Malenica B., Kapitanovic S., Gagro A. Monocyte response to LPS after exposure to corticosteroids and chloroquine with implications for systemic lupus erythematosus. Scand. J. Immunol. 2010;72:434–443. doi: 10.1111/j.1365-3083.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 42.Park J., Kwon D., Choi C., Oh J.W., Benveniste E.N. Chloroquine induces activation of nuclear factor-kappaB and subsequent expression of pro-inflammatory cytokines by human astroglial cells. J. Neurochem. 2003;84:1266–1274. doi: 10.1046/j.1471-4159.2003.01623.x. [DOI] [PubMed] [Google Scholar]
- 43.Eperon S., Jungi T.W. The use of human monocytoid lines as indicators of endotoxin. J. Immunol. Methods. 1996;194:121–129. doi: 10.1016/0022-1759(96)00073-7. [DOI] [PubMed] [Google Scholar]
- 44.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray S.M., Down C.M., Boulware D.R., Stauffer W.M., Cavert W.P., Schacker T.W., Brenchley J.M., Douek D.C. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J. Virol. 2010;84:12082–12086. doi: 10.1128/JVI.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romanelli F., Smith K.M., Hoven A.D. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr. Pharm. Des. 2004;10:2643–2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 47.Aguirre-Cruz L., Torres K.J., Jung-Cook H., Fortuny C., Sánchez E., Soda-Mehry A., Sotelo J., Reyes-Terán G. Short communication: preferential concentration of hydroxychloroquine in adenoid tissue of HIV-infected subjects. AIDS Res. Hum. Retroviruses. 2010;26:339–342. doi: 10.1089/aid.2009.0129. [DOI] [PubMed] [Google Scholar]


