Abstract
Tespa1 has been recently reported to be a critical molecule in T-cell development, however, the precise molecular mechanisms of Tespa1 remain elusive. Here, we demonstrate that Tespa1 shows amino-acid sequence homology to KRAS-induced actin-interacting protein (KRAP), an inositol 1,4,5-trisphosphate receptor (IP3R) binding protein, and that Tespa1 physically associates with IP3R in T and B lymphocytes. Two-consecutive phenylalanine residues (Phe185/Phe186) in Tespa1, which are conserved between Tespa1 and KRAP, are indispensable for the association between Tespa1 and IP3R. These findings suggest that Tespa1 plays critical roles in the immune system through the regulation of the IP3R.
Keywords: Tespa1, IP3R, Protein–protein interaction, Thymus, Spleen, Lymphocyte
Abbreviations: Tespa1, thymocyte-expressed positive selection-associated 1; IP3R, Inositol 1,4,5-trisphosphate receptor; KRAP, KRAS-induced actin-interacting protein.
Highlights
▸ We identified Tespa1 as a novel IP3R-associated protein in lymphoid tissues. ▸ Tespa1 interacts with multiple subtypes of IP3R in T and B lymphocytes. ▸ Amino-terminal region of IP3R is sufficient for the association with Tespa1. ▸ Two consecutive phenylalanines in Tespa1 are critical for the interaction with IP3R. ▸ Tespa1 is structurally and functionally related to KRAP.
1. Introduction
Intracellular Ca2+ is a versatile, universal second messenger controlling numerous biological processes [1,2]. Three inositol 1,4,5-trisphosphate receptor (IP3R) subtypes are differentially expressed among tissues [3–7] and function as the Ca2+ release channel on endoplasmic reticulum membranes [8–12]. IP3R is regulated by many intracellular modulators, phosphorylation by kinases, and associated proteins [13–17].
KRAS-induced actin-interacting protein (KRAP), originally identified as one of the deregulated expression genes in colorectal cancer [18] and also known as sperm-specific antigen 2 (SSFA2), physiologically participates in the regulation of systemic energy homeostasis [19] and of the exocrine system [20]. We have recently demonstrated that KRAP is involved in the regulation of the proper localization and function of IP3R through the physical molecular interaction in the epithelial cells [21–23]. On the other hand, Tespa1 (thymocyte-expressed positive selection-associated 1) has just recently been reported to play a crucial role in T-cell development in the thymus [24], however, the precise molecular mechanisms of Tespa1 remain elusive.
We herein report that Tespa1 possesses substantial amino acid sequence homology to KRAP. Tespa1 is exclusively expressed in T and B lymphocytes and is functionally related to KRAP; Tespa1 physically interacts with IP3R in T and B lymphocytes. Thus, our finding that IP3R is directly regulated by Tespa1 provides insight into the molecular mechanism underlying the regulation of IP3R in T and B lymphocytes.
2. Materials and methods
2.1. Antibodies
The antibodies used were as follows: anti-actin (A2066) from Sigma, anti-hemagglutinin (HA) (3F10) from Roche, anti-green fluorescent protein (GFP) (632460) from Clontech, anti-IP3R1 (ab5840) from Abcam, anti-IP3R3 (610313) from BD Transduction Laboratories, anti-ERK (K-23) from Santa Cruz Biotechnology, and anti-ATP synthase (3D5) from Abcam. The recombinant human Tespa1 fragment (amino acid residues 2–182) was expressed as a GST fusion protein using the pGEX4T-2 vector (GE Healthcare). The fusion protein was soluble in nondenaturing buffer and was purified with glutathione-Sepharose 4B (Amersham Pharmacia). Antiserum was obtained by injecting the recombinant Tespa1 protein into a Japanese White rabbit followed by booster injection. Antiserum was purified with an affinity column prepared by cross-linking the recombinant protein to CNBr-activated Sepharose 4B (Amersham Pharmacia).
2.2. Animals
All animals used in this study were treated in accordance with the guidelines of Fukuoka University.
2.3. Cell isolation and subcellular fractionation
Positive selection of a specific type of lymphocyte was carried out using MACS microbeads coated with a specific monoclonal antibody (Miltenyi Biotec) as described previously [25]. Subcellular fractions from mouse thymus were obtained as described previously [21].
2.4. Cell culture and transfection
Cell culture and transfection were performed as previously described [21].
2.5. Immunoprecipitations and Western blotting
Immunoprecipitations and Western blotting were performed as previously described [18,21].
2.6. Immunocytochemistry
Immunostaining was performed as described previously [18,21].
2.7. Construction of plasmids
The cDNAs encoding full-length human Tespa1 (residues 2–521) and its deletion mutants (residues 2–201 and 2–182) were cloned into the pCMV-HA vector (Clontech). Site-directed mutants of Tespa1 were generated by utilizing the KOD-Plus-Mutagenesis kit (TOYOBO). The GFP-tagged full-length mouse IP3R1 fusion protein and its deletion mutants were generated as described previously [21]. The cDNA encoding IP3R1 deletion mutants (residues 611–2749, 2216–2749, 1–230 fused to 2216–2749, and 231–610 fused to 2216–2749) were cloned into the pEGFP-N1 vector (Clontech).
3. Results and discussion
3.1. Identification of Tespa1 as a KRAP-related protein
To explore whether there are structurally and functionally KRAP-related proteins, we used the N-terminal amino acid sequences of KRAP (residues 1–203 of mouse KRAP or residues 1–201 of human KRAP) as the query sequences for the protein BLAST program (National Center for Biotechnology Information, http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome), and this search revealed that the gene Tespa1 encodes a KRAP-related protein (Supplementary data, Fig. S1). NH2-terminal amino acid residues 1–300 of Tespa1, which are conserved between human and mouse species, showed 50% amino acid sequence similarity with those of KRAPs (Supplementary data, Fig. S1).
To examine the exact tissue distribution of the mouse Tespa1 protein, Western blot analysis and immunoprecipitation experiments for the Tespa1 protein in the adult mouse tissues were performed (Fig. 1A). Strong expressions of the Tespa1 protein were detected in the thymus and spleen, but the protein was rarely detected in the other tissues when total tissue lysates were used as the samples for Western blotting (Fig. 1A, top). Although weak expressions of the Tespa1 protein were also detected in the immunoprecipitates concentrated from lung and skeletal muscle by using anti-Tespa1 antibody (Fig. 1A, bottom), Tespa1 was found to encode an immune system-specific protein. Subsequently, we examined MACS-selected lymphocytes from the adult mouse spleen to determine the cell-type distribution of the Tespa1 protein. Among the lymphocytes isolated from the spleen, Tespa1 was detected in CD19+, CD4+, and CD8+ lymphocytes, but undetectable in CD11b+ lymphocytes (Fig. 1B), demonstrating that the Tespa1 protein is predominantly expressed in B and T lymphocytes but not in other cell types, such as monocytes and macrophages in the spleen. These results are well-consistent with the previous data that mRNA expression of Tespa1 is specifically detected in the lymphoid tissues, thymus, spleen, and lymph nodes, and is detectable in T and B lymphocytes, but not in macrophages [24]. In addition, to examine the subcellular localization of the Tespa1 protein in the mouse thymus, we carried out a biochemical subcellular fractionation assay and showed that Tespa1 was exclusively detected in the P3, microsomal fraction, which was similar to the expression patterns for IP3R1 and IP3R3 proteins (Fig. 1C). It is of note that KRAP was previously found to be also fractionated into the microsomal fraction prepared from the mouse liver [21], which was probably due to the physical association of KRAP with IP3R on the endoplasmic reticulum membranes [21]. Thus, these observations suggested the possibility that the Tespa1 protein may also bind to the lumen of the cytosolic side of the endoplasmic reticulum membranes by interacting with IP3R.
Fig. 1.
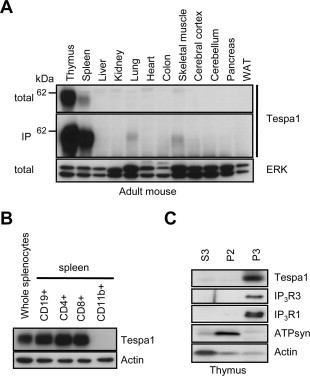
Tespa1 protein exists in the microsomal fraction in lymphocytes. (A) Western blot analysis and immunoprecipitations by using anti-Tespa1 antibody for Tespa1 protein expression in the 12 kinds of adult mouse tissues. ERK is used as a loading control. WAT, white adipose tissue. (B) Western blot analysis for Tespa1 protein expression in CD19+, CD4+, CD8+, and CD11b+ cells isolated from the adult mouse spleen. Actin is used as a loading control. (C) Western blot analysis of Tespa1, IP3R1, IP3R3, ATP synthase (ATP syn), and actin expressions in the subcellular fractions from the adult mouse thymus. S3, soluble cytoplasmic fraction; P2, crude mitochondrial fraction; P3, crude microsomal fraction; total, total lysate; IP, immunoprecipitations.
3.2. Tespa1 interacts with IP3R subtypes in T and B lymphocytes
To examine whether Tespa1 interacts with IP3R, anti-Tespa1 immunoprecipitation and anti-IP3R3 immunoprecipitation experiments were performed using the mouse thymus or spleen, and the results showed that Tespa1 interacts with IP3R1 and IP3R3 (Fig. 2A and B). In addition, co-immunoprecipitation studies using B220+ lymphocytes, CD4+ lymphocytes, a human T-cell leukemia line (Jurkat), and a mouse T-cell leukemia line (EL4) confirmed the association between Tespa1 and IP3Rs (Fig. 2C and D). Furthermore, endogenous Tespa1 is well-colocalized with IP3R3 in Jurkat cells (Fig. 2E). These results showed that the Tespa1 protein binds multiple subtypes of IP3R both in tissues and in cultured cells. It is of note that Tespa1 efficiently associated with both the subtype IP3R1 and the subtype IP3R3 in the thymus (Fig. 2A), whereas Tespa1 preferentially associated with IP3R3 over IP3R1 in the spleen (Fig. 2A–D).
Fig. 2.
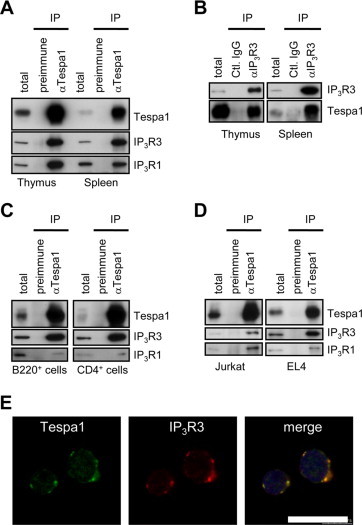
Physical association of Tespa1 with IP3R subtypes. (A) Immunoprecipitations with anti-Tespa1 (αTespa1) or control (preimmune) antibodies were performed using the mouse thymus or spleen, followed by Western blotting with anti-Tespa1, anti-IP3R1, or anti-IP3R3 antibodies. (B) Immunoprecipitations with IP3R3 (αIP3R3) or control (Ctl.IgG) antibodies were performed using the mouse thymus or spleen, followed by Western blotting with anti-Tespa1 or anti-IP3R1 antibodies. (C and D) Immunoprecipitations with anti-Tespa1 (αTespa1) or control (preimmune) antibodies were performed using B220+–B lymphocytes or CD4+–T lymphocytes isolated from the adult mouse spleen (C), or using T-cell leukemia lines, Jurkat or EL4 (D), followed by Western blotting with anti-Tespa1, anti-IP3R1, or anti-IP3R3 antibodies. IP, Immunoprecipitations; total, total lysate. (E) Confocal immunostaining with anti-Tespa1 and anti-IP3R3 antibodies in Jurkat cells. Blue, 4′,6-Diamidino-2-phenylindole (DAPI) staining. Scale bar, 15 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Identification of the critical region of IP3R for the association with Tespa1
To identify the critical region of IP3Rs for the association with Tespa1, we examined the interactions of several GFP-tagged IP3R1 deletion mutants (Fig. 3A, MI-a–i) with the full-length HA-tagged Tespa1. The NH2-terminal amino acid residues 1–610, which were conserved among IP3R subtypes, bound to Tespa1, but the suppressor domain (MI-e) or ligand binding domain (MI-f) did not bind to Tespa1 (Fig. 3B and C). These results indicate that amino acid residues 1–610 spanning over these two domains were necessary for the interaction with Tespa1 (Fig. 3B and C). Because we previously reported that the interaction of IP3Rs with KRAP is also mediated by the NH2-terminal amino acid residues 1–610 of IP3Rs [21], the molecular mechanism underlying the formations of the KRAP–IP3R complex and Tespa1–IP3R complex would be well-conserved.
Fig. 3.
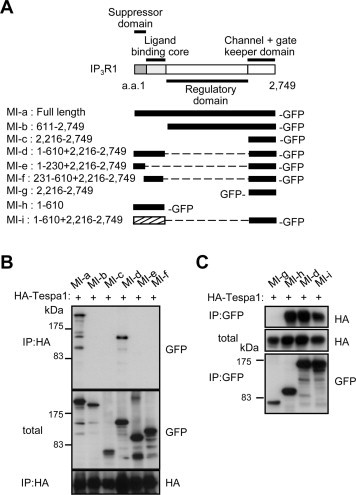
Critical region of IP3R for the association with Tespa1. (A) A schematic illustration of the functional domains of mouse IP3R1 (top) and GFP-tagged IP3R1-deletion mutants used in this study (bottom). Black ribbon is shown for IP3R1 sequence. Hatched ribbon is shown for IP3R3 sequence. (B and C) Co-immunoprecipitations using full-length HA-tagged Tespa1 and deletion mutants of IP3R1. a.a., amino acids; IP, immunoprecipitations; total, total lysate.
3.4. Identification of the critical region of Tespa1 for the association with IP3R
To determine the region of Tespa1 that is critical for the association with IP3R, we constructed several HA-tagged human Tespa1 deletion mutants (Fig. 4A, MT-a–c) and examined their interaction with the full-length of GFP-tagged IP3R1. We found that the MT-b but not the MT-c interacts with IP3R1, indicating that a span of 19 amino acid residues (183–201) of human Tespa1 is essential for the interaction with IP3R1 (Fig. 4B). Interestingly, this critical region is highly conserved between human and mouse Tespa1 as well as between human and mouse KRAP (Fig. 4C). Because we previously found that two consecutive phenylalanine residues (Phe202/Phe203) in mouse KRAP are critical for the association of KRAP with IP3R [23], we exchanged one or both phenylalanine residue(s) at positions 185 and 186 of human Tespa1 for alanine residues (Fig. 4C), and compared the IP3R1-binding activity between the resulting proteins and the wild-type Tespa1 form. Neither the F185A/F186A mutant nor the F185A mutant bound with IP3R1, whereas the F186A mutant showed a weak interaction with IP3R1 (Fig. 4D). Together, these results indicated that a common molecular base, in which two consecutive phenylalanine residues conserved among the KRAP and Tespa1 proteins are critical for the association with IP3Rs, underlies the formations of the KRAP–IP3R complex and Tespa1–IP3R complex.
Fig. 4.
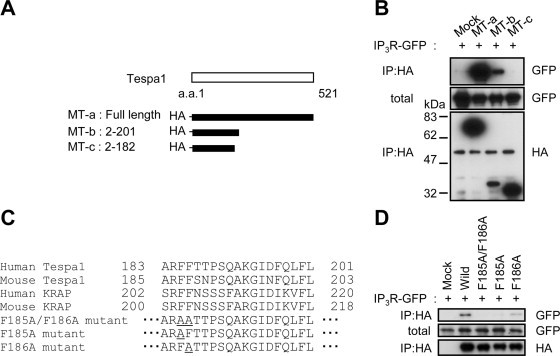
Critical region of Tespa1 for the association with IP3R. (A) A schematic illustration of human Tespa1 (top) and HA-tagged Tespa1-deletion mutants used in this study (bottom). (B) Co-immunoprecipitations using full-length IP3R1 and deletion mutants of Tespa1. (C) The critical region of Tespa1 required for the IP3R interaction (top). Double or single amino acid substitutions from phenylalanine to alanine residue(s) within human Tespa1 (bottom). (D) Co-immunoprecipitations using full-length IP3R and various mutants of Tespa1 (C). a.a., amino acids; IP, immunoprecipitations; total, total lysate.
In this study, we identified Tespa1 as a structurally and functionally KRAP-related protein; Tespa1 has substantial homology to KRAP and is a novel binding partner of IP3Rs in T and B lymphocytes. Recently, Tespa1 was reported to play critical roles in the positive selection of thymocytes in vivo [24], based on the analysis of Tespa1-deficient mice, and also suggested that Tespa1 is involved in the proper assembly of the Lat signalosome through its interaction with PLC-γ1 and Grb2 [24,26–31]. This finding indicates the physiological significance of Tespa1 in T-cell antigen receptor signaling in the immune system. Here, we have clearly demonstrated that Tespa1 is a novel IP3R-interacting protein in T and B lymphocytes, and this finding would explain why the severe impairments of calcium flux in the Tespa1-deficient mice occur [24]. Our finding is well-consistent with a recent hypothesis that Tespa1 participates in T-cell signaling through regulating calcium release from endoplasmic reticulum through interaction with IP3R, after interacting with the Lat signalosome [32].
Because Tespa1 is predominantly expressed both in T lymphocytes and in B lymphocytes, it is likely that Tespa1 would affect calcium signaling through the physical interaction with IP3R in B lymphocytes as well as T lymphocytes. Interestingly, Tespa1 appeared to preferentially interact with IP3R3 over IP3R1 in splenocytes (Fig. 2A–D), whereas Tespa1 efficiently interacted with both the IP3R subtypes in the thymus (Fig. 2A), suggesting that Tespa1 may interact with different subtypes of IP3R according to the cell-type and/or cellular status. If this is the case, the association between Tespa1 and distinct IP3R subtypes would play critical roles in the cell-type-specific and/or cellular status-specific biological processes in the lymphocytes.
In conclusion, we identified Tespa1 as a novel binding partner of IP3R in the T and B lymphocytes, and these findings shed light on the molecular mechanism underlying calcium signaling through the regulation of IP3R in the immune system.
Acknowledgments
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, a Grant-in-Aid for Young Scientists (B) Grant No. 24710265. This work was supported in part by funds (No. 112503) from the Central Research Institute of Fukuoka University. We thank Takami Danno, Aya Fujikane and Yuka Horio for their technical assistance.
Appendix A. Supplementary data
Human Tespa1, mouse Tespa1, human KRAP, and mouse KRAP protein sequences aligned using Clustal W program (http://www.genome.jp/tools/clustalw/). The degree of conservation is denoted by the following symbols: “*” (identical in all sequences), “:” (conserved substitutions), and “.” (semiconserved substitutions).
References
- 1.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Ross C.A., Danoff S.K., Schell M.J., Snyder S.H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc. Natl. Acad. Sci. USA. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp A.H., McPherson P.S., Dawson T.M., Aoki C., Campbell K.P., Snyder S.H. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama T., Yamamoto-Hino M., Miyawaki A., Furuichi T., Mikoshiba K., Hasegawa M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: dynamic aspects of their cell-type specific expression. FEBS Lett. 1994;349:191–196. doi: 10.1016/0014-5793(94)00662-8. [DOI] [PubMed] [Google Scholar]
- 6.Newton C.L., Mignery G.A., Südhof T.C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- 7.Wojcikiewicz R.J. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraman T., Ondriasová E., Ondrias K., Harnick D.J., Marks A.R. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc. Natl. Acad. Sci. USA. 1995;92:6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A.A., Soloski M.J., Sharp A.H., Schilling G., Sabatini D.M., Li S.H., 6et al. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara H., Kurosaki M., Takata M., Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharenberg A.M., Humphries L.A., Rawlings D.J. Calcium signalling and cell-fate choice in B cells. Nat. Rev. Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.deSouza N., Cui J., Dura M., McDonald T.V., Marks A.R. A function for tyrosine phosphorylation of type 1 inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J. Cell Biol. 2007;179:923–934. doi: 10.1083/jcb.200708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson R.L., Boehning D., Snyder S.H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 14.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Foskett J.K., White C., Cheung K.H., Mak D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J. Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Fritz N., Ibarra C., Uhlén P. Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations. Neurochem. Res. 2011;36:1175–1185. doi: 10.1007/s11064-011-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto T., Koyanagi M., Baba I., Nakabayashi K., Kato N., Sasazuki T. Analysis of KRAP expression and localization, and genes regulated by KRAP in a human colon cancer cell line. J. Hum. Genet. 2007;52:978–984. doi: 10.1007/s10038-007-0204-8. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto T., Miyasaka K., Koyanagi M., Tsunoda T., Baba I., Doi K., 6et al. Altered energy homeostasis and resistance to diet-induced obesity in KRAP-deficient mice. PLoS One. 2009;4:e4240. doi: 10.1371/journal.pone.0004240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyasaka K., Fujimoto T., Kawanami T., Takiguchi S., Jimi A., Funakoshi A., 6et al. Pancreatic hypertrophy in Ki-ras-induced actin-interacting protein gene knockout mice. Pancreas. 2011;40:79–83. doi: 10.1097/MPA.0b013e3181f66c22. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto T., Machida T., Tanaka Y., Tsunoda T., Doi K., Ota T., 6et al, Shirasawa . KRAS-induced actin-interacting protein is required for the proper localization of inositol 1,4,5-trisphosphate receptor in the epithelial cells. Biochem. Biophys. Res. Commun. 2011;407:438–443. doi: 10.1016/j.bbrc.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto T., Machida T., Tsunoda T., Doi K., Ota T., Kuroki M., 6et al. KRAS-induced actin-interacting protein regulates inositol 1,4,5-trisphosphate-receptor-mediated calcium release. Biochem. Biophys. Res. Commun. 2011;408:214–217. doi: 10.1016/j.bbrc.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto T., Machida T., Tsunoda T., Doi K., Ota T., Kuroki M., 6et al. Determination of the critical region of KRAS-induced actin-interacting protein for the interaction with inositol 1,4,5-trisphosphate receptor. Biochem. Biophys. Res. Commun. 2011;408:282–286. doi: 10.1016/j.bbrc.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Wang D., Zheng M., Lei L., Ji J., Yao Y., Qiu Y., 6et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat. Immunol. 2012;13:560–568. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 25.Koyanagi M., Nakabayashi K., Fujimoto T., Gu N., Baba I., Takashima Y., 6et al. ZFAT expression in B and T lymphocytes and identification of ZFAT-regulated genes. Genomics. 2008;91:451–457. doi: 10.1016/j.ygeno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Johnson A.L., Aravind L., Shulzhenko N., Morgun A., Choi S.Y., Crockford T.L., 6et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesourne R., Uehara S., Lee J., Song K.D., Li L., Pinkhasov J., 6et al. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu G., Vallée S., Rybakin V., McGuire M.V., Ampudia J., Brockmeyer C., 6et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat. Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick M.S., Oda H., Hayakawa K., Sato Y., Eshima K., Kirikae T. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc. Natl. Acad. Sci. USA. 2009;106:16345–16350. doi: 10.1073/pnas.0908593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakugawa K., Yasuda T., Miura I., Kobayashi A., Fukiage H., Satoh R. A novel gene essential for the development of single positive thymocytes. Mol. Cell Biol. 2009;29:5128–5135. doi: 10.1128/MCB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockmeyer C., Paster W., Pepper D., Tan C.P., Trudgian D.C., McGowan S., 6et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J. Biol. Chem. 2011;286:7535–7547. doi: 10.1074/jbc.M110.201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gascoigne NR., Fu G. Tespa1: another gatekeeper for positive selection. Nat. Immunol. 2012;13:530–532. doi: 10.1038/ni.2315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human Tespa1, mouse Tespa1, human KRAP, and mouse KRAP protein sequences aligned using Clustal W program (http://www.genome.jp/tools/clustalw/). The degree of conservation is denoted by the following symbols: “*” (identical in all sequences), “:” (conserved substitutions), and “.” (semiconserved substitutions).


