Summary
Endocytosis is an essential cellular process in eukaryotic cells that involves concordant functions of clathrin and adaptor proteins, various protein and lipid kinases, phosphatases and the actin cytoskeleton. In Saccharomyces cerevisiae, Ark1p is a member of the serine/threonine protein kinase (SPK) family that affects profoundly the organization of the cortical actin cytoskeleton. To study the function of MoArk1, an Ark1p homologue identified in Magnaporthe oryzae, we disrupted the MoARK1 gene and characterized the ΔMoark1 mutant strain. The ΔMoark1 mutant exhibited various defects ranging from mycelial growth and conidial formation to appressorium‐mediated host infection. The ΔMoark1 mutant also exhibited decreased appressorium turgor pressure and attenuated virulence on rice and barley. In addition, the ΔMoark1 mutant displayed defects in endocytosis and formation of the Spitzenkörper, and was hyposensitive to exogenous oxidative stress. Moreover, a MoArk1‐green fluorescent protein (MoArk1‐GFP) fusion protein showed an actin‐like localization pattern by localizing to the apical regions of hyphae. This pattern of localization appeared to be regulated by the N‐ethylmaleimide‐sensitive factor attachment protein receptor (SNARE) proteins MoSec22 and MoVam7. Finally, detailed analysis revealed that the proline‐rich region within the MoArk1 serine/threonine kinase (S_TKc) domain was critical for endocytosis, subcellular localization and pathogenicity. These results collectively suggest that MoArk1 exhibits conserved functions in endocytosis and actin cytoskeleton organization, which may underlie growth, cell wall integrity and virulence of the fungus.
Introduction
Eukaryotic cells internalize macromolecules and other materials from the extracellular medium through the conserved process of endocytosis. Consequentially, endocytosis serves multiple functions, including the recycling of membrane proteins and lipids, internalization of membrane proteins and lipids for degradation and internalization of signalling molecules (D'Hondt et al., 2000; Emans et al., 2002). In the budding yeast Saccharomyces cerevisiae, the availability of the fluorescent dye FM4‐64 and Ste2p and Ste3p pheromone receptors has allowed for the identification of the endocytic pathway (Toret and Drubin, 2006). Many features of the yeast endocytic pathway are also surprisingly conserved in mammalian systems (Davis et al., 1993; Raths et al., 1993). In yeasts and other fungi, either pathogenic or saprophytic, endocytosis and intracellular transport events are important for cellular functions, including polarity establishment, hyphal growth and/or virulence (Atkinson et al., 2002; Dou et al., 2011; Read and Kalkman, 2003; Shaw et al., 2011; Song et al., 2010; Wang and Shen, 2011). Numerous studies have examined the important roles of specific endocytic components, such as Aspergillus oryzae End4/Sla2, Fusarium graminearum End1, Ustilago maydis Yup1 and mammalian cyclin‐G‐associated kinase (GAK) and adaptor‐associated kinase 1 (AAK1) (Fuchs et al., 2006; Higuchi et al., 2009; Kim et al., 2009; Smythe and Ayscough, 2003). In mammalian cells, members of the Ark/Prk family of kinases, including GAK and AAK1, are important for endocytosis (Smythe and Ayscough, 2003). The roles of endocytic transport in the growth and pathogenicity of U. maydis have also been subject to in‐depth discussions (Fuchs and Steinberg, 2005; Steinberg, 2007). The importance of endocytosis as a physiological pathway and the molecular mechanism of endocytosis have also been reported. For example, Aspergillus nidulans actin and myosin are important factors in endocytosis and polarized cell growth (Kaksonen et al., 2006). In addition, phosphorylation of A. nidulans MyoA regulates events in the endocytic pathway, such as membrane internalization (Hervas‐Aguilar and Penalva, 2010; Idrissi et al., 2008). These reports further highlight that the endocytic pathway is important for engaging in normal physiological functions in filamentous fungi, similar to other well‐defined eukaryotic cells.
In the rice blast fungus Magnaporthe oryzae, infection occurs by conidia that are normally composed of three cells. The germination of conidia involves the production of a germ tube from one or more of these cells (Howard and Valent, 1996). Endocytosis is thought to be important for conidial cells to detect certain external signals, and plays a crucial role in various aspects of hyphal tip growth (Fischer‐Parton et al., 2000). Successful detection for the incorporation of FM4‐64 into the Spitzenkörper body identified this organelle as being involved in endocytic membrane trafficking (Fischer‐Parton et al., 2000; Harris et al., 2005; Verdin et al., 2009). Despite these findings, the physiological importance and molecular process of the endocytic process in filamentous fungi remain largely unaddressed, and little is known regarding the exocytotic transport of enzymes and other cell wall lytic materials in host tissue‐invading processes (Gow et al., 2002). Previously, we have characterized MoSec22 and MoVam7 as two members of the secretory soluble N‐ethylmaleimide‐sensitive factor attachment protein receptor (SNARE) complex in M. oryzae, and have found that they play important roles in membrane trafficking, as well as in cellular growth, stress tolerance and pathogenicity (Dou et al., 2011; Song et al., 2010). SNARE proteins are a family of conserved proteins involved in the intracellular transport of membrane‐coated cargo from one subcellular compartment to another (Pelham, 1999). As a part of our continuing research efforts to examine the roles of membrane trafficking in fungal pathogenesis, we analysed the physiological significance of MoArk1, the S. cerevisiae actin‐regulating kinase 1 (Ark1) homologue in M. oryzae. In the present study, we demonstrated that MoArk1 regulates endocytosis and is required for conidial development, stress resistance, membrane trafficking and pathogenicity.
Results
Identification and characterization of MoARK1
Examination of the M. oryzae genome database at the Broad Institute (http://www.broad.mit.edu/annotation/genome/magnaporthe_grisea/Home.html) revealed that MGG_11326.6 shares high amino acid sequence homology to S. cerevisiae Ark1p (ScArk1p), and we thus named it MoArk1. MoArk1 consists of 1021 amino acids and appears to be encoded by a single‐copy gene. Similar to ScArk1p, MoArk1 contains a putative serine/threonine kinase (S_TKc) domain in its N‐terminus, and three phosphorylation sites and a conserved proline‐rich motif RPTAPPKP in its C‐terminus (Fig. S1A, see Supporting Information). MoArk1 shares 56% amino acid sequence identity with ScArk1p and 58% with the N. crassa Ark1 homologue. In contrast, MoArk1 is less similar to human Gak1 (17%) and Drosophila melanogaster auxilin (15%) (Fig. S1B). To address the function of MoArk1 in M. oryzae, the targeted gene deletion vector pMD‐MoARK1KO was introduced into the wild‐type strain (Fig. S2A, see Supporting Information), and two ΔMoark1 mutants were confirmed by reverse transcription‐polymerase chain reaction (RT‐PCR) and Southern blot analysis (Fig. S2B, C).
MoARK1 affects endocytosis and formation of the Spitzenkörper body
Because ScArk1p is involved in endocytosis and the actin cytoskeleton, we first evaluated whether the loss of MoARK1 also resulted in defects in endocytosis and intracellular transport. We performed FM4‐64 staining to examine vacuolar membrane internalization. In wild‐type cells, visible internal staining suggested rapid uptake of the FM4‐64 dye and functional endocytosis. In these cells, FM4‐64 was internalized within 15 min of incubation, resulting in various bright ring‐like stained structures representing endosomes and vesicles. In contrast, no definitive staining pattern was observed in the hyphal cell of the ΔMoark1 mutant, either at the plasma membrane or in the cytosol, up to 30–45 min after exposure to the dye. Reintroduction of the MoARK1 gene to the ΔMoark1 mutant restored the staining pattern (Fig. 1A). These results indicate that the ΔMoark1 mutant is altered in endocytosis.
Figure 1.
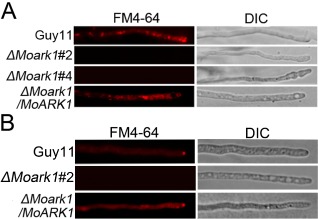
MoArk1 has a role in the formation of the Spitzenkörper and endocytosis. (A) FM4‐64 staining revealed that the ΔMoark1 mutant was defective in endocytosis. Strains were grown for 2 days on complete medium (CM)‐overlaid microscope slides before the addition of FM4‐64 and photographs were taken after 15 min of exposure to FM4‐64. Camera exposure is indicated in seconds (800 ms). (B) The wild‐type and complemented strains showed the presence of an intact Spitzenkörper at the tips of the hyphae, which was missing in the ΔMoark1 mutants after exposure to FM4‐64 staining for 15 min. Strains were grown for 2 days on CM‐overlaid microscope slides before staining. DIC, differential interference contrast image.
The Spitzenkörper body is a dynamic structure present at the tips of hyphal cells with a single highly polarized growth site. It is closely associated with cellular morphogenesis and polar growth and is only present at actively growing sites (Virag and Harris, 2006). Recent studies have suggested that the Spitzenkörper is involved in both exocytosis and endocytosis. To examine whether the endocytosis defect of the ΔMoark1 mutation also affected Spitzenkörper formation, we observed Spitzenkörper formation using FM4‐64 staining. The Spitzenkörper was readily visible within 15 min of incubation in the wild‐type strain, but not in the ΔMoark1 mutants, even after extended incubation (Fig. 1B), suggesting that disruption of MoARK1 results in defects in Spitzenkörper formation.
Growth defects of ΔMoark1 mutants on various media
Given that ScArk1p was identified as relevant to the growth characteristics of the yeast cell (Smythe and Ayscough, 2003), we investigated whether MoArk1 had a similar role. After incubation at 28 °C for 7 days in the dark, the ΔMoark1 mutant showed reduced growth rates, sparse aerial mycelia and abnormal pigmentation on various media, including complete medium (CM), oatmeal agar medium (OM), straw decoction and corn agar (SDC) and V8 (Fig. 2A). This suggests that the loss of MoArk1 may reduce the fitness of the ΔMoark1 mutant. The dry weight of the mycelium, which was incubated in CM for 48 h, was reduced significantly by >30% when compared with the wild‐type, indicating that MoArk1 is important for growth (Fig. 2B). To determine whether the deletion of MoArk1 affected growth on complex media, wild‐type Guy11, the ΔMoark1 mutant and complemented strains were cultured on GMM [1% glucose minimal medium: 0.52 g/L KCl, 0.52 g/L MgSO4·7H2O, 1.52 g/L KH2PO4, 10 g/L glucose, 0.001% (W/V) thiamine and 0.1% (W/V) trace elements] or FMM [1% fructose minimal medium: 0.52 g/L KCl, 0.52 g/L MgSO4·7H2O, 1.52 g/L KH2PO4, 10 g/L fructose, 0.001% (W/V) thiamine and 0.1% (W/V) trace elements] containing different carbon and nitrogen sources, such as vitamins (VA), casamino acids (CA), peptone (PE), yeast extract (YE), yeast nitrogen base without amino acids (YN‐AA), NaNO3 and (NH4)2SO4. The results showed that the ΔMoark1 mutant grew much more slowly than the wild‐type and complemented strains on GMM + VA, GMM + CA, GMM + PE, GMM + NaNO3 and FMM + NaNO3 media (Fig. 2C, D). In contrast, no significant difference was observed between the wild‐type and ΔMoark1 mutant on GMM + YE, GMM + YE‐AA and FMM + (NH4)2SO4 media (Fig. 2C, D). These results indicate that MoArk1 plays a crucial role in nutrient uptake.
Figure 2.
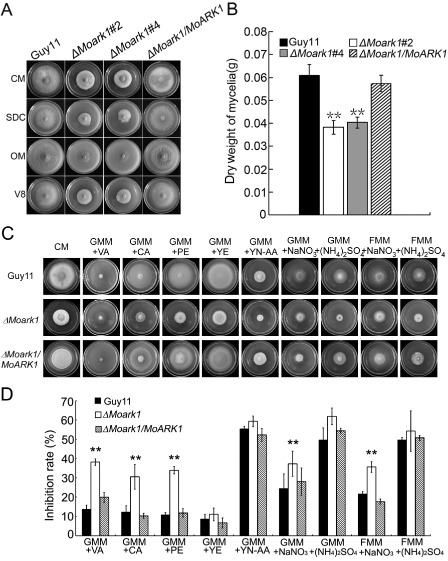
MoArk1 is required for normal mycelial growth on various media. (A) The ΔMoark1 mutants displayed reduced mycelial growth on complete medium (CM), straw decoction and corn agar (SDC), oatmeal agar (OM) and V8 medium following incubation of plates at 28 °C for 7 days in the dark. (B) Mycelium dry weight assessment of cultures of ΔMoark1 mutants, wild‐type and complemented strains (ΔMoark1/MoARK1). (C) Supplements, such as vitamins (VA), casamino acids (CA), peptone (PE), yeast extract (YE) and yeast nitrogen base without amino acids (YN‐AA), were added in the same amounts to GMM [1% glucose minimal medium: 0.52 g/L KCl, 0.52 g/L MgSO4·7H2O, 1.52 g/L KH2PO4, 10 g/L glucose, 0.001% (W/V) thiamine and 0.1% (W/V) trace elements; containing 10 mM NH4 +] to determine what component(s) of CM remediated ΔMoark1 growth. In addition, 100 m m NaNO3 or 50 m m (NH4)2SO4 was added to 1% glucose or 1% fructose to compare the different monosaccharide absorption differences. (D) Statistical analyses of the inhibition rate of wild‐type Guy11, ΔMoark1 mutant and the complemented strains on different sole nitrogen and carbon sources. Error bars represent the standard deviations and asterisks denote statistical significances (P < 0.01).
MoArk1 disruption results in a reduction in conidia formation and abnormal conidium morphology
Conidia play an important role in the disease cycle of rice blast (Lee et al., 2006). To examine the role of MoArk1 in conidium production, conidiation of the wild‐type strain (Guy11), ΔMoark1 mutants (#2 and #4) and the complemented strain (ΔMoark1/MoARK1) from 7‐day‐old SDC culture was examined. Conidia formation was reduced by approximately two‐ to three‐fold in the ΔMoark1 mutants compared with the wild‐type and complemented strains (Fig. 3A–C). Some of the conidia formed by ΔMoark1 mutants also showed abnormal morphology. Calcofluor white (CFW) staining showed that 30% of the mutant conidia had only one or two cells, in contrast with the mostly (∼95%) three‐celled conidia of the wild‐type (Fig. 3D). Surprisingly, we found that ΔMoark1 mutants showed no obvious defect in conidium germination and appressorium formation. These results suggest that the loss of MoArk1 affects conidiogenesis and conidial morphology.
Figure 3.
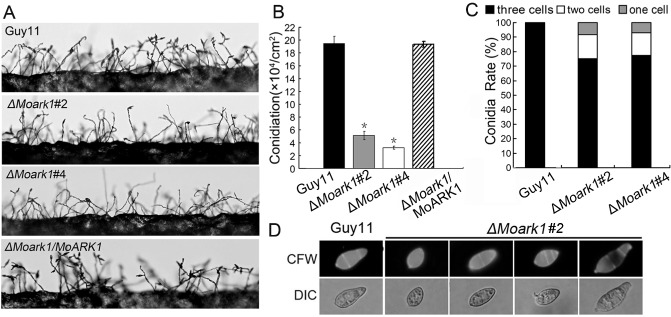
The ΔMoark1 deletion mutants show abnormal conidial morphology and reduced conidiation. (A) Development of conidia on conidiophores is affected by MoARK1 disruption. Strains grown on straw decoction and corn agar (SDC) medium for 7 days were examined by light microscopy. (B) Statistical analysis of conidia production. The conidia produced by the wild‐type strain (Guy11), mutants and complemented strains grown on SDC medium for 10 days were collected, counted and analysed by Duncan analysis (P < 0.01). Asterisks indicate significant differences among Guy11, the ΔMoark1 mutant and complemented strains. Three independent experiments yielded similar results. Error bars represent standard deviation (n = 3). (C) The percentage of each type of abnormal conidia (n = 100). Three independent experiments were performed and similar results were obtained. (D) Conidia shape comparison. The conidia were stained with calcofluor white and photographed.
MoArk1 directly or indirectly contributes to the pathogenicity of M. oryzae
To determine whether MoArk1 is involved in pathogenesis, conidial suspensions (5 × 104 spores/mL) from the wild‐type, two independently generated ΔMoark1 mutants and complemented strains were sprayed onto susceptible rice seedlings (CO‐39 cv. oryzae) and detached barley leaves. After 7 days, the ΔMoark1 mutants still caused disease lesions, but a significant reduction in lesion number was found compared with those produced by the controls (Fig. 4A). Disease on rice was also quantified using a ‘lesion‐type’ scoring assay (Valent et al., 1991), which showed that, although lesion types 1–3 were produced by both the ΔMoark1 mutants and wild‐type, lesion types 4 and 5 (severe, coalescing) were not produced by the mutant (Fig. 4B). On detached barley leaves, which were inoculated with three different concentrations of conidial suspension (1 × 105, 1 × 104 and 1 × 103 spores/mL), lesions produced by the ΔMoark1 mutants were less severe (Fig. 4C). Thus, a loss of MoArk1 function reduces the colonization of leaves by the rice blast fungus.
Figure 4.
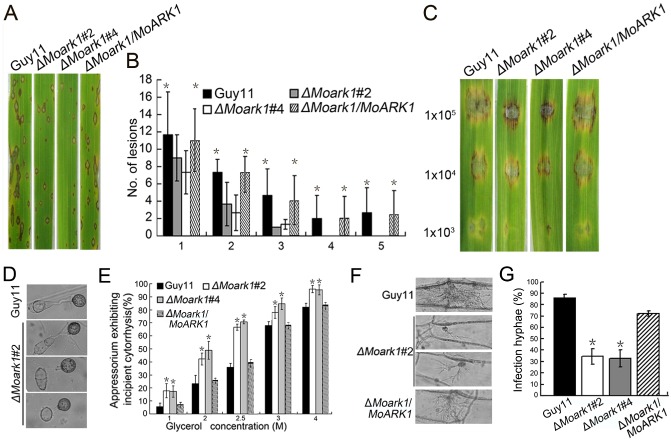
MoARK1 deletion attenuated pathogenicity. (A) The MoARK1 deletion attenuated pathogenicity on rice leaves. (B) Quantification of lesion type (0, no lesion; 1, pinhead‐sized brown specks; 2, 1.5‐mm brown spots; 3, 2–3‐mm grey spots with brown margins; 4, many elliptical grey spots longer than 3 mm; 5, coalesced lesions infecting 50% or more of the leaf area) reveals no difference in lesion types 1–3; however, the ΔMoark1 mutants do not make any lesions of types 4 and 5. Lesions were photographed and measured or scored at 7 days post‐inoculation (dpi) and experiments were repeated twice with similar results. n = 35 for each mutant. (C) Conidia containing different concentrations of ΔMoark1 mutants were drop inoculated onto barley and show a virulence defect compared with the wild‐type strain (Guy11) and complemented strains, as manifested by smaller lesions at 5 dpi. (D) Appressoria formation is altered in the ΔMoark1 mutant. (E) Collapsed appressoria were observed in the wild‐type Guy11, ΔMoark1 mutant and complemented strains. For each glycerol concentration, at least 100 appressoria were observed and the numbers of collapsed appressoria were counted. (F) The ΔMoark1 mutants exhibited reduced host penetration on onion epidermis after 48 h in contrast with control strains. (G) Quantification of hyphae infection on onion epidermis after 48 h. Three independent biological experiments yielded similar results. Error bars represent standard deviation, and asterisks represent significant difference between Guy11 and mutants (P < 0.01).
ΔMoark1 mutants are altered in the generation of appressorium turgor pressure
To penetrate the rice leaf cuticle and cause infection, a high appressorium internal turgor pressure is required (Talbot and Foster, 2001; Thines et al., 2000). Appressorium turgor can be measured using an incipient cytorrhysis assay, which uses hyperosmotic concentrations of a solute to collapse the appressoria (DeJong et al., 1997). As appressorial formation appears to be normal in the ΔMoark1 mutants (Fig. 4D), we hypothesized that the reduced pathogenicity may be a result of a defect in appressorium turgor pressure. The appressoria of the ΔMoark1 mutants showed an increased collapse rate even in 1 m glycerol solution (Fig. 4E), validating our hypothesis. We further inoculated the conidial suspensions onto onion epidermis and found that only a small number of appressoria were developed after 48 h (approximately 30%, compared with 95% in the wild‐type) (Fig. 4F). The wild‐type strain exhibited an approximately 85% penetration rate, whereas the penetration rate of the ΔMoark1 mutants was only 30%. The percentage of infectious hyphae in the wild‐type was also distinct from that of the ΔMoark1 mutant (Fig. 4G). These results indicate that the reduced pathogenicity of the ΔMoark1 mutant is caused, at least in part, by defects in appressorium turgor pressure and subsequent host penetration.
ΔMoark1 mutants are defective in infectious hyphal growth on the plant
To further explore the reasons why ΔMoark1 mutants show reduced virulence on host plants, we examined the invasive hyphal growth on both barley leaves and rice leaf sheaths. After incubation with the spore suspensions for 48 h, four types (type 1, no penetration; type 2, with penetration peg; type 3, with a single invasive hypha; type 4, with extensive hyphal growth) of invasive hyphae were observed in barley tissues (Fig. 5A). In the wild‐type Guy11 and the complemented strains, 60% of cells showed type 4 and 18% showed type 1 and type 2 invasive hyphal growth. In contrast, less than 10% of cells showed type 4 and more than 80% showed type 1 and type 2 invasive hyphal growth in the ΔMoark1 mutants. In addition, in the proline‐rich domain deletion mutant, 40% of cells showed type 4 and 20% showed type 1 and type 2 (Fig. 5A, B). A similar result was observed in rice leaf sheaths, with the wild‐type Guy11 and complemented strains displaying faster hyphal growth extension to neighbouring cells and the ΔMoark1 mutants showing lower hyphal growth limited to one cell (Fig. 5C). These results indicate that MoArk1 plays an important role in infectious hyphal growth and plant colonization.
Figure 5.
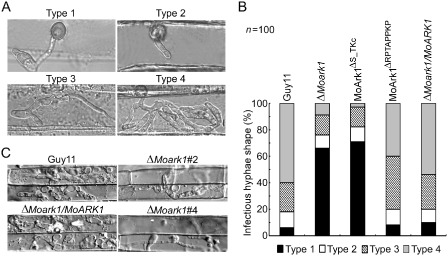
Colonization of ΔMoark1 mutant on plants. (A) Close observation of infectious growth on barley. Excised barley leaves from 7‐day‐old barley seedlings were inoculated with conidial suspension (1 × 104 spores/mL of each strain). Infectious growth was observed at 24 h post‐inoculation (hpi). (B) Statistical analysis for each type of infectious hyphal shape, for each tested strain; 100 infecting hyphae (n = 100) were counted per replicate and the experiment was repeated three times. (C) Excised rice sheath from 4‐week‐old rice seedlings was inoculated with conidial suspension (1 × 104 spores/mL of each strain). Infectious growth was observed at 48 hpi.
ΔMoark1 mutants are less sensitive to salt and osmotic stress
To investigate the role of MoArk1 in stress responses, such as sensitivity to salt and osmotic adaptation, the wild‐type Guy11, ΔMoark1 and complemented strains were exposed to various conditions, including ion stress (Na+ and K+) and osmotic stress (sorbitol). The ΔMoark1 mutant exposed to 0.7 m NaCl, 1 m sorbitol and 1.2 m KCl showed 20%, 8% and 45% lower growth inhibition, respectively, than the wild‐type strain (Fig. S3A, B, see Supporting Information). This demonstrates that the lack of MoArk1 affects salt and osmotic adaptation.
Cell wall integrity is altered in the ΔMoark1 mutants
As the fungal cell wall plays an essential role in maintaining hyphal morphology and adaptation to the environment, the structural integrity of the ΔMoark1 mutant cell wall and membrane was examined. Mycelial growth was measured on CM containing various concentrations of cell wall stressors, including CFW, sodium dodecylsulphate (SDS) and Congo Red (CR). In growth assays with three different concentrations of CFW, the ΔMoark1 mutant showed slight resistance to CFW with an average 8%, 10% and 12% lower growth inhibition than the wild‐type and complemented strains, which were 30%, 40% and 50%, respectively (Fig. S4A, B, see Supporting Information). The responses to SDS and CR were slightly different. At lower concentrations, the ΔMoark1 mutants showed sensitivity to these stress inducers, but, at higher concentrations, the mutants showed more resistance (Fig. S4C–E). The results were largely supportive of our hypothesis that MoArk1 plays a role in cell wall integrity.
Deletion of MoARK1 alters chitin distribution
Previous studies have shown that ΔMovam7 and ΔMosec22 mutants exhibit abnormal chitin distributions, which are not restricted to growing apices, but also occur on lateral walls along hyphal axes. In the wild‐type M. oryzae strain, CFW fluorescence was mostly distributed at the septa and tips where chitin, one of the main components of the fungal cell wall, is actively synthesized. In the ΔMoark1 mutants, patches of bright fluorescence were observed on the lateral wall of hyphae in addition to septa and hyphal tips (Fig. 6A). This abnormal distribution of cell wall components was restored by the reintroduction of wild‐type MoArk1 (Fig. 6A). As a result of continuous tip elongation in filamentous fungi, these organisms may need to recycle certain components, such as cell wall‐building enzymes, to the tip region. As CFW intercalates with nascent chitin chains, this result suggests that the altered distribution of chitin on the ΔMoark1 mutant cell wall could be caused by aberrant cell wall synthesis activities.
Figure 6.
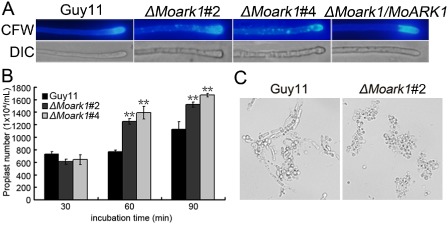
Mycelial calcofluor white (CFW) staining and protoplast release assay. (A) Disruption of MoARK1 altered the distribution of chitin on the cell wall. Wild‐type and mutant hyphae were stained with 10 μg/mL CFW for 5 min without light before being photographed. The experiment was repeated three times with triplicates, and displayed the same results. DIC, differential interference contrast image. (B) Protoplast release assay of Guy11 and ΔMoark1 mutants. Asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.01, according to Duncan's range test. (C) Light microscopic examination of protoplast release after 40 min.
In addition, we examined the effects of lytic enzymes (10 mg/mL lysing enzymes) on the ΔMoark1 mutant. More protoplasts were found in the ΔMoark1 mutant than in the controls after treatment for 60 and 90 min (Fig. 6B). When observed at 60 min, the ΔMoark1 mutant showed a greater number of protoplasts and almost no mycelial fragments were observed, in contrast with the wild‐type, where mycelial fragments were still found (Fig. 6C).
ΔMoark1 mutants are less sensitive to oxidative stress and exhibit higher extracellular peroxidase activities
In fungi, secreted peroxidases are regarded as an important component to help pathogens detoxify host‐derived reactive oxygen species during plant–microbe interactions (Guo et al., 2010). To investigate whether MoArk1 was involved in the oxidative stress response, wild‐type Guy11, ΔMoark1 mutants and the complemented strains were exposed to hydrogen peroxide (H2O2). The mycelial growth of the ΔMoark1 mutants was affected slightly (Fig. S5A, see Supporting Information). Exposure to 2.5 and 5 mm H2O2 led to an average 5% (2.5 mm) and 13% (5 mm) decrease, respectively, in growth inhibition compared with the wild‐type strain (Fig. S5B).
We next evaluated the influence of H2O2 on CR degradation, as indicated by the presence of discolored halos surrounding the colony. Discolored halos were observed in the ΔMoark1 mutants and the control strain (Fig. S5C). The discoloration appeared to be more solid than in the wild‐type, suggesting that MoArk1 might also affect peroxidase activity. Indeed, enzyme activity assays using extracellular culture filtrates and 2,2′‐Azino‐bis(3‐ethyl benzothiazoline‐6‐sulfonic acid) diammonium salt (ABTS) as a substrate revealed that the ΔMoark1 mutant exhibited stronger peroxidase activity (Fig. S5D). We further examined the transcriptional levels of four peroxidase‐encoding genes, all of which possess a signal peptide (MGG_04545.6, MGG_13291.6, MGG_08730.6 and MGG_07790.6). We found that the expression levels for three of these genes were increased in the ΔMoark1 mutants (Fig. S5D). These data suggest that MoArk1 is involved in controlling extracellular peroxidase activities by negative feedback regulation.
A proline‐rich motif is responsible for the subcellular localization of MoArk1
In S. cerevisiae, Ark1p was found to affect the organization of the cortical actin cytoskeleton. Actin plays multiple complex roles in cell growth and cell shape (Heath et al., 2003). Recently, actin patches, which represent sites of endocytosis, have been demonstrated to be present in a subapical collar at the growing tips of hyphae and germ tubes of filamentous fungi. This zone of endocytosis is now known to be necessary for filamentous growth to proceed (Kaksonen et al., 2003). A ScArk1p‐green fluorescent protein (ScArk1p‐GFP) fusion protein was visible in the cortical patch structure with a very similar appearance and behaviour to actin patches. When GFP and actin were both visualized by indirect immunofluorescence, they were found to co‐localize in patches at the cell cortex (Smythe and Ayscough, 2003). To investigate whether MoArk1 exhibits the actin‐like distribution pattern, we detected the subcellular localization of MoArk1 using a MoARK1‐GFP fusion protein. In ΔMoark1 expressing MoArk1‐GFP, fluorescence was detected mainly in vacuole‐like patches in vegetative hyphal tips. Meanwhile, the hyphae were stained with rhodamine–phalloidin which binds actin after 48 h of static culture, which showed red fluorescence co‐localizing with MoArk1‐GFP fluorescence (Fig. 7A). During appressoria development, fluorescent signals were also detected at the periphery of appressoria and in vacuoles. In mature appressoria (24 h), GFP signals were mainly localized to globular structures at the base of mature appressoria (Fig. 7B).
Figure 7.
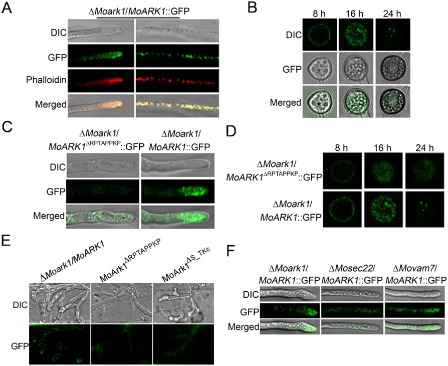
Cellular localization of MoArk1‐green fluorescent protein (MoArk1‐GFP) and MoArk1 Δ RPTAPPKP‐GFP. (A) Vegetative hyphal tips were observed under laser scanning confocal microscopy. The hyphae were stained with the actin dye rhodamine‐phalloidin after 48 h of static culture. DIC, differential interference contrast image. (B) ΔMoark1/ MoArk1‐GFP fluorescence observation during appressoria formation process after 8, 16 and 24 h under laser scanning confocal microscopy. (C) Vegetative hyphal tips of ΔMoark1/MoArk1 Δ RPTAPPKP‐GFP were observed under laser scanning confocal microscopy. (D) ΔMoark1/MoArk1 Δ RPTAPPKP‐GFP fluorescence observation during appressoria formation process after 8, 16 and 24 h under laser scanning confocal microscopy. (E) Invasive hyphae produced by the MoARK1‐GFP, MoARK1 Δ RPTAPPKP‐GFP and MoARK1 Δ S _ TKc‐GFP transformants in onion epidermal cells at 24 hpi. (F) Vegetative hyphal tips of ΔMoark1/MoARK1, ΔMosec22/MoARK1 and ΔMovam7/MoARK1 under laser scanning confocal microscopy.
Proline‐rich (KPxPPPKP) regions are known to mediate protein–protein interactions and can be bound by Src‐homology (SH3) domains (Cope et al., 1999). Several interactions involving proline‐rich motifs and SH3 domains are found among proteins in yeast cortical actin patches. MoArk1 may also participate in such associations. We generated the MoARK1 ΔRPTAPPKP‐GFP construct and transformed it into ΔMoark1 protoplasts. In the resulting transformants, the GFP fluorescence was weaker and the Ark1 localization pattern was not observed in hyphae and mature appressoria, compared with the MoARK1‐GFP transformants (Fig. 7C, D). We also examined the GFP signal during infection. The MoARK1‐GFP transformant showed an actin‐like localization pattern in the plant cells. However, only dispersed GFP signal was observed in the MoARK1 ΔRPTAPPKP‐GFP and MoARK1 ΔS_TKc‐GFP transformants (Fig. 7E). The above results indicate that the proline‐rich region is responsible for the subcellular localization of MoArk1, as well as pathogenicity.
MoArk1 subcellular localization involves functions of SNARE proteins MoVam7 and MoSec22
SNARE proteins ensure the specific recognition and transportation of vesicles and target membrane‐mediated fusions (Fukuda et al., 2000). Our previous studies identified two SNARE proteins, MoVam7 and MoSec22, which are involved in cell membrane transport and endocytosis (Dou et al., 2011; Song et al., 2010). To determine whether MoVam7 and MoSec22 were involved in the subcellular localization of MoArk1, we transformed MoARK1‐GFP into the ΔMosec22 and ΔMovam7 mutants. We found that GFP fluorescence was clustered at the hyphal tip in the ΔMoark1/MoARK1 complemented strain. In contrast, the fluorescence was primarily internal in the hyphae of ΔMosec22/MoARK1‐GFP and ΔMovam7/MoARK1‐GFP mutants (Fig. 7F). This suggests that the lack of MoSec22 and MoVam7 disturbs vesicle transport, which affects the normal localization of MoArk1.
Functional characterization of different domains of MoARK1
MoARK1 has an S/TKc domain and a RPTAPPKP motif, similar to ScArk1p. We also generated mutant alleles of MoARK1‐GFP (Fig. 8A). Transformants expressing the MoArkΔS_TKc‐GFP showed similar phenotypes to ΔMoark1, which exhibited reduction in plant infection and altered endocytosis (Fig. 8B, C). This indicates that both the S/TKc domain and the RPTAPPKP motif are essential for the role of MoArk1 in endocytosis and virulence.
Figure 8.
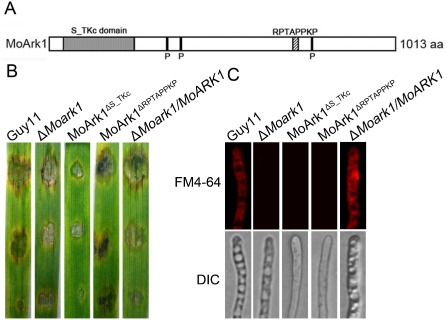
Function of serine/threonine kinase (S/TKc) domain and RPTAPPKP motif. (A) Prediction domains of MoArk1. (B) Pathogenicity of mutants MoArk1 Δ S / TKc and MoArk1 Δ RPTAPPKP. (C) FM4‐64 staining of mutants MoArk1 Δ S / TKc and MoArk1 Δ RPTAPPKP revealed that they were defective in endocytosis. DIC, differential interference contrast image.
Discussion
Endocytosis is the process by which eukaryotic cells internalize macromolecules containing plasma membrane lipids and associated proteins in vesicles that fuse with the endosomal system. Subsequent sorting into different endosomal domains determines whether a given cargo recycles to the plasma membrane, traffics to the Golgi or follows the endocytic pathway to the vacuolar lumen, undergoing degradation (Penalva, 2010). Saccharomyces cerevisiae has played a key role in our understanding of the endocytic internalization process, and ScArk1p is one of the proteins that regulates endocytosis through binding to other cortical actin cytoskeleton proteins. As a serine/threonine kinase, ScArk1p initiates phosphorylation cycles that control the endocytic machinery (Galletta and Copper, 2009). Here, we characterized MoARK1, a ScArk1 homologue from M. oryzae, and found that it too plays a role in the regulation of endocytosis and the actin cytoskeleton. We confirmed that such important functions endow MoArk1 with a role in growth, cell wall integrity and virulence.
MoArk1 plays a role in the cell wall integrity of M. oryzae
In M. oryzae, the cell wall undergoes considerable changes during morphogenesis (Jeon et al., 2008). Previous studies have revealed that most cell wall‐defective mutants with breached cell wall integrity exhibit various developmental defects and are nonpathogenic (Jeon et al., 2008; Li et al., 2006). In addition, the polarized growth of filamentous fungi has been speculated to require the endocytic uptake and recycling of cell wall components, such as chitin (Harris, 2006). Multiple signalling pathways allow organisms to respond to different extracellular stimuli and to adjust their cellular machinery according to changes in their environment. Sensing changes in environmental osmolarity is vital for cell survival. Several cell wall integrity‐associated genes, such as MoMCK1, MoPDEH and MoRGS1, have been characterized in M. oryzae (Jeon et al., 2008; Zhang et al., 2011a, b), which have been described as being essential for cell wall integrity and pathogenicity. Consistent with these studies, we found that the weakened cell walls and membranes were coupled with the abnormal distribution of chitins in the ΔMoark1 mutants. Thus, the disruption of normal membrane trafficking by MoArk1 may negatively affect the secretory transport of chitosomes containing chitins and the enzymes that synthesize chitin. Meanwhile, the ΔMoark1 mutant exhibited some resistance to cell wall stress‐inducing agents, such as CFW, at higher concentrations, which may result from changes in cell wall metabolism/composition or compensatory function by other serine/threonine kinases.
MoSec22 and MoVam7 are SNARE homologues in M. oryzae (Dou et al., 2011; Song et al., 2010). Targeted deletion of MoSec22 and MoVam7 resulted in numerous developmental defects, including cell wall integrity, which were very similar to the ecology phenotype of the ΔMoark1 mutants. Thus, endocytosis signalling may exhibit cross‐talk with mitogen‐activated protein kinase (MAPK) signalling in the regulation of cell wall integrity. In A. oryzae, the homologue of S. cerevisiae End4/Sla2 is AoEnd4. Hyphae grown under AoEND4‐repressed conditions displayed marked growth defects and irregularly shaped colonies as a result of apical growth defects, endocytic defects in FM4‐64 and abnormal cell wall synthesis. CFW staining revealed that chitin accumulated in aberrant invagination structures under AoEND4‐repressed conditions (Higuchi et al., 2009). These results suggest that proteins involved in cell wall synthesis, such as chitin synthases, are probably not recycled to the tip region and therefore accumulate in the aberrant invagination structures. Overall, this is consistent with the crucial role of endocytosis in the physiology of hyphal growth and the maintenance of cell wall integrity.
Effects of MoArk1 in endocytosis and pathogenicity
Studies in S. cerevisiae have demonstrated the importance of intracellular transport in the growth and differentiation of lower eukaryotic organisms. As secretory properties, in particular, have the propensity to be linked to virulence, intracellular transport in medically important pathogenic fungi, such as Candida albicans and Cryptococcus neoformans, has been the focus of several studies (Chaffin et al., 1998). In C. neoformans, deletion of the endocytic protein Cin1 resulted in mutant strains with reduced pathogenicity and pleiotropic defects, including reduced melanin production (Shen et al., 2010). In M. oryzae, defects in cell wall composition can influence appressorium formation and impair the successful infection of rice (Skamnioti et al., 2007). During hyphal growth and appressorium formation, endocytic markers were actively internalized by both conidia and conidial germlings on rice leaves, suggesting that endocytosis might play a major role in spore germination and growth of the germ tube.
FM4‐64 staining revealed that endocytosis was blocked by the disruption of MoARK1, and the ΔMoark1 mutants also failed to display the Spitzenkörper body, the structure associated with rapidly growing hyphal tips in filamentous fungi. Meanwhile, the ΔMoark1 mutant showed infection defects on plants. During the extreme polarized growth of fungal hyphae, secretory vesicles are thought to accumulate in a subapical region called the Spitzenkörper. In ΔMoark1 mutants, the secretory vesicles and endosome may have failed to accumulate because of the lack of the Spitzenkörper body, as well as abnormal appressorium formation, which is a terminal cell required for the penetration of the host that is formed from the germ tube tip. This indicates that growth and differentiation were compromised in the ΔMoark1 mutants, which would probably have reduced pathogenicity on host plants as a result of a loss of endocytosis and abnormal appressoria.
The balance between endocytosis and exocytosis in M. oryzae
The apical recycling model predicts that a balance between exocytic and endocytic processes must be present at the hyphal tip to allow for growth and, when exocytosis and endocytosis are in balance, the resulting cell shape is a hypha (Shaw et al., 2011). Accordingly, the conidium shape of the ΔMoark1 mutants displayed abnormal morphology, indicating that exocytosis and endocytosis may function in overlapping, but distinct, pathways that regulate the intracellular trafficking in M. oryzae. When endocytosis was down‐regulated or abolished, as in the slaB, fimA and arfB mutants in Aspergillus, the cell was unable to maintain polarity (Hervas‐Aguilar and Penalva, 2010; Lee et al., 2008; Upadhyay and Shaw, 2008). In contrast, when endocytosis was unaffected, actin patches were present at the cell apex, and hyphal growth ceased as the polarization machinery was cycled back into the cell. As a precise balance between exocytosis and endocytosis exists at the hyphal tip, one can further predict that the fungal cell could alter this balance to undergo morphogenetic changes. In M. oryzae, both the ΔMovam7 and the ΔMosec22 mutants are defective in conidiogenesis and appressorium formation; they failed to colonize plant tissue and were unable to form conidiophores, suggesting that vesicular trafficking mediated by different SNAREs is dependent on the stage of conidiogenesis. Meanwhile, the SNARE proteins MoVam7 and MoSec22 are involved in the subcellular localization of MoArk1. Future studies on the intracellular trafficking of the endocytic pathway and the balance between endocytosis and exocytosis are warranted.
In summary, we found that MoArk1 is a multifunctional protein required for conidiogenesis and pathogenicity in M. oryzae. MoArk1 participates in the maintenance of cell wall integrity and intracellular trafficking. Although the precise role of MoArk1 in the regulation of endocytosis remains the subject of future studies, the multifunction of MoArk1 underscores the importance of endocytic proteins in the physiology and pathogenicity of fungi. Further studies to dissect the proteins that interact with MoArk1 and to delineate the significance of these interactions will reveal the importance of endocytosis in virulence mechanisms of M. oryzae.
Experimental Procedures
Strains and culture conditions
Magnaporthe oryzae strain Guy11 was used as the parental strain, and all of the strains were cultured on CM at 28 °C unless stated otherwise. Liquid CM was used to prepare the mycelia for DNA and RNA extraction. For conidiation, strain blocks were maintained on SDC (100 g of straw, 40 g of corn powder, 15 g of agar in 1 L of distilled water) at 28 °C for 7 days in the dark, followed by 3 days of continuous illumination under fluorescent light. For medium containing cell wall‐perturbing agents, the final concentrations were 0.005, 0.01 and 0.02% for SDS, 200, 400 and 600 μg/mL for CR, and 200, 400 and 600 μg/mL for CFW.
MoARK1 cloning and sequence analysis
A full‐length cDNA fragment for the MoARK1 gene was isolated from the Guy11 strain. cDNA was cloned into the pMD19 T‐vector (TaKaRa, Dalian, China) to generate pMD‐MoARK1 and verified by sequencing. Amino acid sequence alignments were performed using the Clustal_W program and the calculated phylogenetic tree was viewed using the Mega3.1 Beta program.
Disruption of MoARK1 and ΔMoark1 mutant complementation
The vector pMD‐MoARK1 was constructed for targeted gene deletion by inserting the hygromycin resistance HPH marker gene cassette into the two flanking sequences of the MoARK1 gene (Fig. S2A). A 1.0‐kb upstream flanking sequence and a 1.1‐kb downstream flanking sequence were amplified with the primer pairs FL8678/FL8679 and FL8680/FL8681, respectively. Two PCR fragments were linked by overlap PCR with primer pair FL8678/FL8681, and the linked sequence was cloned into the pMD19 T‐vector. The HPH gene cassette, amplified with primers FL1111/FL1112, was inserted into pMD‐MoARK1 at the PmeI site to generate the final pMD‐MoARK1KO. A 3.5‐kb fragment containing the MoARK1 disruption allele was amplified using primers FL8678/FL8681, purified and used to transform Guy11 according to established protocols. Table S1 (see Supporting Information) lists all of the primers used.
Putative ΔMoark1 mutants were screened by PCR and confirmed by Southern blotting analysis (Fig. S2B, C). Further confirmation was performed through RT‐PCR with primers FL8682/FL8683. For complementation, a 4.9‐kb PCR product containing the full‐length MoARK1 coding region and the 1.5‐kb upstream region, was amplified using primers FL9653/FL9654 and subcloned into pYF11, generating pYF11‐MoARK1R. The resulting transformants were first screened by phenotype characterization, followed by PCR amplification, and verified by fully restored growth.
Assays for dry weight, protoplast release, vegetative growth, conidiation and appressorium formation
For vegetative growth, mycelial plugs of 3 mm × 3 mm were transferred from 7‐day‐old CM plates and inoculated onto fresh media (CM, V8, OM and SDC) (Zhang et al., 2010), followed by incubation at 28 °C. The radial growth was measured after incubation for 6 days. All experiments were repeated three times, each with three replicates. For conidia production, mycelia were grown in the dark on SDC medium at 28 °C for 7 days, followed by constant illumination for 3–4 days. Conidia were collected by washing with double‐distilled water (ddH2O), filtered through three‐layer lens paper and concentrated by centrifugation (6000 g) for 10 min. The final spores were suspended in 2 mL of ddH2O and counted using a haemacytometer.
Appressorium formation was measured on Gel Bond film (FMC Bioproducts, Rockland, ME, USA), as described previously (Zhang et al., 2011c). Appressoria were observed through direct microscopic examination and percentages were obtained from at least 100 conidia per replicate at 24 and 48 h in at least three experiments.
Light microscopy studies
To examine hyphal morphology, strains were grown on a thin layer of CM agar on the microscope slides. After 2 days in a 28 °C growth chamber, the hyphae were observed under an Olympus BH‐2 microscope (Olympus, Tokyo, Japan). The cell wall, hyphal septum and conidia were visualized by CFW (10 μg/mL, Sigma, St. Louis, MO, USA) staining, as described by Harris et al. (1994). FM4‐64 staining was conducted following the procedures described previously (Fischer‐Parton et al., 2000). Fluorescence microscopy was performed using laser scanning confocal microscopy.
Pathogenicity assay
Conidia were harvested from 10‐day‐old SDC agar cultures, filtered through three layers of lens paper and resuspended to a concentration of 5 × 104 spores/mL in a 0.2% (w/v) gelatin solution. For the detached leaf assay, leaves from 7‐day‐old barley (cv. Four‐arris) seedlings were used. Two‐week‐old seedlings of rice (Oryza sativa cv. CO39) and 7‐day‐old seedlings of barley were used for infection assays. Three 20‐μL droplets were placed onto the upper side of the barley leaves maintained on 4% (w/v) water agar (WA) plates. Photographs were taken 5 days after incubation at 25 °C. For spray inoculation, 5 mL of a conidial suspension of each treatment were sprayed onto rice with a sprayer. Inoculated plants were kept in a growth chamber at 25 °C with 90% humidity and in the dark for the first 24 h, followed by a 12 h/12 h light/dark cycle. Lesion formation was observed daily and photographed 7 days after inoculation (Guo et al., 2011).
Measurement of the chitin content
The chitin (N‐acetylglucosamine, GlcNAc) content was determined as described by Bulik et al. (2003). Briefly, for each sample, 5 mg of freeze–dried mycelia were resuspended in 1 mL of 6% KOH and heated at 80 °C for 90 min. Samples were centrifuged (16 000 g, 10 min) and the pellets were washed with phosphate‐buffered saline in three cycles of centrifugation and suspension (16 000 g, 10 min), before final suspension in 0.5 mL of McIlvaine's buffer (pH 6). An aliquot of 100 μL (13 units) of Streptomyces plicatus chitinase (Sigma) was added and incubation was carried out for 16 h at 37 °C with gentle mixing; 100‐μL samples were then combined with 100 μL of 0.27 m sodium borate (pH 9), heated for 10 min at 100 °C, and 1 mL of freshly diluted (1:10) Ehrlich's reagent (10 g p‐dimethylaminobenzaldehyde in 1.25 mL of HCl and 8.75 mL of glacial acetic acid) was added. After incubation at 37 °C for 20 min, 1 mL of the sample was transferred to a 2.5‐mL plastic cuvette (Greiner, Bahlingen, Germany) and the absorbance at 585 nm was recorded. Standard curves were prepared with GlcNAc (Sigma). The experiment was repeated three times.
Supporting information
Fig. S1 Magnaporthe oryzae MoArk1 is a fungal actin‐regulating kinase 1 (Ark1) protein. (A) Prediction of domains of MoArk1 using the SMART website. (B) Phylogenetic analysis of MoArk1 and other Ark1 homologues, including MoArk1 (Magnaporthe oryzae, XP_003713889.1), Neurospora crassa (XP_962918.1), Fusarium graminearum (EGU75522.17), Aspergillus nidulans (XP_661798.1), Saccharomyces cerevisiae (NP_014378.1), Saccharomyces cerevisiae (NP_012171.1), Candida albicans (XP_446476.1), Ustilago maydis (XP_759228.1) and Saccharomyces cerevisiae (NP_009615.1). Sequence alignments were performed using the Clustal_W program, and the calculated phylogenetic tree was viewed using the Mega3.1 Beta program. Neighbour‐joining tree with 1000 bootstrap replicates of phylogenetic relationships among fungal Ark1 homologues. Numbers above the branches represent amino acid substitution.
Fig. S2 Targeted gene replacement and complementation. (A) Restriction map of the MoARK1 genomic region. Disruption construct containing the homologous sequences flanking the HPH cassette to replace a part of MoARK1. (B) Expression analysis of MoARK1 in the wild‐type, ΔMoark1 mutant and complemented strains using Actin as a control. (C) Southern blot analysis of the ΔMoark1 mutant (lanes 1 and 2) and the wild‐type (lane 3) strains using MoARK1‐ or HPH‐specific probes. Genomic DNA was digested with EcoRI.
Fig. S3 Assessment of tolerance to salt and osmotic stresses. (A) The ΔMoark1 mutant, wild‐type and complemented strains were incubated on complete medium (CM) plates containing KCl, NaCl and sorbitol at various concentrations at 28 °C for 7 days. (B) Colony inhibition rate measurement. Inhibition rate = [(diameter of untreated strain—diameter of treated strain)/diameter of untreated strain] × 100%. Error bars represent the standard deviations and asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.05, according to Duncan's range test.
Fig. S4 Responses of wild‐type, ΔMoark1 and ΔMoark1/MoARK1 strains to cell wall‐disturbing agents. (A, C) The ΔMoark1 mutant, wild‐type and complemented strains were incubated on complete medium (CM) plates supplemented with various stress inducers for 7 days at 28 °C. (B, D, E) Statistical analysis of the growth inhibition rate of mycelia on CM supplemented with cell wall‐disturbing agents. Double asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.01, and single asterisk indicates a significant difference at P < 0.05.
Fig. S5 MoARK1 is related to tolerance to H2O2 and the activity of extracellular peroxidase. (A) ΔMoark1 mutants were less sensitive to a higher concentration of H2O2. The Guy11, ΔMoark1 mutants and complemented strain were incubated on complete medium (CM) plates supplemented with 2.5 and 5 mm H2O2 for 6 days before being photographed. (B) Statistical analysis of the growth inhibition rate of mycelial growth on CM supplemented with H2O2. Asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.01, according to Duncan's range test. (C) The discoloration of Congo Red was tested on CM plates containing 200 μg/mL of the dye at a final concentration. Discoloration was observed on day 7 after inoculation at 28 °C. (D) Peroxidase activity was measured using the ABTS oxidizing test under normal and H2O2‐supplemented conditions. (E) Expression profiles of five genes encoding predicted peroxidases under normal or oxidative stress‐induced conditions.
Table S1 Primers used in this study.
Acknowledgements
This research was supported by the National Basic Research Program of China (Grant No: 2012CB114000 to ZZ), Natural Science Foundation of China (Grant No: 31271998 to ZZ, 30971890 to XZ), the Fundamental Research Funds for the Central Universities (Grant No: KYZ201105 to ZZ) and the Project of Jiangsu of China (Grant No: Sx (2009) 54 to XZ). Research in Ping Wang's laboratory was supported by US grants (NIH/NIAID AI054958 and AI074001).
References
- Atkinson, H.A. , Daniels, A. and Read, N.D. (2002) Live‐cell imaging of endocytosis during conidial germination in the rice blast fungus, Magnaporthe grisea . Fungal Genet. Biol. 37, 233–244. [DOI] [PubMed] [Google Scholar]
- Bulik, D.A. , Olczak, M. , Lucero, H.A. , Osmond, B.C. , Robbins, P.W. and Specht, C.A. (2003) Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell, 2, 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin, W.L. , Lopez‐Ribot, J.L. , Casanova, M. , Gozalbo, D. and Martinez, J.P. (1998) Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62, 130–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope, M.J. , Yang, S. , Shang, C. and Drubin, D.G. (1999) Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J. Cell Biol. 144, 1203–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, N.G. , Horecka, J.L. and Sprague, G.F. Jr (1993) Cis‐ and trans‐acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 122, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong, J.C. , MCCormack, B.J. , Smirnoff, N. and Talbot, N.J. (1997) Glycerol generates turgor in rice blast. Nature, 389, 244–245. [Google Scholar]
- D'Hondt, K. , Heese‐Peck, A. and Riezman, H. (2000) Protein and lipid requirements for endocytosis. Annu. Rev. Genet. 34, 255–295. [DOI] [PubMed] [Google Scholar]
- Dou, X.Y. , Wang, Q. , Qi, Z.Q. , Song, W.W. , Wang, W. , Guo, M. , Zhang, H.F. , Zhang, Z.G. , Wang, P. and Zheng, X.B. (2011) MoVam7, a conserved SNARE involved in vacuole assembly, is required for growth, endocytosis, ROS accumulation, and pathogenesis of Magnaporthe oryzae . PLoS ONE, 6, e16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans, N. , Zimmermann, S. and Fischer, R. (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell, 14, 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer‐Parton, S. , Parton, R.M. , Hickey, P.C. , Dijksterhuis, J. , Atkinson, H.A. and Read, N.D. (2000) Confocal microscopy of FM4‐64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. Oxf. 198, 246–259. [DOI] [PubMed] [Google Scholar]
- Fuchs, U. and Steinberg, G. (2005) Endocytosis in the plant‐pathogenic fungus Ustilago maydis . Protoplasma, 226, 75–80. [DOI] [PubMed] [Google Scholar]
- Fuchs, U. , Hause, G. , Schuchardt, I. and Steinberg, G. (2006) Endocytosis is essential for pathogenic development in the corn smut fungus Ustilago maydis . Plant Cell, 18, 2066–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, R. , McNew, J.A. , Weber, T. , Parlati, F. , Engel, T. , Nickel, W. , Rothman, J.E. and Sollner, T.H. (2000) Functional architecture of an intracellular membrane t‐SNARE. Nature, 407, 198–202. [DOI] [PubMed] [Google Scholar]
- Galletta, B.J. and Cooper, J.A. (2009) Actin and endocytosis: mechanisms and phylogeny. Curr. Opin. Cell Biol. 21, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow, N.A.R. , Brown, A.J.P. and Odds, F.C. (2002) Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5, 366–371. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Guo, W. , Chen, Y. , Dong, S.M. , Zhang, X.B. , Zhang, H.F. , Song, W.W. , Wang, W. , Lv, R.L. , Zhang, Z.G. , Wang, Y.C. and Zheng, X.B. (2010) The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 1053–1068. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Chen, Y. , Du, Y. , Dong, Y.H. , Guo, W. , Zhai, S. , Zhang, H.F. , Dong, S.M. , Zhang, Z.G. , Wang, Y.C. , Wang, P. and Zheng, X.B. (2011) The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae . PLoS Pathog. 7, e1001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S.D. (2006) Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 251, 41–77. [DOI] [PubMed] [Google Scholar]
- Harris, S.D. , Morrell, J.L. and Hamer, J.E. (1994) Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics, 136, 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S.D. , Read, N.D. , Roberson, R.W. , Shaw, B. , Seiler, S. , Plamann, M. and Momany, M. (2005) Polarisome meets Spitzenkörper: microscopy, genetics, and genomics converge. Eukaryot. Cell, 4, 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, I.B. , Bonham, M. , Akram, A. and Gupta, G.D. (2003) The interrelationships of actin and hyphal tip growth in the ascomycete Geotrichum candidum . Fungal Genet. Biol. 38, 85–97. [DOI] [PubMed] [Google Scholar]
- Hervas‐Aguilar, A. and Penalva, M.A. (2010) Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans . Eukaryot. Cell, 9, 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, Y. , Shoji, J.Y. , Arioka, M. and Kitamoto, K. (2009) Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae . Eukaryot. Cell, 8, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, R.J. and Valent, B. (1996) Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea . Annu. Rev. Microbiol. 50, 491–512. [DOI] [PubMed] [Google Scholar]
- Idrissi, F.Z. , Grotsch, H. , Fernandez‐Golbano, I.M. , Presciatto‐Baschong, C. , Riezman, H. and Geli, M.I. (2008) Distinct acto/myosin‐I structures associate with endocytic profiles at the plasma membrane. J. Cell Biol. 180, 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, J. , Goh, J. , Yoo, S. , Chi, M.H. , Choi, J. , Rho, H.S. , Park, J. , Han, S.S. , Kim, B.R. , Park, S.Y. , Kim, S. and Lee, Y.H. (2008) A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae . Mol. Plant–Microbe Interact. 21, 525–534. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M. , Sun, Y. and Drubin, D.G. (2003) A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell, 115, 475–487. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M. , Toret, C.P. and Drubin, D.G. (2006) Harnessing actin dynamics for clathrin‐mediated endocytosis. Nat. Rev. Mol. Cell Biol. 7, 404–414. [DOI] [PubMed] [Google Scholar]
- Kim, J.H. , Kim, H.W. , Heo, D.H. , Chang, M. , Baek, I.J. and Yun, C.W. (2009) FgEnd1 is a putative component of the endocytic machinery and mediates ferrichrome uptake in Fusarium graminearum . Curr. Genet. 55, 593–600. [DOI] [PubMed] [Google Scholar]
- Lee, K. , Singh, P. , Chung, W.C. , Ash, J. , Kim, T.S. , Hang, L. and Park, S. (2006) Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae . Fungal Genet. Biol. 43, 694–706. [DOI] [PubMed] [Google Scholar]
- Lee, S.C. , Schmidtke, S.N. , Dangott, L.J. and Shaw, B.D. (2008) Aspergillus nidulans ArfB plays a role in endocytosis and polarized growth. Eukaryot. Cell, 7, 1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S.J. , Myung, K. , Guse, D. , Donkin, B. , Proctor, R.H. , Grayburn, W.S. and Calvo, A.M. (2006) FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides . Mol. Microbiol. 62, 1418–1432. [DOI] [PubMed] [Google Scholar]
- Pelham, H.R. (1999) SNAREs and the secretory pathway—lessons from yeast. Exp. Cell Res. 247, 1–8. [DOI] [PubMed] [Google Scholar]
- Penalva, M.A. (2010) Endocytosis in filamentous fungi: Cinderella gets her reward. Curr. Opin. Microbiol. 13, 684–692. [DOI] [PubMed] [Google Scholar]
- Raths, S. , Rohrer, J. , Crausaz, F. and Riezman, H. (1993) end3 and end4: two mutants defective in receptor‐mediated and fluid‐phase endocytosis in Saccharomyces cerevisiae . J. Cell Biol. 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, N.D. and Kalkman, E.R. (2003) Does endocytosis occur in fungal hyphae? Fungal Genet. Biol. 39, 199–203. [DOI] [PubMed] [Google Scholar]
- Shaw, B.D. , Chung, D.W. , Wang, C.L. , Quintanilla, L.A. and Upadhyay, S. (2011) A role for endocytic recycling in hyphal growth. Fungal. Biol. 115, 541–546. [DOI] [PubMed] [Google Scholar]
- Shen, G. , Whittington, A. , Song, K. and Wang, P. (2010) Pleiotropic function of intersectin homologue Cin1 in Cryptococcus neoformans. Mol. Microbiol., 76, 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamnioti, P. , Henderson, C. , Zhang, Z. , Robinson, Z. and Gurr, S.J. (2007) A novel role for catalase B in the maintenance of fungal cell‐wall integrity during host invasion in the rice blast fungus Magnaporthe grisea . Mol. Plant–Microbe Interact. 20, 568–580. [DOI] [PubMed] [Google Scholar]
- Smythe, E. and Ayscough, K.R. (2003) The Ark1/Prk1 family of protein kinases. Regulators of endocytosis and the actin skeleton In: EMBO Rep. 4, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.W. , Dou, X.Y. , Qi, Z.Q. , Wang, Q. , Zhang, X. , Zhang, H.F. , Guo, M. , Dong, S.M. , Zhang, Z.G. , Wang, P. and Zheng, X.B. (2010) R‐SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of Magnaporthe oryzae . PLoS ONE, 5, e13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, G. (2007) On the move: endosomes in fungal growth and pathogenicity. Nat. Rev. Microbiol. 5, 309–316. [DOI] [PubMed] [Google Scholar]
- Talbot, N.J. and Foster, A.J. (2001) Genetics and genomics of the rice blast fungus Magnaporthe grisea: developing an experimental model for understanding fungal diseases of cereals. Adv. Bot. Res. 34, 263–287. [Google Scholar]
- Thines, E. , Weber, R.W. and Talbot, N.J. (2000) MAP kinase and protein kinase A‐dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea . Plant Cell, 12, 1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toret, C.P. and Drubin, D.G. (2006) The budding yeast endocytic pathway. J. Cell Sci. 119, 4585–4587. [DOI] [PubMed] [Google Scholar]
- Upadhyay, S. and Shaw, B.D. (2008) The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans . Mol. Microbiol. 68, 690–705. [DOI] [PubMed] [Google Scholar]
- Valent, B. , Farrall, L. and Chumley, F.G. (1991) Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics, 127, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin, J. , Bartnicki‐Garcia, S. and Riquelme, M. (2009) Functional stratification of the Spitzenkörper of Neurospora crassa . Mol. Microbiol. 74, 1044–1053. [DOI] [PubMed] [Google Scholar]
- Virag, A. and Harris, S.D. (2006) The Spitzenkörper: a molecular perspective. Mycol. Res. 110, 4–13. [DOI] [PubMed] [Google Scholar]
- Wang, P. and Shen, G. (2011) The endocytic adaptor proteins of pathogenic fungi: charting new and familiar pathways. Med. Mycol. 49, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.F. , Liu, K.Y. , Zhang, X. , Song, W.W. , Zhao, Q. , Dong, Y.H. , Guo, M. , Zheng, X.B. and Zhang, Z.G. (2010) A two‐component histidine kinase, MoSLN1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae . Curr. Genet. 56, 517–528. [DOI] [PubMed] [Google Scholar]
- Zhang, H.F. , Liu, K.Y. , Zhang, X. , Tang, W. , Wang, J.S. , Guo, M. , Zhao, Q. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2011a) Two phosphodiesterase genes, PDEL and PDEH, regulate development and pathogenicity by modulating intracellular cyclic AMP levels in Magnaporthe oryzae . PLoS ONE, 6, e17241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.F. , Tang, W. , Liu, K.Y. , Huang, Q. , Zhang, X. , Yan, X. , Chen, Y. , Wang, J.S. , Qi, Z.Q. , Wang, Z.Y. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2011b) Eight RGS and RGS‐like proteins orchestrate growth, differentiation, and pathogenicity of Magnaporthe oryzae . PLoS Pathog. 7, e1002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.S. , Lv, R.L. , Dou, X.Y. , Qi, Z.Q. , Hua, C.L. , Zhang, H.F. , Wang, Z.Y. , Zheng, X.B. and Zhang, Z.G. (2011c) The function of MoGlk1 in integration of glucose and ammonium utilization in Magnaporthe oryzae . PLoS ONE, 6, e22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Magnaporthe oryzae MoArk1 is a fungal actin‐regulating kinase 1 (Ark1) protein. (A) Prediction of domains of MoArk1 using the SMART website. (B) Phylogenetic analysis of MoArk1 and other Ark1 homologues, including MoArk1 (Magnaporthe oryzae, XP_003713889.1), Neurospora crassa (XP_962918.1), Fusarium graminearum (EGU75522.17), Aspergillus nidulans (XP_661798.1), Saccharomyces cerevisiae (NP_014378.1), Saccharomyces cerevisiae (NP_012171.1), Candida albicans (XP_446476.1), Ustilago maydis (XP_759228.1) and Saccharomyces cerevisiae (NP_009615.1). Sequence alignments were performed using the Clustal_W program, and the calculated phylogenetic tree was viewed using the Mega3.1 Beta program. Neighbour‐joining tree with 1000 bootstrap replicates of phylogenetic relationships among fungal Ark1 homologues. Numbers above the branches represent amino acid substitution.
Fig. S2 Targeted gene replacement and complementation. (A) Restriction map of the MoARK1 genomic region. Disruption construct containing the homologous sequences flanking the HPH cassette to replace a part of MoARK1. (B) Expression analysis of MoARK1 in the wild‐type, ΔMoark1 mutant and complemented strains using Actin as a control. (C) Southern blot analysis of the ΔMoark1 mutant (lanes 1 and 2) and the wild‐type (lane 3) strains using MoARK1‐ or HPH‐specific probes. Genomic DNA was digested with EcoRI.
Fig. S3 Assessment of tolerance to salt and osmotic stresses. (A) The ΔMoark1 mutant, wild‐type and complemented strains were incubated on complete medium (CM) plates containing KCl, NaCl and sorbitol at various concentrations at 28 °C for 7 days. (B) Colony inhibition rate measurement. Inhibition rate = [(diameter of untreated strain—diameter of treated strain)/diameter of untreated strain] × 100%. Error bars represent the standard deviations and asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.05, according to Duncan's range test.
Fig. S4 Responses of wild‐type, ΔMoark1 and ΔMoark1/MoARK1 strains to cell wall‐disturbing agents. (A, C) The ΔMoark1 mutant, wild‐type and complemented strains were incubated on complete medium (CM) plates supplemented with various stress inducers for 7 days at 28 °C. (B, D, E) Statistical analysis of the growth inhibition rate of mycelia on CM supplemented with cell wall‐disturbing agents. Double asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.01, and single asterisk indicates a significant difference at P < 0.05.
Fig. S5 MoARK1 is related to tolerance to H2O2 and the activity of extracellular peroxidase. (A) ΔMoark1 mutants were less sensitive to a higher concentration of H2O2. The Guy11, ΔMoark1 mutants and complemented strain were incubated on complete medium (CM) plates supplemented with 2.5 and 5 mm H2O2 for 6 days before being photographed. (B) Statistical analysis of the growth inhibition rate of mycelial growth on CM supplemented with H2O2. Asterisks indicate a significant difference between the mutants and wild‐type strain at P < 0.01, according to Duncan's range test. (C) The discoloration of Congo Red was tested on CM plates containing 200 μg/mL of the dye at a final concentration. Discoloration was observed on day 7 after inoculation at 28 °C. (D) Peroxidase activity was measured using the ABTS oxidizing test under normal and H2O2‐supplemented conditions. (E) Expression profiles of five genes encoding predicted peroxidases under normal or oxidative stress‐induced conditions.
Table S1 Primers used in this study.


