Abstract
Pulmonary vascular loss is an early feature of chronic obstructive pulmonary disease. Biomarkers of inflammation and of metabolic syndrome, predicts loss of lung function in World Trade Center Lung Injury (WTC-LI). We investigated if other cardiovascular disease (CVD) biomarkers also predicted WTC-LI.
This nested case-cohort study used 801 never smoker, WTC exposed firefighters with normal pre-9/11 lung function presenting for subspecialty pulmonary evaluation (SPE) before March, 2008. A representative sub-cohort of 124/801 with serum drawn within six months of 9/11 defined CVD biomarker distribution. Post-9/11/01 FEV1 at subspecialty exam defined cases: susceptible WTC-LI cases with FEV1≤77% predicted (66/801) and resistant WTC-LI cases with FEV1≥107% (68/801). All models were adjusted for WTC exposure intensity, BMI at SPE, age at 9/11, and pre-9/11 FEV1.
Susceptible WTC-LI cases had higher levels of Apo-AII, CRP, and MIP-4 with significant RRs of 3.85, 3.93, and 0.26 respectively with an area under the curve (AUC) of 0.858. Resistant WTC-LI cases had significantly higher sVCAM and lower MPO with RRs of 2.24, and 2.89 respectively; AUC 0.830.
Biomarkers of CVD in serum six-month post-9/11 predicted either susceptibility or resistance to WTC-LI. These biomarkers may define pathways producing or protecting subjects from pulmonary vascular disease and associated loss of lung function after an irritant exposure.
Keywords: Airway Inflammation, Cytokines, Pulmonary Funtion Testing
Introduction
One of the hallmarks of particulate matter (PM) exposure is systemic inflammation, endothelial dysfunction, and subsequent end-organ damage. (1–5) Epidemiologic investigation has documented associations between increased ambient PM, lung disease, and cardiovascular disease (CVD). (6–12) Inflammation and remodeling are key features of airflow obstruction in asthma and chronic obstructive pulmonary disease (COPD). (13, 14) High ambient PM exposures significantly decrease forced expiratory volume in one second (FEV1) even after five to seven days. (15–17) Systemic inflammation produces vascular endothelial injury and subsequent CVD. (18–20) Recent studies associate systemic vascular involvement with lung disease (21, 22) and prospective studies have demonstrated an association between impaired lung function and central arterial stiffness even before the development of CVD, with systemic inflammation contributing to this association. (23–25)
Recently, we have shown that biomarkers of inflammation (GM-CSF, MDC) and of the metabolic syndrome, observed in serum drawn within six months of WTC exposure, predict the post-9/11 decline in FEV1 in this cohort of WTC-exposed FDNY firefighters. (3, 17) Utilizing a nested case-cohort design, the goal of this study is to investigate if CVD serum biomarkers drawn within six months of 9/11/01 can predict WTC-LI in this longitudinal well phenotyped cohort of FDNY firefighters. We hypothesized that individuals exposed to WTC particulates who went on to develop persistent WTC-LI, would express different levels of CVD biomarkers than those similarly exposed individuals who were resistant to developing WTC-LI.
The collapse of the WTC exposed tens of thousands of people to extremely high PM concentrations. Several cohorts of WTC-exposed individuals have been longitudinally followed. Among exposed workers, volunteers, and lower Manhattan residents, abnormal spirometry has been a common finding. (26) In rescue/recovery workers from the Fire Department of the City of New York (FDNY), WTC exposure led to WTC lung injury (WTC-LI) as evidenced by substantial declines in pulmonary function in the first six months after 9/11 (at a rate twelve times greater than that found prior to 9/11) (27, 28) and these findings persisted over the next 6.5 years. (29)
Pulmonary vascular injury occurs early in smoking related chronic obstructive lung disease (COPD) with pulmonary perfusion abnormalities and reduced blood return to the heart observed prior to development of abnormal FEV1. (30, 31) Similar pathophysiology likely occurs in irritant induced COPD. Pulmonary arteriopathy was present in 58% of lung biopsies from non-FDNY WTC exposed individuals and in 74% with constrictive bronchiolitis after inhalational exposures during military service in Iraq and Afghanistan. (32, 33)
Methods
Study Participants
WTC exposed FDNY firefighters entered the FDNY-Medical Monitoring and Treatment Program (MMTP) and had spirometry at medical monitoring entry (MME). (34, 35) Briefly, FDNY Spirometry was performed according to American Thoracic Society/ European Respiratory Society (ATS/ERS) guidelines using Portascreen Spirometry (S&M Instruments, Doylestown, PA). (36) In order to provide three acceptable spirograms, seated workers wearing nose clips performed up to 8 forced expiratory maneuvers per testing session. Subspecialty pulmonary examination (SPE) pulmonary function tests (PFTs) were performed according to ATS/ERS guidelines using a Jaeger Masterscreen PFT System (Viasys Healthcare, Yorba Linda, CA). All acceptable measures were expressed in absolute values (liters) and as percent predicted of normal
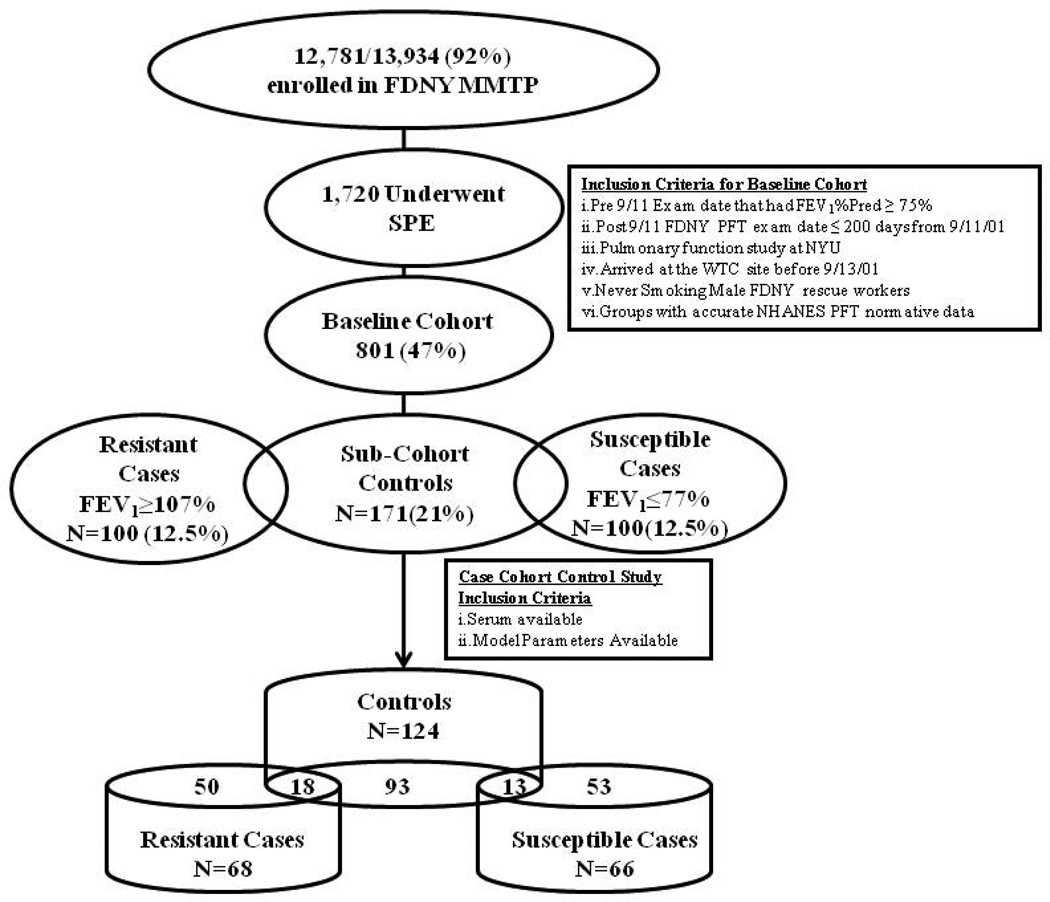
The study cohort was derived from symptomatic subjects referred for SPE (N=1720) between 10/1/2001 and 3/10/2008. (37) The baseline cohort consisted of never-smokers (consistently reported not smoking on all health screens), male, had reliable National Health and Nutrition Examination Survey (NHANES) normative data for predicted FEV1, had post-9/11 FDNY PFTs within 200 days of 9/11/01 and had pre-9/11 FEV1 >75% predicted (801/1720, 47%). All subjects signed informed Institutional Review Board approved consent at the time of enrollment allowing analysis of their information and samples for research (Montefiore Medical Center; #07-09-320 and New York University; #11-00439).
Cases were defined by their FEV1% Predicted at SPE using NHANES III criteria. Those resistant to WTC-LI, were defined as being within one standard deviation (SD) of the highest FEV1% predicted of the study cohort (N=100) had an FEV1≥107%. Those susceptible to WTC-LI were similarly defined as being within one SD of the lowest FEV1% predicted of the cohort (N=100), which was an FEV1≤77%. The sub-cohort controls (N=171) were randomly selected from the study cohort after stratification based on BMI and FEV1% Predicted at MME. Serum biomarkers were available for N=124/171 of the sub-cohort controls, N=68/100 resistant cases, and 66/100 susceptible cases.
Demographics
Age, gender, and years of service at FDNY were obtained from the FDNY-WTC-monitoring database. BMIs were calculated from height and weight measured at the time of MME and SPE. Degree of exposure was self-reported at the first FDNY-WTC-monitoring and was categorized using the FDNY-WTC Exposure Intensity Index (Arrival Time): i. Present on the morning of 9/11/2001 ii. Arrived after noon on 9/11/2001 iii. Arrived on 9/12/2001. Those arriving after day three were excluded from analysis as a result of their low numbers in this sample. (3, 17)
Serum Sampling and Analysis
Blood was drawn at MME, from 10/29/2001-1/312002 for the subjects in this analysis. Samples were allowed to stand for one hour at room temperature before being centrifuged at 1,800 g for ten minutes. Serum was stored at −80°C (Bio-Reference Laboratories, Inc. Elmwood Park, NJ). Serum was thawed once at four degrees and assayed using CVD-1 (HCVD1-67AK), Apolipoproteins (APO-62K), and Neurodegenerative (HNDG2-36K) panels according to manufacturer’s instructions (Millipore, Billerica, MA) on a Luminex 200IS (Luminex Corporation, Austin, TX). Data analyzed with MasterPlex QT software (Version 1.2; MiraiBio, Inc.). Each batch of samples processed contained controls and cases in an approximate 12/7 ratio.
Statistical Analysis
SPSS 19 (IBM, Armonk, NY) used for all database management and statistics. Demographic information and analyte levels were compared by Mann-Whitney U or Kruskal-Wallis where appropriate. We used logistic regression analysis to estimate the CVD biomarkers relative risks for crude and single analyte models adjusted for the confounders of age at 9/11, BMI at SPE, exposure group, and pre-9/11 FEV1% predicted, Table 4. Relative risk was calculated by binary logistic regression. Cases (WTC-LI susceptible or resistant) were compared to the sub-cohort controls as the dichotomous outcome variable and cutpoints of analytes were used as the predictors. MPO <25th percentile, MIP-4 ≥50th percentile, CRP ≥2.45 mg/dl, and Apo-AII, sVCAM ≥75th were defined as cutpoints. (3, 38, 39) Models were adjusted for potential confounders; BMI at SPE, exposure group as categorical variable, age on 9/11, and FEV1% Predicted prior to 9/11 as a continuous variable. Hosmer-Lemeshow goodness-of-fit statistic was used as a statistical test of model adequacy. Receiver operator characteristic area under the curve (ROC AUC) was also quantified for each final model. For all statistical tests significance was assessed by p <0.05.
Table 4.
Relative Risk Models Predicting Resistance or Susceptibility to World Trade Center-Lung Injury
| Analyte | Cutpoint# ¶ | Sample Fraction | Relative Risk(95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Crude+ | Adjusted § | ||||
| RESISTANT | Single Analyte | MPO | ≤105.26 | 31/68 | 31/124 | 2.37(1.26–4.44) | 2.92(1.40–6.08) |
| sVCAM | ≥1568.65 | 30/68 | 31/124 | 2.51(1.34–4.71) | 2.26(1.10–4.64) | ||
| Multi-Analyte | MPO | ≤105.26 | 31/68 | 31/124 | 2.39(1.26–4.56) | 2.89(1.37–6.10) | |
| sVCAM | ≥1568.65 | 30/68 | 31/124 | 2.53(1.34–4.82) | 2.24(1.07–4.70) | ||
| AUC | 0.647(0.564–0.731) | 0.830(0.774–0.887) | |||||
| SUSCEPTIBLE | Single Analyte | CRP | ≥2.45 | 58/66 | 85/124 | 3.33(1.45–7.63) | 3.00(1.20–7.47) |
| MIP-4 | ≥194.90 | 18/66 | 59/124 | 0.45(0.24–0.84) | 0.37(0.18–0.77) | ||
| Apo AI | ≥4220.87 | 33/66 | 30/124 | 3.13(1.66–5.91) | 3.61(1.72–7.61) | ||
| Apo AII | ≥1772.87 | 36/66 | 30/124 | 3.76(1.99–7.10) | 4.04(1.94–8.40) | ||
| Apo CII | ≥398.44 | 29/66 | 31/124 | 2.35(1.25–4.43) | 2.69(1.29–5.62) | ||
| Apo CIII | ≥1058.87 | 29/66 | 31/124 | 2.35(1.25–4.43) | 2.71(1.29–5.70) | ||
| Apo E | ≥268.71 | 33/66 | 31/124 | 3.00(1.60–5.64) | 3.03(1.46–6.30) | ||
| Multi-Analyte | Apo AII | ≥1772.87 | 36/66 | 30/124 | 3.51(1.80–6.87) | 3.85(1.79–8.26) | |
| CRP | ≥2.45 | 58/66 | 85/124 | 4.01(1.65–9.79) | 3.93(1.43–10.79) | ||
| MIP-4 | ≥194.90 | 18/66 | 59/124 | 0.33(0.16–0.66) | 0.26(0.11–0.59) | ||
| AUC | 0.767(0.692–0.842) | 0.858(0.796–0.919) | |||||
MIP-4, sVCAM, and MPO in ng/mL, CRP in mg/dL, Apolipoproteins in µg/mL
Cutpoints were based on the 75th percentile of the Controls for all analytes except MPO (25th percentile) and CRP ≥2.45
Crude represents the risk without adjusting for any confounders
Adjusted: age at 9/11, BMI at SPE, exposure group and pre-9/11 FEV1% Predicted
Abbreviation Dictionary: Area Under the Curve(AUC)
Results
Demographics
We developed a sub-cohort control to represent the full spectrum of lung function response of the baseline cohort and two case definitions represented extremes of response to WTC dust and smoke (Figure 1). The demographics of all groups with serum available are summarized in Table 1. The control and case groups had similar WTC exposure; time from 9/11 to MME, time from 9/11 to SPE, years of service, and age at 9/11. BMI of susceptible cases was higher than controls or resistant cases at MME and SPE.
Figure 1.
Study Design
Table 1.
Demographics and FEV1 of Subspecialty Pulmonary Evaluation Cohorts
| Characteristic | Baseline Cohort N=801 |
Resistant+ N=68 |
Controls+ N=124 |
Susceptible+ N=66 |
|
|---|---|---|---|---|---|
| WTC Arrival Time# | Morning 9/11 | 197 (25) | 13(19) | 21(17) | 18 (27) |
| Afternoon 9/11 | 498 (62) | 48(71) | 88(71) | 36 (55) | |
| Anytime ≥ 9/12 | 106 (13) | 7(10) | 15 (12) | 12(18) | |
| 9/11 to Study, Months¶ | MME | 2.7 (2–3.8) | 2.0(2–3) | 2.5 (2–3) | 2.0 (2–3) |
| SPE | 33.8 (25–57) | 33.0(27–53) | 33.3 (25–55) | 32.0 (21–52) | |
| BMI, kg/m2¶ § | MME | 28.0 (26–30) | 27.3(26–29) | 28.0(26–30) | 29.1 (27–32) |
| SPE | 28.9 (27–31) | 27.6(26–30) | 29.0(27–31) | 30.3 (28–34) | |
| Years of Service at 9/11¶ƒ | 18.3(11–23) | 18.7(10–24) | 18.0 (11–22) | 18.8(14–23) | |
| Age at 9/11¶ | 40.0 (36–45) | 42.0(38–46) | 42.0 (37–46) | 40.5(36–46) | |
Expressed as N (%);
Expressed as Median (Inter Quartile Range);
p<0.05 by one-way ANOVA Kruskal Wallis between Susceptible, Resistant Cases, and Controls
BMI at MME: Resistant N=67
Years of Service: Controls: N=68; Resistant N=26; Susceptible: N=51
Abbreviation Dictionary: Medical Monitoring Entry (MME); Subspecialty Pulmonary Exam (SPE)
Lung Function
Susceptible cases had lower FEV1 and this correlated with DLCO and measures of airflow obstruction, including methacholine and bronchodilator response, Table 2. Lung function in resistant cases and sub-cohort controls increased from the MME to SPE (105% to 113% and 92% to 95% respectively) while the FEV1% predicted of affected cases continued to decline between the two pulmonary function tests (79% to 72%; p<0.001 all comparisons).
Table 2.
Pulmonary Function Testing#
| Resistant | Controls | Susceptible | pƒ | ||
|---|---|---|---|---|---|
| Pre 9/11 FEV1 | 117(107–124) | 102(92–112) | 89(82–96) | <0.001 | |
| MME FEV1 | 105(96–113) | 92(82–99) | 79(72–89) | <0.001 | |
| FEV1 | 113(109–118) | 95(84–103) | 72(67–75) | <0.001 | |
| MCT Slope¶ | 0.039(0.02–0.07) | 0.048(0.03–0.12) | 0.15(0.05–1.8) | <0.001 | |
| SPE | DLCO % Predicted+ | 119(108–135) | 106(99–116) | 95(84–107) | <0.001 |
| BD Response§ | 4(2–8) | 5(2–10) | 15(7–28) | <0.001 | |
Values Expressed as Median (Inter Quartile Range)
MCT Slope: Controls N=94; Resistant Cases N=57; Susceptible Cases N=32
DLCO % Predicted: Controls N=59; Resistant Cases N=24; Susceptible Cases N=44
BD Response : Controls N=64; Resistant Cases N=26; Susceptible Cases=48
Significance assessed by one-way ANOVA Kruskal Wallis between Resistant, Susceptible Cases and Controls
Abbreviation Dictionary: MCT: Methacholine Challenge Test; BD: Bronchodilator; DLCO: Diffusion Capacity of the Lung for Carbon Monoxide; FEV1, Forced Expiratory Capacity in 1 second
CVD Biomarkers
We measured CVD serum biomarkers expressed within six months of 9/11/2001. Compared to sub-cohort controls, cases susceptible to WTC-LI had significant elevations of Apolipoprotein (Apo) AI (4273.98 vs. 2569.94, p=0.003), Apo AII (1830.96 vs. 845.12, p=0.002), Apo CII (305.80 vs. 203.18, p=0.006), Apo CIII (973.58 v 600.91, p=0.005), and Apo E (257.43 vs. 140.71, p=0.001), and significantly lower levels of Macrophage InflammatoryProtein-4 (MIP-4) (145.28 vs. 184.68, p=0.015), Table 3A. Whereas resistant WTC-LI cases had significantly decreased levels of Myeloperoxidase (MPO) (117.94 vs.143.56, p=0.016) compared to sub-cohort controls, Table 3B (median concentration in ng/ml except for CRP is in mg/dL).
Table 3.
| A: Values of Significant Serum Analytes in Single logistic Regression Model | |||
|---|---|---|---|
| Analyte# ¶ | Susceptible (N=66) | Controls (N=124) | p+ |
| CRP | 6.28(3.28–13.16) | 5.10(1.99–11.19) | 0.151 |
| MIP-4 | 145.28(106.56–211.25) | 184.68(109.68–948.20) | 0.008 |
| Apo AI | 4273.98(2039.29–6333.55) | 2569.94(1833.34–4198.31) | 0.003 |
| Apo AII | 1830.96(570.65–2594.51) | 845.12(428.00–1730.77) | 0.002 |
| Apo CII | 263.82(11.59–1794.55) | 165.01(LLOD-757.13) | 0.007 |
| Apo CIII | 305.80(157.92–646.63) | 203.18(112.44–398.44) | 0.005 |
| Apo E | 973.58(454.16–1749.37) | 600.91(311.08–1058.87) | 0.001 |
| B: Values of Significant Serum Analytes in Single logistic Regression Model | |||
|---|---|---|---|
| Analyte# ¶ | Resistant (N=68) | Controls (N=124) | p+ |
| MPO | 117.94(68.01–176.91) | 143.56(105.26–212.31) | 0.016 |
| sVCAM | 1500.83(1126.57–1808.16) | 1349.97(1126.57–1568.65) | 0.103 |
Expressed as Median(Inter Quartile Range);
MIP-4 in ng/mL, CRP in µg/dL, Apolipoproteins in µg/mL
p<0.05 by Mann-Whitney U-test between Susceptible Cases and Controls
Abbreviation Dictionary: CRP: C-Reactive Protein; MIP-4: Macrophage Inhibitory Proten-4; Apo: Apolipoprotein; LLOD: Lower Limit of Detection
Expressed as Median(Inter Quartile Range);
Expressed in ng/mL
p<0.05 by Mann-Whitney U-test between Resistant Cases and Controls
Abbreviation Dictionary: MPO: Myeloperoxidase; sVCAM: soluble Intracellular Adhesion Molecule
Resistant WTC-LI Cases: Development of Relative Risk Models
Univariate Model
We found that MPO <25th%, sVCAM ≥75th% predicted cases resistant to WTC-LI in both crude and confounder adjusted models. Multivariate Model. In a multivariate model that combined sVCAM ≥1568.65 and MPO ≤105.26 both stayed significant predictors of resistance to WTC-LI RR=2.24 (95% CI 1.07–4.70) and RR=2.89 (95% CI 1.37–7.47) respectively, Table 4. The final model for resistance to lung injury had an AUC of 0.830 (0.774–0.887).
Susceptible WTC-LI Cases: Development of Relative Risk Models
Univariate Analysis
We found that CRP≥2.45, ApoAI, ApoAII, ApoCII, ApoCIII, and ApoE≥75th% and MIP-4≥50% as crude value, all significantly predicted the risk of having an FEV1≤77% at SPE in individuals who were exposed to WTC dust. CVD biomarkers predicted the risk of WTC-LI at SPE in both crude and confounder adjusted models, Table 4.
Multivariate Model
We then assessed the biomarkers ability in the final logistic regression model to predict case status after adjusting for confounders. We found that a three analyte model combining Apo AII, CRP, and MIP-4 optimally predicted the susceptible cases with ApoAII≥1772.87 RR=3.85 (95% CI, 1.79–8.26), CRP≥2.45 mg/dL, RR=3.93 (95% CI, 1.43–10.79), and MIP-4>194.90 ng/mL, RR=0.26 (95% CI, 0.11–0.59). The final model produced a receiver operator characteristic area under the curve (AUC) of 0.858 (0.79–0.919), Table 4. Apo-AII was used in the final model because it consistently produced the highest RR of any Apo when combined with another Apo and ApoAII made the other Apolipoproteins insignificant when combined together.
Discussion
We observed serum biomarkers classically associated with CVD to predict lung function after exposure to dust and smoke at the WTC site in FDNY non-smoking firefighters with normal pre-9/11 lung function. Firefighters with elevated sVCAM and low MPO levels within six months of 9/11/2001 recovered lung function returning to pre-9/11 values after an acute decline. Alternately, individuals with elevated ApoAII and CRP levels within six months of 9/11 had significantly increased risk of developing decreased lung function over the subsequent six years while elevated MIP-4 reduced the risk of susceptibility to decreased lung function.
Our observation that risk factors for vascular injury are also predictors of lung dysfunction is consistent with recent reports that perfusion abnormalities and reduced pulmonary blood flow occur prior to development of abnormal FEV1 in smokers at risk for COPD. In other WTC exposed cohorts pulmonary vasculopathy is a prominent feature. Vascular injury may be a prominent feature of irritant exposure since soldiers exposed to sand and products of combustion also had vasculopathy on pathology. CRP is known a marker of acute systemic inflammation and CVD. (40, 41) It is biologically plausible that processes that injure systemic arteries could also injure pulmonary arteries. There is an inverse relationship between serum CRP and FEV1. (42) CRP levels were elevated in individuals with COPD independent of any CVD risks. (43) Pulmonary hypertension another disease of the pulmonary vasculature is associated with CVD biomarkers. (44) This parallels our prior observation that dyslipidemia predicts poor outcome after WTC dust exposure. MIP-4 (CCL18) is an early promoter of regulatory T cell differentiation and may generate an anti-inflammatory counter-regulatory response. (45, 46) Our data demonstrate that low levels of MPO demonstrate less neutrophil activation in patients resistant to the damaging effects of WTC dust exposure. Neutrophil activation is an important mediator of PM induced the pulmonary and cardiovascular vascular injury. (1, 47) Taken together, the data in this and other recent reports emphasize the need to better understand the mechanisms by which inhaled irritants damage pulmonary vessels.
This Case-cohort study design was utilized to assess the predictive abilities of CVD biomarkers in determining the relative risk (RR) of either being protected from or developing WTC-LI. (48–50) Sub-cohort controls, susceptible and resistant cases were all drawn from the larger baseline cohort. The sub-cohort controls contain an overlapping population of those who also met case definitions. The odds ratios approximate the RR of the biomarker-disease relationship of the larger cohort. In addition, case-cohort studies have several advantages. First, comparing multiple case definitions to the same sub-cohort control group facilitates the identification of biomarkers of susceptibility and resistance to WTC-LI. Second, the case-cohort studies are cost-effective and logistically efficient since biomarkers only needed to be assayed for a sample of the entire cohort. Lastly, the study design minimized batch bias and freeze-thaw problems associated with biomarker discovery. (48)
The study cohort was nested within a larger intensively evaluated, longitudinally followed symptomatic firefighters who presented for subspecialty pulmonary evaluation prior to March 2008. We chose to exclude ever-smokers to eliminate the confounding effect of smoking on lung function and CVD risk. We narrowed the baseline cohort of 801 to produce a sub-cohort control of 136 patients with serum available, a group large enough to be representative of the baseline cohort but small enough to allow cost effective measurement of biomarker expression. Since the control group represented the larger cohort, comparing biomarkers expression in cases to that in the sub cohort control permitted relative risk measures produced by the biomarkers. We used FEV1 for case definition because it is the best single measure of lung function available on the entire FDNY cohort at each point of interest. Cases resistant to WTC-LI were defined by FEV1 in the top 12.5 percentile while susceptible cases were defined by FEV1 in the lowest 12.5 percentile at SPE. This cohort had serial pulmonary function pre- and post-9/11/2001 thereby allowing us to define resistance or susceptibility to WTC-LI after a severe irritant exposure. Another unique aspect of the study is biomarker expression was measured within six months after 9/11/01 and prior to recovery from injury or the development of persistent WTC-LI.
Cases susceptible or resistant to WTC-LI and the sub-cohort controls experienced similar exposure to smoke and dust, which led to declines in their FEV1 within six months post 9/11. This suggests a similar response to acute irritant exposure. However, protected cases and cohort controls maintained normal lung function while affected cases developed abnormal FEV1 by SPE. One explanation for this may be that the WTC-LI cases had significantly lower pre-9/11 FEV1. Cases therefore needed a smaller relative decline to drop below 77% post-9/11. We adjusted for this potential confounder by adding pre-9/11 FEV1 to all models. Predictors of resistance and susceptibility were not strongly affected by pre-9/11 FEV1 demonstrating the serum biomarker effect was independent of the pre-exposure lung function. Similarly, increased BMI is well known to be associated with reduced FEV1, but adjusting for this potential confounder did not alter the ability of the serum biomarkers to predict recovery or decline in lung function. This demonstrates an inflammatory process in susceptible lung injury cases that produces persistent loss of lung function for years after the insult. On the other hand, resistant cases showed long-term protection from WTC-LI.
There are several limitations to this study. It’s a single unique cohort of FDNY firefighters with serum samples available from 10/29/2001-1/31/2002. This limits the generalizability of these finding to other WTC exposed cohorts who likely include individuals with pre-existing lung disease or other risk factors such as smoking. Definitions of susceptible and resistant cases were based on FEV1 measured at SPE. We believe that low FEV1 is the single best outcome measure to define lung injury in the FDNY cohort because FEV1 has been longitudinally measured starting three years prior to 9/11/2001 and continues to be performed at every FDNY-WTC-MMTP. However, using FEV1 as a single measure of lung function could lead to non-differential misclassification. Since FEV1 is reduced in both restriction and obstruction low FEV1 doesn’t distinguish between the two. In prior investigation, we have observed that obstruction caused the vast majority of abnormal FEV1 in WTC exposed fire fighters. (37) In addition, patients with accelerated decline in FEV1 who will progress to disease but currently have a normal FEV1 will be misclassified as controls. While misclassification may occur when using FEV1<77% as a single measure of abnormal lung function, heterogeneity of disease(s) produced by this single measure will bias the results toward the null. In spite of the potential for non-differential information bias, using low FEV1 has yielded strong biomarkers-disease associations. (3, 39) Since all the FDNY firefighters were exposed to WTC dust, we do not have unexposed control group to compare and therefore we cannot determine if WTC exposure is necessary for the observed effect. Replication of these findings in other longitudinally followed populations with and without PM exposure will be important to demonstrate the generalizability of these findings.
Utilizing a nested case-cohort design, we were able to identify CVD serum biomarkers drawn within six months of 9/11/01 that predicted if an exposed individual was likely to recover lung function or progress to lung disease. These biomarkers were expressed during disease evolution and so reflect processes leading to WTC-LI susceptibility or resistance. The observation that CVD biomarkers predict changes in lung function is consistent with a growing body of evidence that pulmonary vascular disease occurs early in COPD. This study emphasizes the utility of serum stored in the aftermath of a disaster. This insight on protein expression may guide future mechanistic and therapeutic studies designed to blunt the impact of the world wide COPD epidemic.
Acknowledgements
We are thankful to the FDNY rescue workers for their selfless dedication.
Funding: K23HL084191 (AN), K24A1080298 (MDW), RO1HL057879; (MDW), HL090316, Al080298A, TL1RR029892; T32 ES007267 (BN, SJC); U01CA008617, RO1HL090316 (WNR), NIOSH/CDC (U10-OH008243, U10-OH008242), and 1 UL1RR029893.
Footnotes
Disclosures: The authors of this manuscript have no actual or potential conflicts of interest to disclose.
References
- 1.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. The New England journal of medicine. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. Epub 2007/09/15. [DOI] [PubMed] [Google Scholar]
- 2.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. American journal of respiratory and critical care medicine. 2007;176(4):395–400. doi: 10.1164/rccm.200606-872OC. Epub 2007/04/21. [DOI] [PubMed] [Google Scholar]
- 3.Naveed B, Weiden MD, Kwon S, Gracely EJ, Comfort AL, Ferrier N, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. American journal of respiratory and critical care medicine. 2012;185(4):392–399. doi: 10.1164/rccm.201109-1672OC. Epub 2011/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61(1):17–22. doi: 10.1136/thx.2005.041996. Epub 2005/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. Epub 2004/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 7.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, et al. Exposure to traffic and the onset of myocardial infarction. The New England journal of medicine. 2004;351(17):1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 8.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke; a journal of cerebral circulation. 2005;36(12):2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 9.Wellenius GA, Yeh GY, Coull BA, Suh HH, Phillips RS, Mittleman MA. Effects of ambient air pollution on functional status in patients with chronic congestive heart failure: a repeated-measures study. Environ Health. 2007;6:26. doi: 10.1186/1476-069X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellenius GA, Coull BA, Batalha JR, Diaz EA, Lawrence J, Godleski JJ. Effects of ambient particles and carbon monoxide on supraventricular arrhythmias in a rat model of myocardial infarction. Inhalation toxicology. 2006;18(14):1077–1082. doi: 10.1080/08958370600945473. [DOI] [PubMed] [Google Scholar]
- 11.Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. The American journal of cardiology. 2006;97(3):404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 12.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. Epub 2006/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. The New England journal of medicine. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. Epub 2009/06/06. [DOI] [PubMed] [Google Scholar]
- 14.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. The Journal of allergy and clinical immunology. 2003;111(2):215–225. doi: 10.1067/mai.2003.128. quiz 26. Epub 2003/02/18. [DOI] [PubMed] [Google Scholar]
- 15.Hoek G, Brunekreef B. Acute effects of a winter air pollution episode on pulmonary function and respiratory symptoms of children. Archives of environmental health. 1993;48(5):328–335. doi: 10.1080/00039896.1993.9936721. Epub 1993/09/01. [DOI] [PubMed] [Google Scholar]
- 16.Brunekreef B, Hoek G. The relationship between low-level air pollution exposure and short-term changes in lung function in Dutch children. Journal of exposure analysis and environmental epidemiology. 1993;3(Suppl 1):117–128. Epub 1993/01/01. [PubMed] [Google Scholar]
- 17.Kumar S, Aldrich K. Overcoming barriers to electronic medical record (EMR) implementation in the US healthcare system: A comparative study. Health informatics journal. 2010;16(4):306–318. doi: 10.1177/1460458210380523. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 18.Koenig W. Inflammation and coronary heart disease: an overview. Cardiology in review. 2001;9(1):31–35. doi: 10.1097/00045415-200101000-00007. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmeister A, Rothenbacher D, Bazner U, Frohlich M, Brenner H, Hombach V, et al. Role of novel markers of inflammation in patients with stable coronary heart disease. The American journal of cardiology. 2001;87(3):262–266. doi: 10.1016/s0002-9149(00)01355-2. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 20.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. Epub 2003/03/26. [DOI] [PubMed] [Google Scholar]
- 21.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, et al. An animal model of autoimmune emphysema. American journal of respiratory and critical care medicine. 2005;171(7):734–742. doi: 10.1164/rccm.200409-1275OC. Epub 2004/11/26. [DOI] [PubMed] [Google Scholar]
- 22.Voelkel N, Taraseviciene-Stewart L. Emphysema: an autoimmune vascular disease? Proceedings of the American Thoracic Society. 2005;2(1):23–25. doi: 10.1513/pats.200405-033MS. Epub 2005/08/23. [DOI] [PubMed] [Google Scholar]
- 23.Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, et al. Reduced pulmonary function is associated with central arterial stiffness in men. American journal of respiratory and critical care medicine. 2001;164(12):2181–2185. doi: 10.1164/ajrccm.164.12.2107137. Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]
- 24.Tockman MS, Pearson JD, Fleg JL, Metter EJ, Kao SY, Rampal KG, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. American journal of respiratory and critical care medicine. 1995;151(2 Pt 1):390–398. doi: 10.1164/ajrccm.151.2.7842197. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 25.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. Epub 2008/06/27. [DOI] [PubMed] [Google Scholar]
- 26.Friedman SM, Maslow CB, Reibman J, Pillai PS, Goldring RM, Farfel MR, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. American journal of respiratory and critical care medicine. 2011;184(5):582–589. doi: 10.1164/rccm.201011-1909OC. Epub 2011/06/07. [DOI] [PubMed] [Google Scholar]
- 27.Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, Christodoulou V, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. American journal of respiratory and critical care medicine. 2006;174(3):312–319. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. The New England journal of medicine. 2002;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 29.Sikora AL, Wilson DJ, Aldrich CC, Blanchard JS. Kinetic and inhibition studies of dihydroxybenzoate-AMP ligase from Escherichia coli. Biochemistry. 2010;49(17):3648–3657. doi: 10.1021/bi100350c. Epub 2010/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Roca J, Barbera JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 2009;106(6):1902–1908. doi: 10.1152/japplphysiol.00085.2009. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 31.Liebow AA. Pulmonary emphysema with special reference to vascular changes. The American review of respiratory disease. 1959;80(1, Part 2):67–93. doi: 10.1164/arrd.1959.80.1P2.67. Epub 1959/07/01. [DOI] [PubMed] [Google Scholar]
- 32.Caplan-Shaw CE, Yee H, Rogers L, Abraham JL, Parsia SS, Naidich DP, et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med. 2011;53(9):981–991. doi: 10.1097/JOM.0b013e31822fff60. Epub 2011/08/24. [DOI] [PubMed] [Google Scholar]
- 33.King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr, Nian H, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222–230. doi: 10.1056/NEJMoa1101388. Epub 2011/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. Epub 2005/09/02. [DOI] [PubMed] [Google Scholar]
- 35.Herbert R, Moline J, Skloot G, Metzger K, Baron S, Luft B, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. Epub 2005/08/02. [DOI] [PubMed] [Google Scholar]
- 37.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, Gustave J, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574. doi: 10.1378/chest.09-1580. Epub 2009/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. The Journal of clinical investigation. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. Epub 2003/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, Kwon S, et al. Inflammatory Biomarkers Predict Airflow Obstruction After Exposure to World Trade Center Dust. Chest. 2011 doi: 10.1378/chest.11-1202. Epub 2011/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. The New England journal of medicine. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. Epub 2004/04/09. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. Epub 2000/03/25. [DOI] [PubMed] [Google Scholar]
- 42.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. American journal of epidemiology. 2002;155(9):842–848. doi: 10.1093/aje/155.9.842. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 43.Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61(1):23–28. doi: 10.1136/thx.2005.042200. Epub 2005/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuditskaya S, Tumblin A, Hoehn GT, Wang G, Drake SK, Xu X, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113(5):1122–1128. doi: 10.1182/blood-2008-03-142604. Epub 2008/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vulcano M, Struyf S, Scapini P, Cassatella M, Bernasconi S, Bonecchi R, et al. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170(7):3843–3849. doi: 10.4049/jimmunol.170.7.3843. Epub 2003/03/21. [DOI] [PubMed] [Google Scholar]
- 46.Azzaoui I, Yahia SA, Chang Y, Vorng H, Morales O, Fan Y, et al. CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood. 2011;118(13):3549–3558. doi: 10.1182/blood-2011-02-338780. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 47.Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. American journal of respiratory and critical care medicine. 1999;159(3):702–709. doi: 10.1164/ajrccm.159.3.9709083. Epub 1999/03/02. [DOI] [PubMed] [Google Scholar]
- 48.Rundle AG, Vineis P, Ahsan H. Design options for molecular epidemiology research within cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1899–1907. doi: 10.1158/1055-9965.EPI-04-0860. Epub 2005/08/17. [DOI] [PubMed] [Google Scholar]
- 49.Miettinen O. Design options in epidemiologic research. An update. Scand J Work Environ Health. 1982;8(Suppl 1):7–14. Epub 1982/01/01. [PubMed] [Google Scholar]
- 50.Prentice RL. On the design of synthetic case-control studies. Biometrics. 1986;42(2):301–310. Epub 1986/06/01. [PubMed] [Google Scholar]